Evaluation of Celastrol Antiviral Activity Against Equid Alphaherpesvirus Type 8 Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, EHV-8 Strains, and Reagents
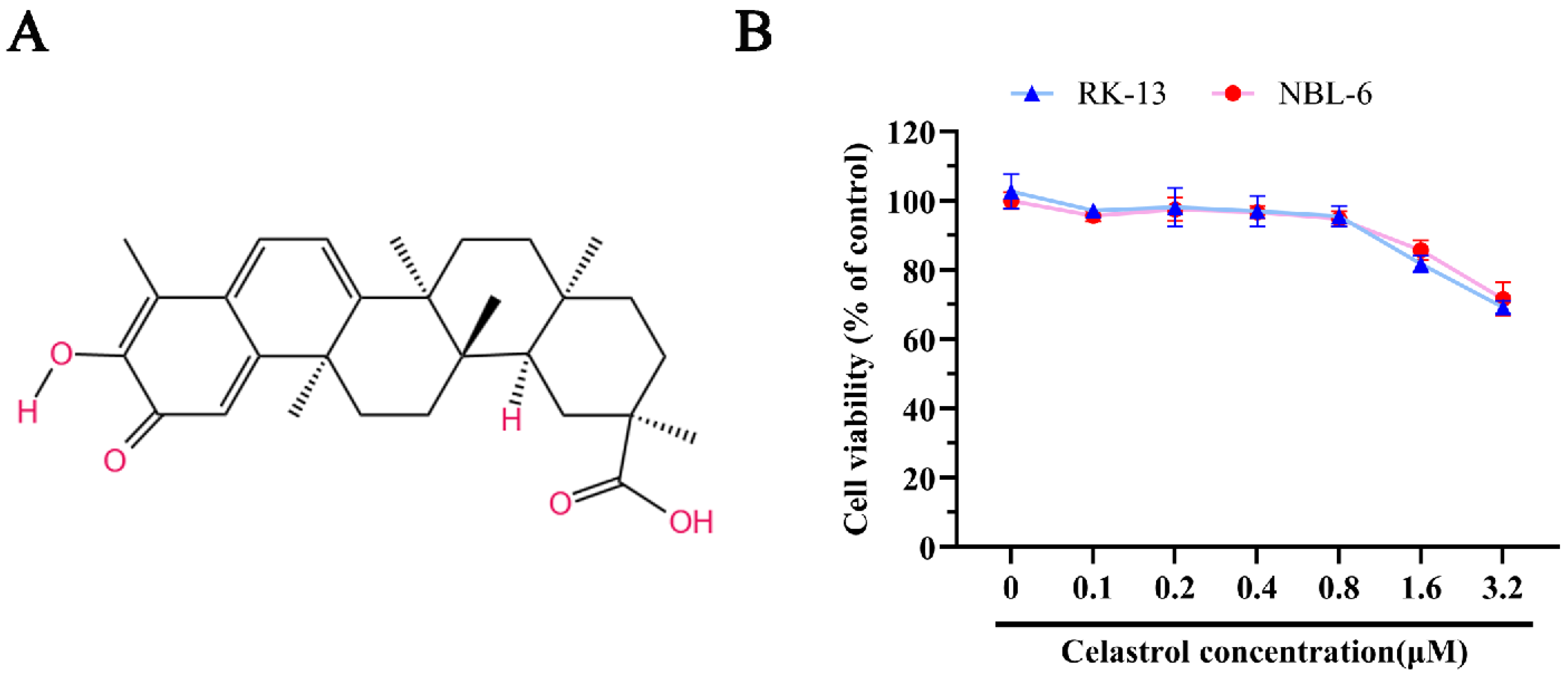
2.2. Cell Viability Detection
2.3. TCID50 Detection
2.4. RNA/DNA Extraction and qPCR Analysis
2.5. Western Blot Assay
2.6. The Viral Infection Inhibition Analysis
2.7. Indirect Immunofluorescence Assay (IFA)
2.8. Time Course Analysis of Celastrol’s Activity Against EHV-8
2.9. Direct Virus Inactivation Assay
2.10. siRNA Knockdown Assay
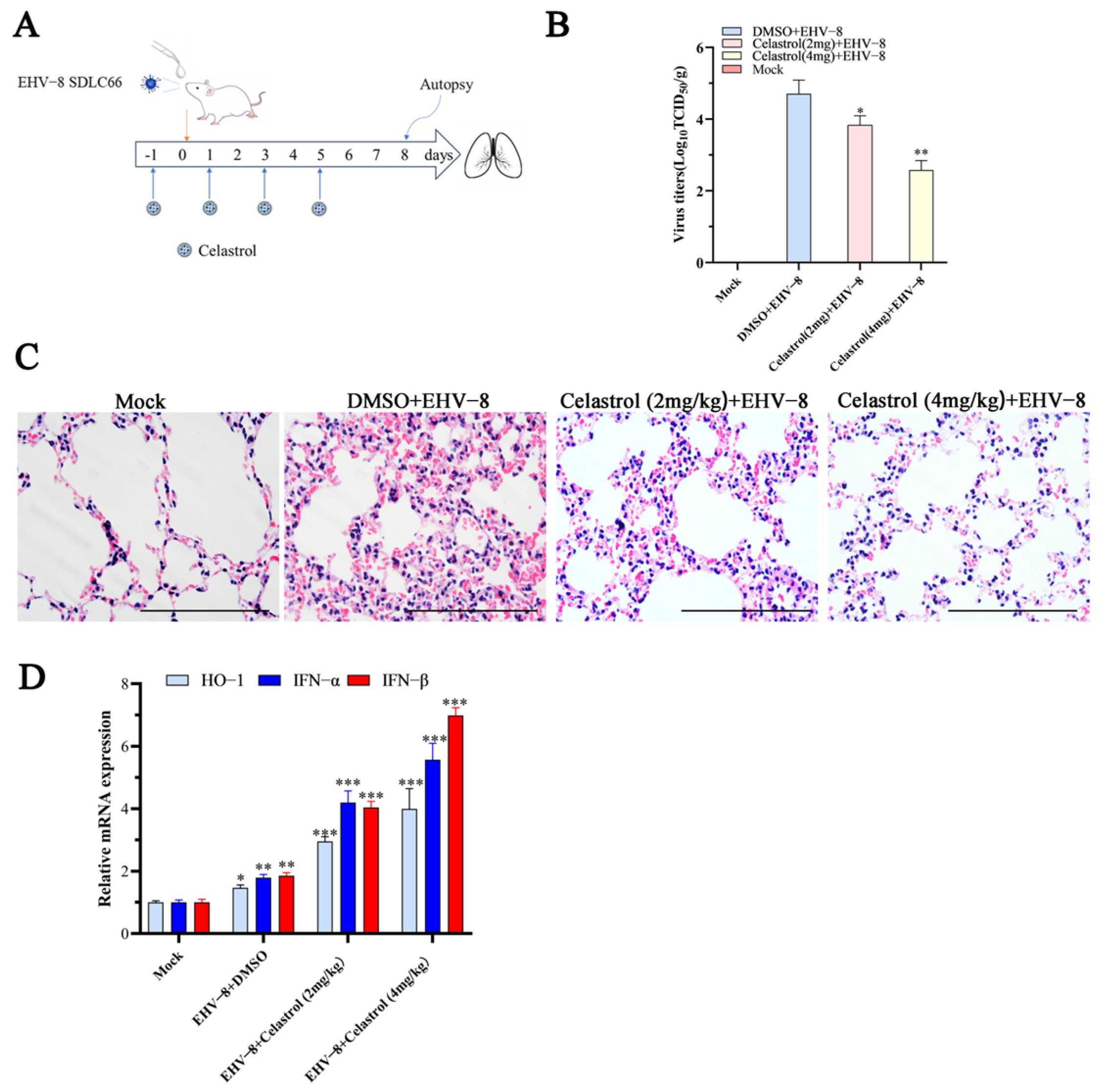
2.11. Anti-EHV-8 Efficacy Assay in Vivo
2.12. Histopathological Evaluation
2.13. Statistical Analysis
3. Results
3.1. The Detection of Celastrol’s Cellular Viability in EHV-8 Susceptible Cell
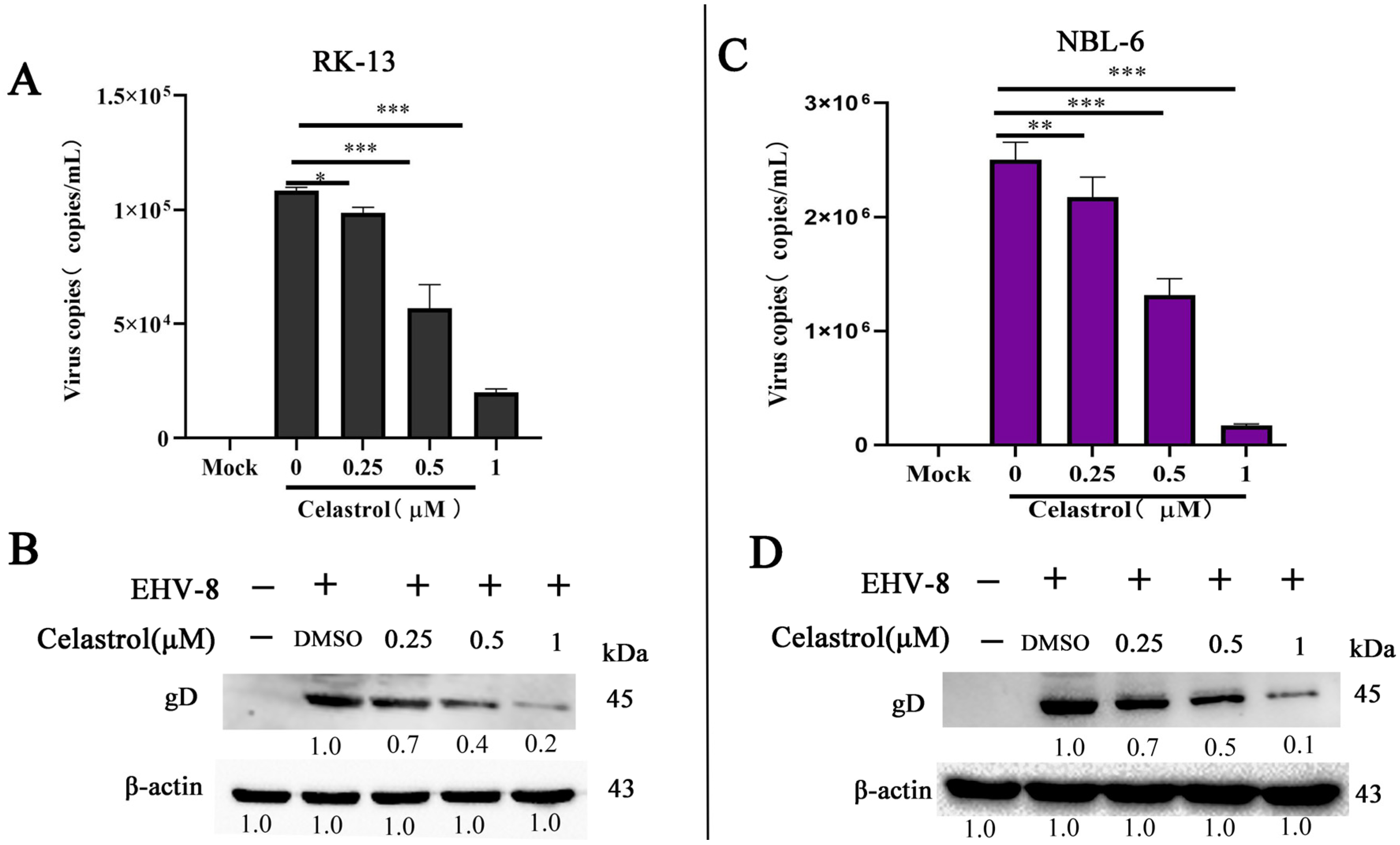
3.2. Inhibition of EHV-8 Infection by Celastrol in Vitro
3.3. Antiviral Activity of Celastrol Against Diverse EHV-8 Strains
3.4. Celastrol Inhibits EHV-8 Infection at Multiple Stages
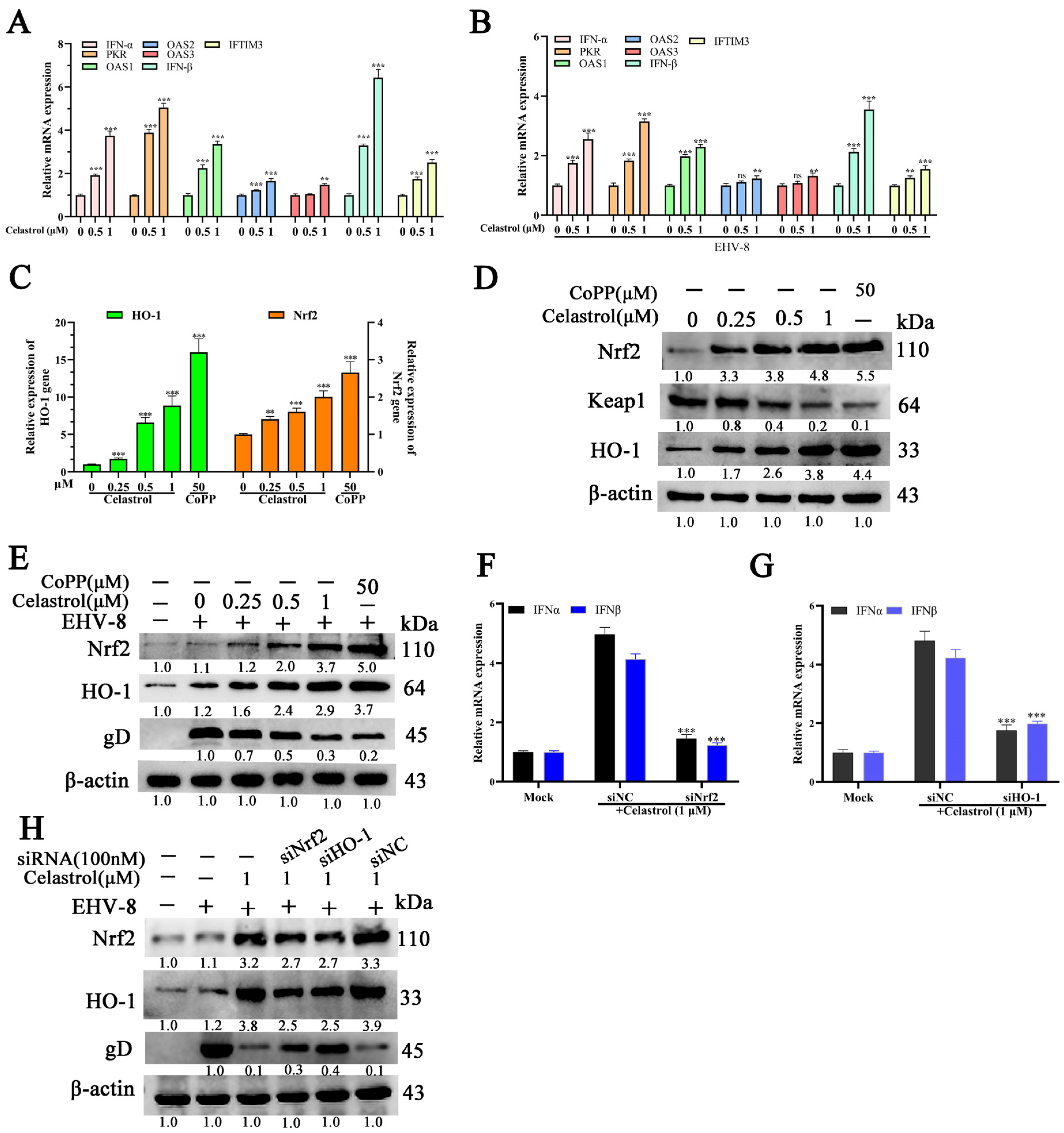
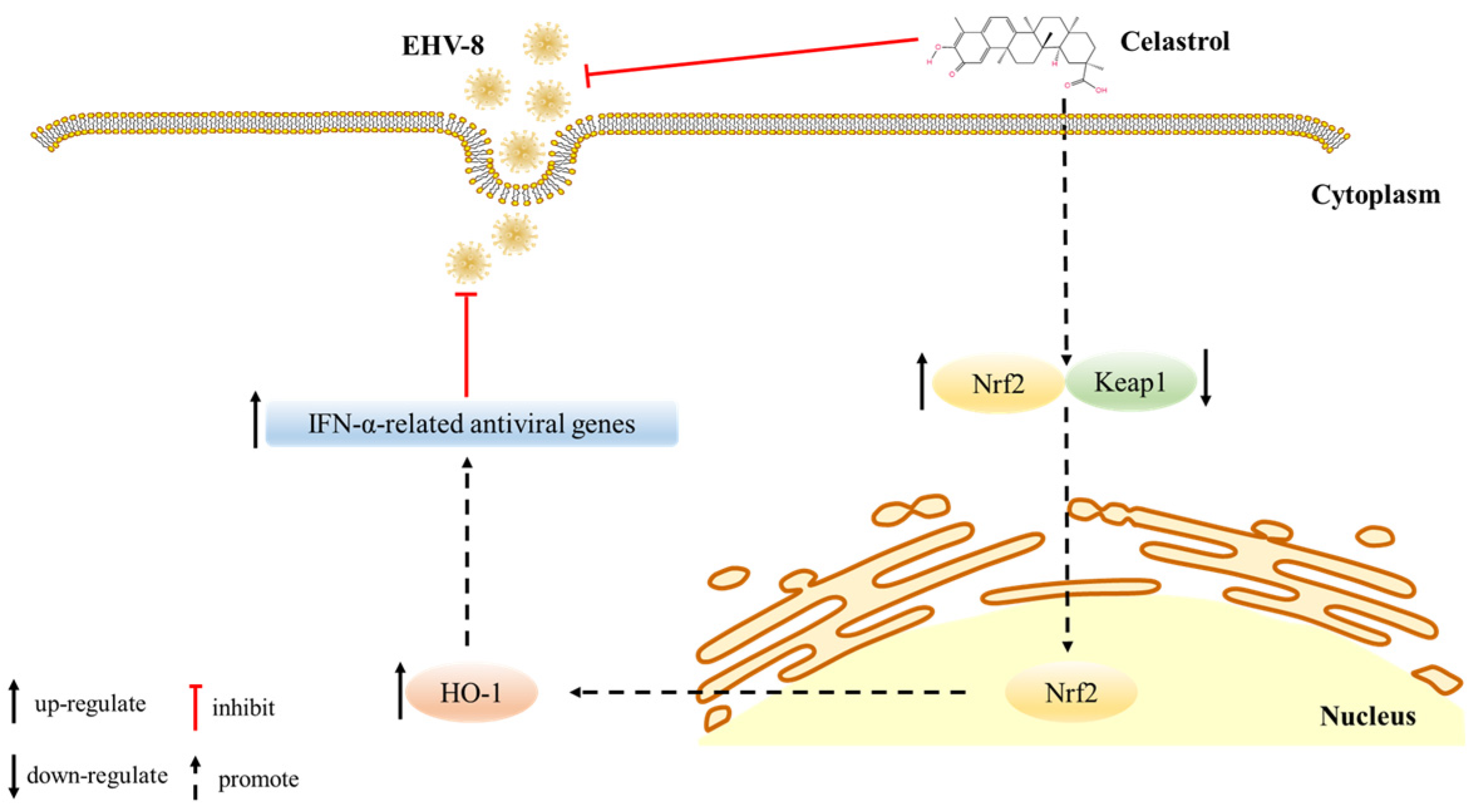
3.5. Celastrol Induces Antiviral IFN Responses via Nrf2/HO-1 Activation
3.6. Celastrol Inhibits EHV-8 Infection in Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garvey, M.; Suarez, N.M.; Kerr, K.; Hector, R.; Moloney-Quinn, L.; Arkins, S.; Davison, A.J.; Cullinane, A. Equid herpesvirus 8: Complete genome sequence and association with abortion in mares. PLoS ONE 2018, 13, e0192301. [Google Scholar] [CrossRef]
- Liu, C.; Guo, W.; Lu, G.; Xiang, W.; Wang, X. Complete genomic sequence of an equine herpesvirus type 8 Wh strain isolated from China. J. Virol. 2012, 86, 5407. [Google Scholar] [CrossRef]
- Schvartz, G.; Edery, N.; Moss, L.; Hadad, R.; Steinman, A.; Karniely, S. Equid Herpesvirus 8 Isolated From an Adult Donkey in Israel. J. Equine Vet. Sci. 2020, 94, 103247. [Google Scholar] [CrossRef] [PubMed]
- Browning, G.F.; Ficorilli, N.; Studdert, M.J. Asinine herpesvirus genomes: Comparison with those of the equine herpesviruses. Arch. Virol. 1988, 101, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, L.; Wang, Y.; Liu, W.; Liu, G.; Zhu, M.; Zhang, W.; Wang, C.; Ren, H.; Li, L. Identification of equine herpesvirus 8 in donkey abortion: A case report. Virol. J. 2022, 19, 10. [Google Scholar] [CrossRef]
- Wang, T.; Hu, L.; Liu, M.; Wang, T.; Hu, X.; Li, Y.; Liu, W.; Li, Y.; Wang, Y.; Ren, H.; et al. The Emergence of Viral Encephalitis in Donkeys by Equid Herpesvirus 8 in China. Front. Microbiol. 2022, 13, 840754. [Google Scholar] [CrossRef]
- Wang, T.; Xi, C.; Yu, Y.; Liu, W.; Akhtar, M.F.; Li, Y.; Wang, C.; Li, L. Characteristics and epidemiological investigation of equid herpesvirus 8 in donkeys in Shandong, China. Arch. Virol. 2023, 168, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Du, C.; Li, H.; Zhou, K.; Luan, Z.; Chang, Y.; Liu, S.; Wei, Y. Autophagy in the pharmacological activities of celastrol. Exp. Ther. Med. 2023, 25, 268. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell. Mol. Med. 2020, 24, 941–953. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, S.; Zhang, Q.; Yi, C.; He, J.; Ye, X.; Liu, M.; Lu, W. Celastrol is a novel selective agonist of cannabinoid receptor 2 with anti-inflammatory and anti-fibrotic activity in a mouse model of systemic sclerosis. Phytomedicine 2020, 67, 153160. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-beta-glucoside by Bacillus glycosyltransferases. J. Biosci. Bioeng. 2021, 131, 176–182. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Youn, G.S.; Kwon, D.J.; Ju, S.M.; Rhim, H.; Bae, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014, 280, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Fuzo, C.A.; Martins, R.B.; Fraga-Silva, T.F.C.; Amstalden, M.K.; Canassa De Leo, T.; Souza, J.P.; Lima, T.M.; Faccioli, L.H.; Okamoto, D.N.; Juliano, M.A.; et al. Celastrol: A lead compound that inhibits SARS-CoV-2 replication, the activity of viral and human cysteine proteases, and virus-induced IL-6 secretion. Drug Dev. Res. 2022, 83, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Wu, Y.H.; Hsieh, C.L.; Lee, J.C. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antivir. Res. 2017, 137, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Hsu, S.P.; Lin, C.K.; Wu, Y.H.; Lee, J.C.; Young, K.C. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antivir. Res. 2017, 146, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Li, Z.Q.; Xu, J.; Ding, M.Y.; Shan, Y.M.; Cheng, Y.C.; Zhang, G.X.; Sun, Y.W.; Wang, Y.Q.; Wang, Y. Celastrol attenuates hepatitis C virus translation and inflammatory response in mice by suppressing heat shock protein 90beta. Acta Pharmacol. Sin. 2023, 44, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Chen, L.; Ren, Q.; Akhtar, M.F.; Liu, W.; Wang, C.; Cao, S.; Liu, W.; Zhao, Q.; et al. Diltiazem HCl suppresses porcine reproductive and respiratory syndrome virus infection in susceptible cells and in swine. Vet. Microbiol. 2024, 292, 110054. [Google Scholar] [CrossRef]
- Li, S.; Xi, C.; Geng, Y.; Tian, W.; Li, L.; Wang, T.; Zhao, J. Pathogenicity and host cytokines response of EqHV-8 infection in C57BL/6J mice. Microb. Pathog. 2024, 186, 106506. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Li, W.; Yu, Y.; Sun, Q.; Chen, W.; Zhou, H.; Wang, C.; Li, L.; Xu, M.; et al. Rutin prevents EqHV-8 induced infection and oxidative stress via Nrf2/HO-1 signaling pathway. Front. Cell. Infect. Microbiol. 2024, 14, 1386462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, L.; Li, R.; Ren, H.; Li, S.; Sun, Q.; Ding, X.; Li, Y.; Wang, C.; Li, L. Hyperoside inhibits EHV-8 infection via alleviating oxidative stress and IFN production through activating JNK/Keap1/Nrf2/HO-1 signaling pathways. J. Virol. 2024, 98, e0015924. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Hu, Q.; Wang, T.; Zhu, G.; Zhao, Q.; Zhou, E.M. Identification of MYH9 Key Domain Involved in the Entry of PRRSV Into Permissive Cells. Front. Microbiol. 2022, 13, 865343. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, X.; Yu, Y.; Sun, Q.; Li, W.; Li, Y.; Li, S.; Chen, L.; Khan, M.Z.; Wang, C.; et al. Blebbistatin as a novel antiviral agent targeting equid herpesvirus type 8. Front. Vet. Sci. 2024, 11, 1390304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, L.; Yang, S.; Liang, Y.; Zhang, Y.; Liu, X.; Pan, X.; Li, J. Pyrogallol protects against influenza A virus-triggered lethal lung injury by activating the Nrf2-PPAR-gamma-HO-1 signaling axis. MedComm (2020) 2024, 5, e531. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, X.; Tian, H.; He, Y.; Li, Y.; Jiang, X.; Zheng, H.; Xiao, S. Induction of HOXA3 by Porcine Reproductive and Respiratory Syndrome Virus Inhibits Type I Interferon Response through Negative Regulation of HO-1 Transcription. J. Virol. 2022, 96, e0186321. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dai, S.; Zhao, X.; Zhang, Y.; Gong, L.; Fu, K.; Ma, C.; Peng, C.; Li, Y. Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies. Biomed. Pharmacother. 2023, 163, 114882. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, J.; Ruan, L.; Chen, L.; Khan, M.Z.; You, A.; Wang, C.; Li, L.; Ren, H.; Wang, T.; et al. Evaluation of Celastrol Antiviral Activity Against Equid Alphaherpesvirus Type 8 Infection. Viruses 2025, 17, 347. https://doi.org/10.3390/v17030347
Yu Y, Wang J, Ruan L, Chen L, Khan MZ, You A, Wang C, Li L, Ren H, Wang T, et al. Evaluation of Celastrol Antiviral Activity Against Equid Alphaherpesvirus Type 8 Infection. Viruses. 2025; 17(3):347. https://doi.org/10.3390/v17030347
Chicago/Turabian StyleYu, Yue, Jiayu Wang, Lian Ruan, Li Chen, Muhammad Zahoor Khan, Anrong You, Changfa Wang, Liangliang Li, Huiying Ren, Tongtong Wang, and et al. 2025. "Evaluation of Celastrol Antiviral Activity Against Equid Alphaherpesvirus Type 8 Infection" Viruses 17, no. 3: 347. https://doi.org/10.3390/v17030347
APA StyleYu, Y., Wang, J., Ruan, L., Chen, L., Khan, M. Z., You, A., Wang, C., Li, L., Ren, H., Wang, T., & Liu, W. (2025). Evaluation of Celastrol Antiviral Activity Against Equid Alphaherpesvirus Type 8 Infection. Viruses, 17(3), 347. https://doi.org/10.3390/v17030347






