Establishment of a Visual Gene Chip Method for the Simultaneous Detection of Seven Waterfowl Virus Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains
2.2. Primers and Probes
2.3. Construction of Plasmid Standards
2.4. Multiplex PCR Amplification Design and Optimisation
2.4.1. Optimisation of the Annealing Temperature for the Sevenfold PCR Amplification Reaction
2.4.2. Optimisation of the Extension Times for the Sevenfold PCR Amplification Reactions
2.4.3. Optimisation of Primer Concentrations for the Sevenfold PCR Amplification Reactions
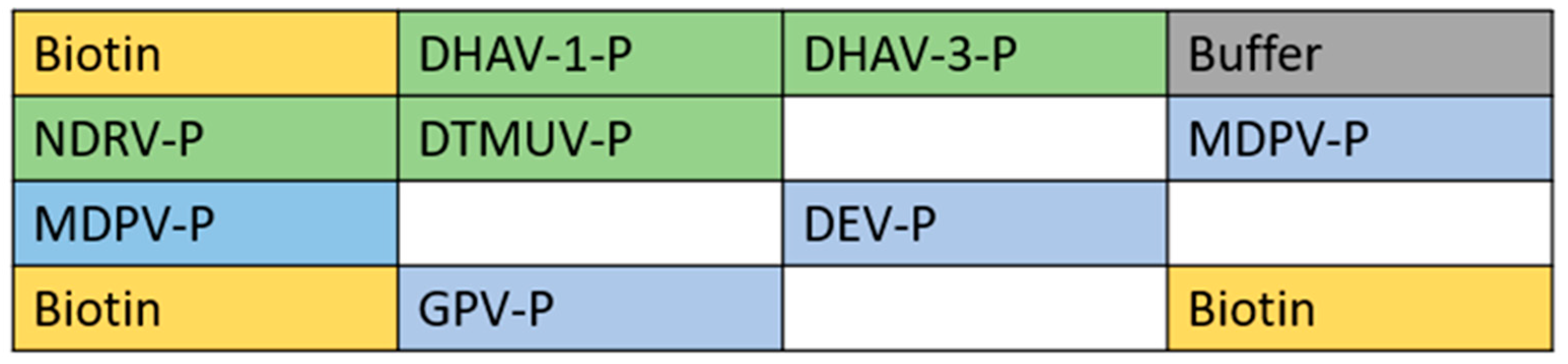
2.5. Preparation of Gene Chip
2.6. Determination of Test Results
2.7. Gene Chip Hybridisation Condition Optimisation
2.7.1. Gene Chip Hybridisation Time Optimisation
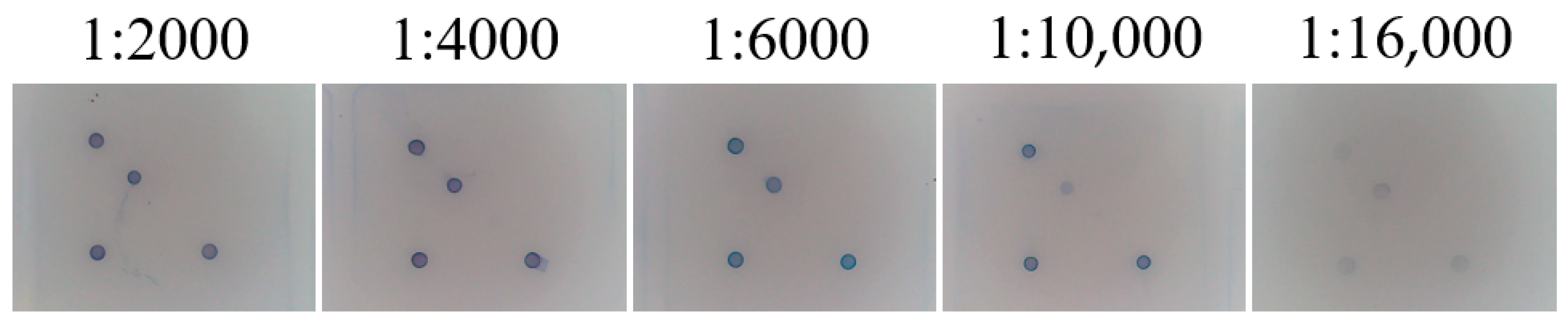
2.7.2. Optimisation of SA-HRP Dilution Concentration
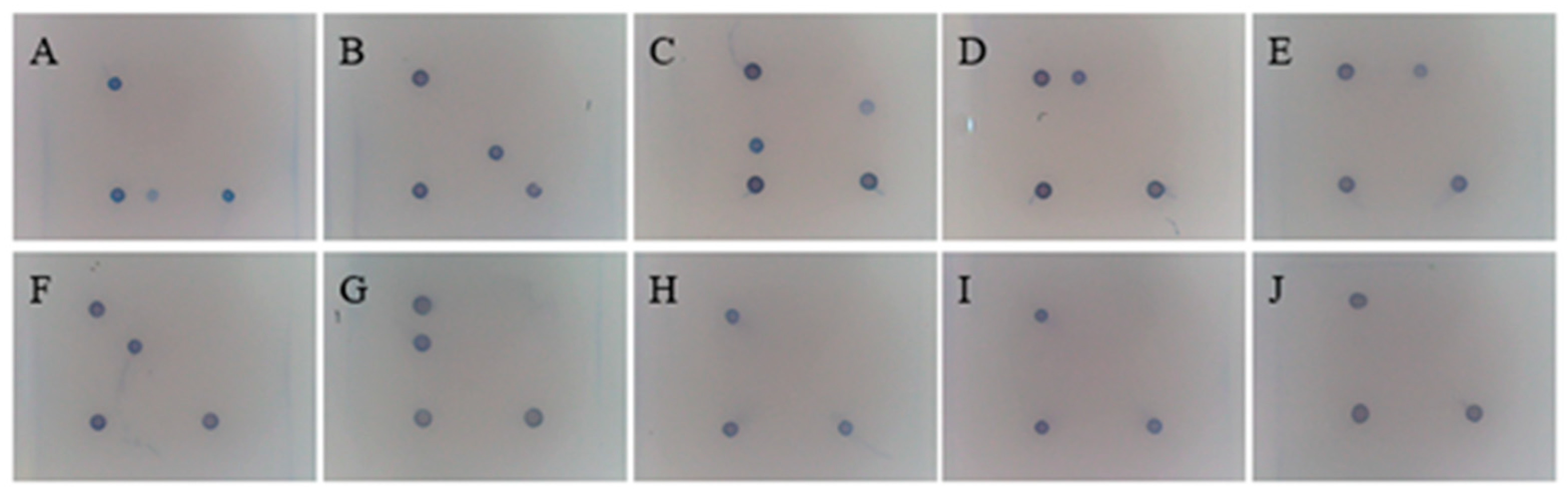
2.8. Specificity Testing of the Gene Chip
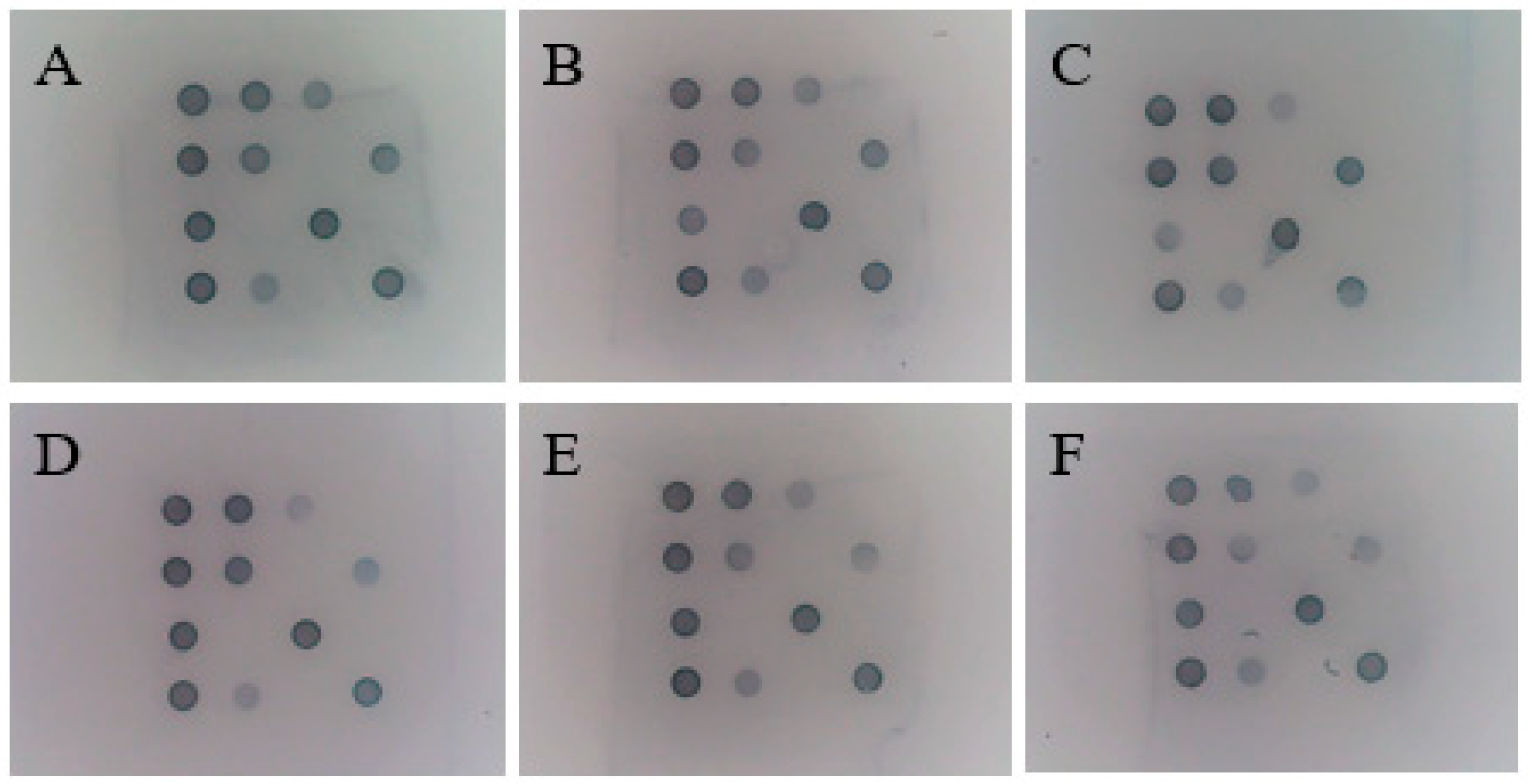
2.9. Sensitivity of Single Positive Plasmids in Gene Chip
2.10. Sensitivity of Gene Chip to Mixed-Positive Plasmids
2.11. Gene Chip Reproducibility Testing
2.12. Gene Chip Stability Testing
2.13. Clinical Sample Detection
3. Results
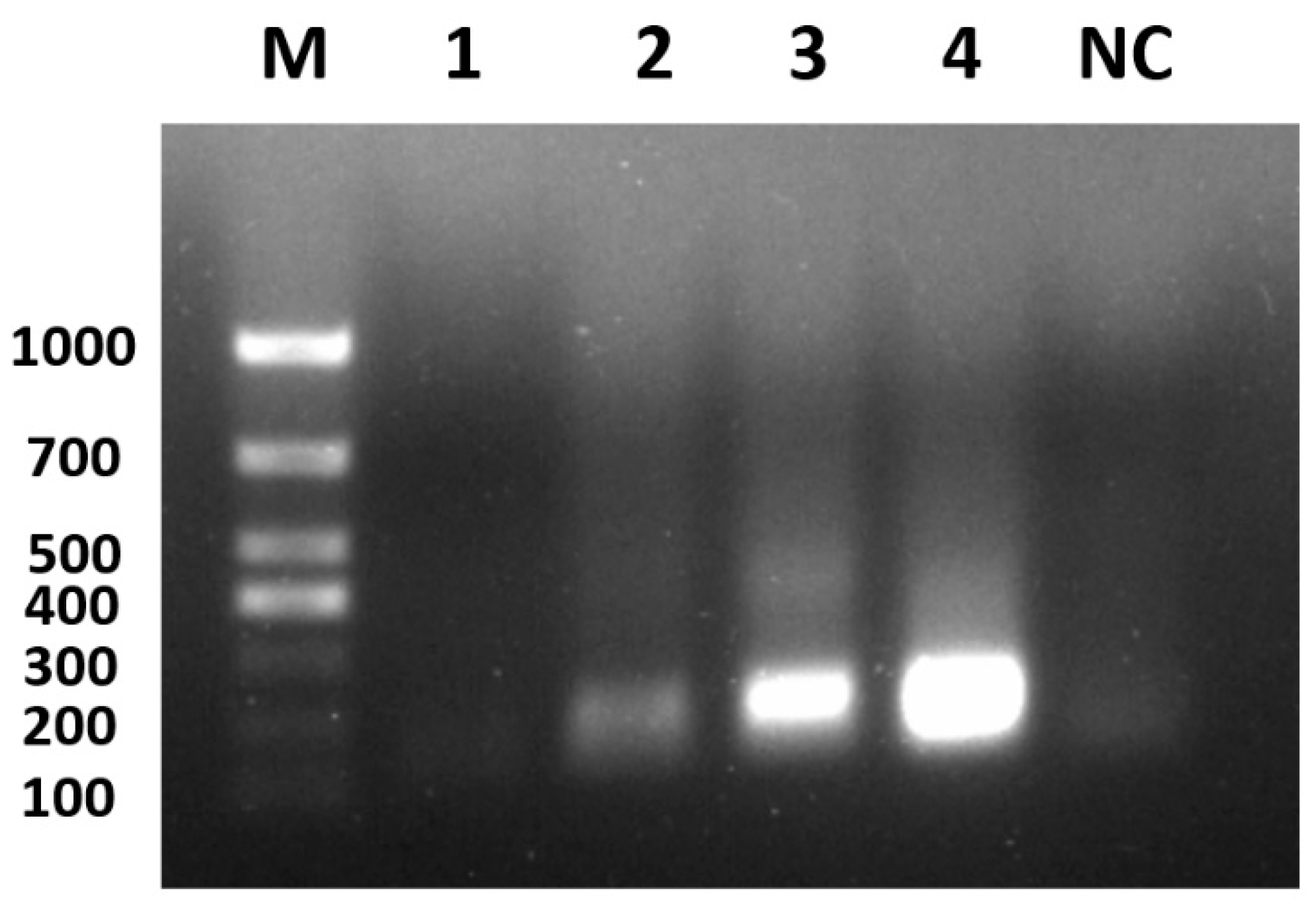
3.1. Recombinant Positive Plasmid Construction
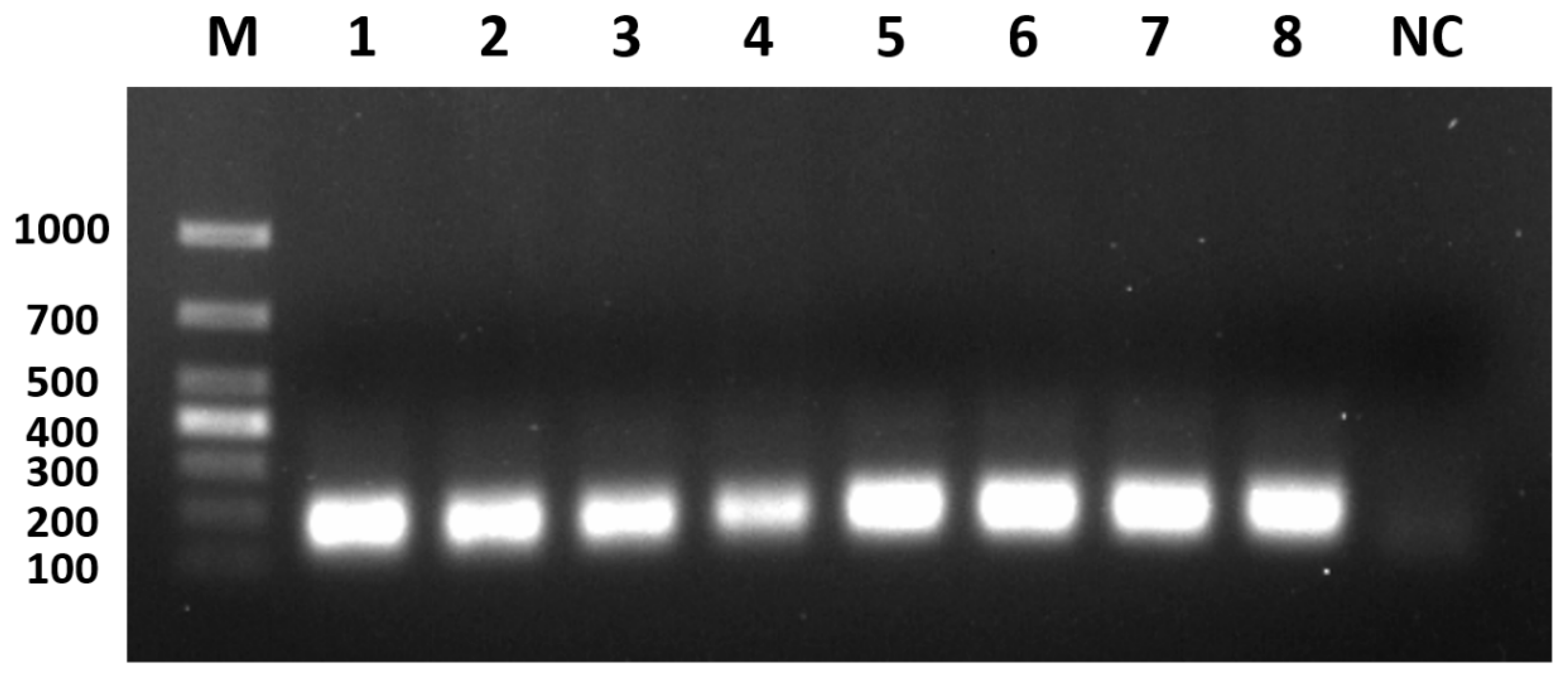
3.2. Optimisation of PCR Amplification Annealing Temperature
3.3. Optimisation of PCR Amplification Extension Times
3.4. Optimisation of Primer Concentrations for PCR Amplification
3.5. Optimisation of Hybridisation Times for Gene Chips
3.6. Optimisation of SA-HRP Concentration for Gene Chips
3.7. Specificity of the Gene Chip
3.8. Sensitivity of Single Plasmid Standards for Gene Chips
3.9. Sensitivity of Mixed Plasmid Standards for Gene Chips
3.10. Reproducibility of the Gene Chip
3.11. Stability of the Gene Chip
3.12. Comparison of Gene Chip Detection Methods with Real-Time PCR Methods
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Sabry, M.I.; Almasri, O. Global Waterfowl Production: Stocking Rate Is a Key Factor for Improving Productivity and Well-Being–A Review. Trop. Anim. Health Prod. 2023, 55, 419. [Google Scholar] [CrossRef] [PubMed]
- Zádori, Z.; Stefancsik, R.; Rauch, T.; Kisary, J. Analysis of the Complete Nucleotide Sequences of Goose and Muscovy Duck Parvoviruses Indicates Common Ancestral Origin with Adeno-Associated Virus 2. Virology 1995, 212, 562–573. [Google Scholar] [CrossRef]
- Takehara, K.; Hyakutake, K.; Imamura, T.; Mutoh, K.; Yoshimura, M. Isolation, Identification, and Plaque Titration of Parvovirus from Muscovy Ducks in Japan. Avian Dis. 1994, 38, 810–815. [Google Scholar] [CrossRef]
- Dhama, K.; Kumar, N.; Saminathan, M.; Tiwari, R.; Karthik, K.; Kumar, M.A.; Palanivelu, M.; Shabbir, M.Z.; Malik, Y.S.; Singh, R.K. Duck Virus Enteritis (Duck Plague)—A Comprehensive Update. Vet. Q. 2017, 37, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhu, D.; Cheng, A.; Wang, M.; Chen, S.; Jia, R.; Liu, M.; Sun, K.; Zhao, X.; Yang, Q.; et al. Molecular Epidemiology of Duck Hepatitis a Virus Types 1 and 3 in China, 2010–2015. Transbound. Emerg. Dis. 2018, 65, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xia, L.; Chen, J.; Gong, Y.; Zhang, L.; Li, P.; Liu, H.; Xie, Z.; Jiang, S. Molecular Epidemiology and Genetic Diversity of Duck Hepatitis a Virus Type 3 in Shandong Province of China, 2012–2014. Acta Virol. 2017, 61, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, S.; Hu, X.; Yu, X.; Wang, Y.; Liu, P.; Lu, X.; Zhang, G.; Hu, X.; Liu, D.; et al. Duck Egg-Drop Syndrome Caused by BYD Virus, a New Tembusu-Related Flavivirus. PLoS ONE 2011, 6, e18106. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, Y.; Zhang, X.; Xu, D.; Dai, X.; Teng, Q.; Yan, L.; Zhou, J.; Ji, X.; Zhang, S.; et al. An Infectious Disease of Ducks Caused by a Newly Emerged Tembusu Virus Strain in Mainland China. Virology 2011, 417, 1–8. [Google Scholar] [CrossRef]
- Varga-Kugler, R.; Marton, S.; Thuma, Á.; Szentpáli-Gavallér, K.; Bálint, Á.; Bányai, K. Candidate “Avian Orthoreovirus B”: An Emerging Waterfowl Pathogen in Europe and Asia? Transbound. Emerg. Dis. 2022, 69, e3386–e3392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, D.; Ning, K.; Liang, T.; Wang, M.; Jiang, M.; Zhang, D. A Duck Reovirus Variant with a Unique Deletion in the Sigma C Gene Exhibiting High Pathogenicity in Pekin Ducklings. Virus Res. 2016, 215, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Y.; Li, C.; Liu, G. Outbreak-Associated Novel Duck Reovirus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Cheng, L.; Chen, C.; Liu, R.; Shi, S.; Fu, G.; Chen, H.; Fu, Q.; Huang, Y. A Duplex PCR Assay for the Simultaneous Detection and Differentiation of Muscovy Duck Parvovirus and Goose Parvovirus. Mol. Cell. Probes 2019, 47, 101439. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Hong, W.; Wang, A.; Zuo, W. A Multiplex PCR for Detection of Six Viruses in Ducks. J. Virol. Methods 2017, 248, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; An, T.; Chen, H.; Wang, Y.; Zhu, L.; Yu, C.; Xia, C.; Zhang, H. Development and Application of Quadruplex Real Time Quantitative PCR Method for Differentiation of Muscovy Duck Parvovirus, Goose Parvovirus, Duck Circovirus, and Duck Adenovirus 3. Front. Cell. Infect. Microbiol. 2024, 14, 1448480. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.W.; Xiong, C.; Shi, K.C.; Xie, S.Y.; Long, F.; Li, J.; Zheng, M.; Wei, X.K.; Feng, S.; Qu, S.; et al. Development and Application of a Multiplex qPCR Assay for the Detection of Duck Circovirus, Duck Tembusu Virus, Muscovy Duck Reovirus, and New Duck Reovirus. Virus Genes 2023, 59, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xiong, C.; Shi, K.; Long, F.; Feng, S.; Qu, S.; Lu, W.; Huang, M.; Lin, C.; Sun, W.; et al. Multiplex Digital PCR: A Superior Technique to qPCR for the Simultaneous Detection of Duck Tembusu Virus, Duck Circovirus, and New Duck Reovirus. Front. Vet. Sci. 2023, 10, 1222789. [Google Scholar] [CrossRef]
- Bier, F.F.; von Nickisch-Rosenegk, M.; Ehrentreich-Förster, E.; Reiss, E.; Henkel, J.; Strehlow, R.; Andresen, D. DNA Microarrays. Adv. Biochem. Eng. Biotechnol. 2008, 109, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, E.R.; Srivastava, L.K.; Quirion, R. DNA Microarrays in Neuropsychopharmacology. Trends Pharmacol. Sci. 2001, 22, 426–436. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Golub, T.R. DNA Microarrays in Clinical Oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Shaw-Smith, C.; Redon, R.; Rickman, L.; Rio, M.; Willatt, L.; Fiegler, H.; Firth, H.; Sanlaville, D.; Winter, R.; Colleaux, L.; et al. Microarray Based Comparative Genomic Hybridisation (Array-CGH) Detects Submicroscopic Chromosomal Deletions and Duplications in Patients with Learning Disability/Mental Retardation and Dysmorphic Features. J. Med. Genet. 2004, 41, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.T.; Lai, L.; Li, H. Microarray Fabrication Techniques for Multiplexed Bioassay Applications. Anal. Biochem. 2023, 683, 115369. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Marla, S.S.; Bao, P.; Hagenow, S.; Mehta, H.; Lucas, A.; Garimella, V.; Patno, T.; Buckingham, W.; Cork, W.; et al. Gold Nanoparticle-Based Detection of Genomic DNA Targets on Microarrays Using a Novel Optical Detection System. Biosens. Bioelectron. 2004, 19, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Xu, Q.; Zhang, R.-H.; Yang, L.; Li, J.-X.; Xie, Z.-J.; Zhu, Y.-L.; Jiang, S.-J.; Si, X.-K. Improved Duplex RT-PCR Assay for Differential Diagnosis of Mixed Infection of Duck Hepatitis a Virus Type 1 and Type 3 in Ducklings. J. Virol. Methods 2013, 192, 12–17. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Bi, Z.; Liu, Y.; Meng, C.; Zhu, J.; Liu, G.; Li, C. Simultaneous Detection of Novel Goose Parvovirus and Novel Duck Reovirus by SYBR Green I-Based Duplex Real-Time Quantitative Polymerase Chain Reaction. 3 Biotech 2024, 14, 288. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Cheng, X.; Xiao, S.; Chen, X.; Chen, S.; Chen, S.; Yu, F. Development of a Duplex SYBR Green I-Based Quantitative Real-Time PCR Assay for the Rapid Differentiation of Goose and Muscovy Duck Parvoviruses. Virol. J. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Westin, L.; Miller, C.; Vollmer, D.; Canter, D.; Radtkey, R.; Nerenberg, M.; O’Connell, J.P. Antimicrobial Resistance and Bacterial Identification Utilizing a Microelectronic Chip Array. J. Clin. Microbiol. 2001, 39, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, X.; Xie, X.; Xu, Y.; Zhao, Z. Gene Chip Array for Differentiation of Mycobacterial Species and Detection of Drug Resistance. Chin. Med. J. 2012, 125, 3292–3297. [Google Scholar] [PubMed]
- Debouck, C.; Goodfellow, P.N. DNA Microarrays in Drug Discovery and Development. Nat. Genet. 1999, 21, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, D.L.; Jensen, R.V.; Gullans, S.R. Better Therapeutics through Microarrays. Nat. Genet. 2002, 32 (Suppl. 4), 547–551. [Google Scholar] [CrossRef]
- Gumanova, N.G.; Bogdanova, N.L.; Metelskaya, V.A. Proteomic Biomarker Evaluation Using Antibody Microarrays: Association between Analytical Methods Such as Microarray and ELISA. Lab. Med. 2024, 55, 325–333. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Yoon, C.W.; Lee, H.D.; Park, M.; Park, J.W. Efficient Protein-Ligand Interaction by Guaranteeing Mesospacing between Immobilized Biotins. Chem. Commun. Camb. Engl. 2004, 11, 1316–1317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krieg, R.; Halbhuber, K.J. Recent Advances in Catalytic Peroxidase Histochemistry. Cell. Mol. Biol. 2003, 49, 547–563. [Google Scholar]





| Virus | Primer and Probe | Sequence (5′→3′) | Product Size (bp) |
|---|---|---|---|
| GPV | Forward | AATTGTTCYCATCAGTYGCTC | 153 |
| Reverse | ARTTTGCYTTCTCACATTCCATAC | ||
| Probe | CCTGTGACTCCTCAGAACTCCCCT | ||
| DEV | Forward | GGCCAGGGAGTTTATAATTCGG | 161 |
| Reverse | GCTATATGTCGTGCATCTAACCC | ||
| Probe | CTGCCATACGWCAAATCCAGGCGAC | ||
| MDPV | Forward | AAGCTACAACAACCACATSTAC | 119 |
| Reverse | GGCAGTGRAATCTGTTGAAAT | ||
| Probe | ATCACAAGCGGAACAAACCCAGAC | ||
| DHAV-1 | Forward | TATGGGYCTCAAAAAGCCAGC | 192 |
| Reverse | CTAAGGCAAAATCTGAAGTRCCC | ||
| Probe | TTCCACTCCCTGCTCCCACYTCC | ||
| DHAV-3 | Forward | TATTCTGTTACRCCBTTACGCCC | 156 |
| Reverse | TGRTGCAGGCARTGGRAA | ||
| Probe | CGACCCATGCCAGYRTYTCAGGG | ||
| DTMUV | Forward | ACTGGTTTCATGAYCTCAACTTACC | 197 |
| Reverse | CATTTCCARTTTGYTTCCAGAGT | ||
| Probe | ACRGGGTCATCAGCGGGGACG | ||
| NDRV | Forward | CGCCTGATACTTTCCCTCCT | 124 |
| Reverse | GGCGTCTCAACACCACCAC | ||
| Probe | TCAAATCCCTCCAAAGCG |
| Sevenfold System Components | μL/Portion |
|---|---|
| ddH2O | 5.85 |
| GPV-F (100 μM) | 0.1 |
| GPV-R (100 μM) | 0.1 |
| DEV-F (100 μM) | 0.1 |
| DEV-R (100 μM) | 0.1 |
| MDPV-F (100 μM) | 0.1 |
| MDPV-R (100 μM) | 0.1 |
| DHAV-1-F (100 μM) | 0.1 |
| DHAV-1-R (100 μM) | 0.1 |
| DHAV-3-F (100 μM) | 0.1 |
| DHAV-3-R (100 μM) | 0.1 |
| DTMUV-F (100 μM) | 0.1 |
| DTMUV-R (100 μM) | 0.1 |
| NDRV-F (100 μM) | 0.1 |
| NDRV-R (100 μM) | 0.1 |
| 2 × One Step Mix | 12.5 |
| One Step Enzyme Mix | 1.23 |
| Template | 4 |
| Total volume | 25 |
| Batch Code | 1.0 × 103 | 1.0 × 102 | 1.0 × 101 | Negative Control | Total | Compliance Rate |
|---|---|---|---|---|---|---|
| CH240620001 | 9 | 9 | 9 | 9 | 36 | 100% |
| CH240718001 | 9 | 9 | 9 | 9 | 36 | |
| CH240828001-1 | 3 | 3 | 3 | 3 | 12 | |
| CH240828001-2 | 3 | 3 | 3 | 3 | 12 | |
| CH240828001-3 | 3 | 3 | 3 | 3 | 12 |
| Virus | qPCR | Gene Chip | Compliance Rate |
|---|---|---|---|
| GPV | 3/210 | 3/210 | 100% |
| DEV | 7/210 | 10/210 | 98.6% |
| MDPV | 15/210 | 17/210 | 98.6% |
| DHAV-1 | 20/210 | 24/210 | 98.1% |
| DHAV-3 | 14/210 | 14/210 | 100% |
| DTMUV | 6/210 | 8/210 | 100% |
| NDRV | 13/210 | 15/210 | 99% |
| DHAV-3 + NDRV | 4/210 | 4/210 | 100% |
| DHAV-1 + DHAV-3 | 8/210 | 9/210 | 99.5% |
| MDPV + NDRV | 3/210 | 3/210 | 100% |
| DTMUV + NDRV | 1/210 | 2/210 | 99.5% |
| DEV + MDPV + DHAV-3 + NDRV | 1/210 | 2/210 | 99.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Song, Y.; Zhai, T.; Qiu, Q.; Wang, J.; Liu, J.; Lv, D.; Huang, X.; Cao, H.; Yang, C.; et al. Establishment of a Visual Gene Chip Method for the Simultaneous Detection of Seven Waterfowl Virus Pathogens. Viruses 2025, 17, 358. https://doi.org/10.3390/v17030358
Yan L, Song Y, Zhai T, Qiu Q, Wang J, Liu J, Lv D, Huang X, Cao H, Yang C, et al. Establishment of a Visual Gene Chip Method for the Simultaneous Detection of Seven Waterfowl Virus Pathogens. Viruses. 2025; 17(3):358. https://doi.org/10.3390/v17030358
Chicago/Turabian StyleYan, Linjie, Yafen Song, Tianshu Zhai, Qian Qiu, Jia Wang, Jinming Liu, Daiyue Lv, Xiaojie Huang, Huabin Cao, Chenghuai Yang, and et al. 2025. "Establishment of a Visual Gene Chip Method for the Simultaneous Detection of Seven Waterfowl Virus Pathogens" Viruses 17, no. 3: 358. https://doi.org/10.3390/v17030358
APA StyleYan, L., Song, Y., Zhai, T., Qiu, Q., Wang, J., Liu, J., Lv, D., Huang, X., Cao, H., Yang, C., & Mao, Y. (2025). Establishment of a Visual Gene Chip Method for the Simultaneous Detection of Seven Waterfowl Virus Pathogens. Viruses, 17(3), 358. https://doi.org/10.3390/v17030358






