Abstract
Torque Teno Virus (TTV) is a highly prevalent non-pathogenic DNA virus whose plasma levels may be related to the host’s immune status. TTV gained attention about 25 years ago, but its replication is not fully understood, nor is its relationship with the host's immune system. Despite this lack of knowledge, TTV is currently being investigated as a functional biomarker of the immune system in patients with immunological damage and inflammatory diseases. Monitoring TTV viral load over time may help clinicians in making therapeutic decisions regarding immunosuppression as well as the likelihood of infectious complications. This review summarizes what we do and do not know about this enigmatic virus.
1. Introduction
It was in 1997 when a paper entitled “A novel DNA virus (TTV) associated with elevated transaminase levels in post-transfusion hepatitis of unknown etiology” was published by Nishizawa and coworkers [1]. First identified as a new hepatitis virus, TT virus (TTV) was named after the initials of a Japanese patient with unexplained post-transfusion hepatitis. In 2004, the meaning of TTV was revised to stand for Torque Teno Virus, derived from the Latin words “torques” and “tenuis,” meaning necklace and thin, respectively, in reference to the structure of its genome. This renaming followed the International Committee on Taxonomy of Viruses (ICTV) rule prohibiting official virus names from being based on a person’s name [2]. As medical history has taught us that viruses have always been associated with the development of disease, researchers scrambled to assign an illness to this new virus. But, TTV seemed to be a mysterious virus, orphaned by disease.
With the advent of DNA sequencing showcasing a significant amount of viral “dark matter”, TTV emerged from being a novel hepatitis virus to an orphan disease agent, ultimately becoming a key element of the human virome [3]. Since the publication of the first paper in 2002 [4], the field of virome research has significantly advanced. As researchers define the virome as the viral component of the human microbiome, the extensive realm of the human virome is gradually being deciphered, including our understanding of TTV.
The current picture is that TTV is a pantropic and ubiquitous virus present in up to 98% of the population [5]. Importantly, the emphasis lies not on positivity but on the concentration of the virus. Plasma viral loads span a range that is considered “normal”. Deviations from this range, whether increased or decreased, signal a pathological condition in which TTV is not the causative agent but acts as an indicator of immune system performance.
Since TTV was first identified in 1997, many similar viruses have been found and classified within the family Anelloviridae, which are found in most healthy humans. Of particular interest is the fact that co-infections with multiple unique lineages are also common, which together constitute a “personal anellome”, a kind of fingerprint [6,7,8,9]. The full extent of human anellovirus (AV) diversity, the “global anellome”, and the mechanism(s) contributing to its diversification remain largely uncharacterized [10,11].
TTV has evolved from a novel hepatitis virus to an orphan pathogen and, ultimately, to a key element of the human virome [6].
Present in the plasma of most of the world’s population, TTV is now considered a long-lived human passenger. However, there are still many unanswered questions.
2. A Step Back: From the Clinic to Basic Virology
TTV has been described in the published literature as a small, non-enveloped, and icosahedral virus with a diameter of 30 to 32 nm and a single-stranded, negative-sense, and circular DNA genome of 3.5 to 3.9 kilobases (kb). Its replication occurs via a rolling-circle mechanism, common to other circular DNA viruses, and employs host polymerases [12]. Further examination of the viral genome reveals a highly conserved non-coding region (UTR) of 1.2 kb, which is responsible for replication and expression control, while the remaining 2.6 kb represents the coding region sequence (CDS) [12,13]. In addition, at least four partially overlapping open reading frames (ORFs) have been identified [14,15,16], along with small ORFs of unknown function, including a hypervariable region (HVR) and an N-terminal poly-A-arginine fragment sequence [17]. ORF1 (700 aa) encodes the capsid protein and is thought to be involved in TTV spin-circle replication, and its core domain appears to be a mediator of binding to genomic DNA and transport to the nucleus [18]. In contrast to the other proteins, ORF1 possesses hypervariable regions (HVRs), where mutations leading to amino acid changes occur more frequently, suggesting the potential for immune evasion by hypermutation [19,20]. Zheng et al. investigated the function of ORF2 (200 aa), revealing its role in suppressing the NF-κB pathway, thereby exerting regulatory effects on both innate and adaptive immunity, as well as the inflammatory response [15]. ORF3, which is conserved within a limited group of the heterogeneous TTV population, encodes a protein called TTV-derived apoptosis-inducing protein (TAIP), which is known to be able to induce p53-independent apoptosis in various human hepatocellular carcinoma cell lines [21]. Sequence analysis predicts the presence of additional potential ORFs that may encode poorly known proteins (Figure 1). In human TTV, a fourth ORF (ORF4) has been observed to encode 70 amino acids with a conserved motif (EX8RX2RX6PX12-19FX1L), though its function remains to be elucidated [13].
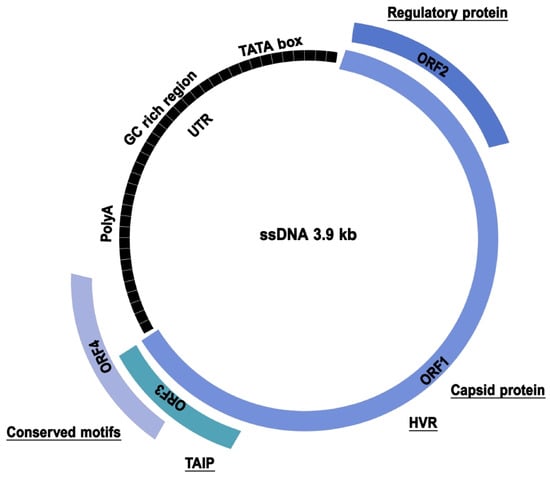
Figure 1.
TTV genome organization. The conserved non-coding region (UTR) and the coding region sequence are illustrated. ORF1 is responsible for the transcription of the capsid protein and contains hypervariable regions (HVRs). ORF2 exerts regulatory effects on both innate and adaptive immunity, as well as the inflammatory response. ORF3 is responsible for the transcription of the TTV-derived apoptosis-inducing protein (TAIP), while ORF4 is a protein whose function remains to be elucidated.
It is evident that the comprehension of the TTV genome has been and continues to be of fundamental importance, given that most studies on TTV are primarily based on the research and quantification of the viral genome in different samples using molecular methods.
In 1999, it was hypothesized that TTV replicates in the liver, given the higher concentration of TTV DNA found in bile compared to peripheral blood [18]. Concurrently, it was proposed that TTV can infect and replicate in bone marrow cells, as the amount of TTV DNA in serum samples decreases to the point of being undetectable during the myelosuppression period following bone marrow transplantation in most TTV-infected patients [22,23]. Subsequent studies concentrated on the tropism of TTV for T and B lymphocytes, monocytes, and natural killer lymphocytes, as well as granulocytes and other polymorphonuclear cells [24,25].
Indeed, TTV genomes have been identified in a cohort of blood transfusion patients who were subject to close monitoring, as well as in metagenomic datasets from a variety of other sample types, including stool [26], urine [27], bone marrow [28], liver [29,30], lungs [6], lymphoid tissue [6], and oropharyngeal and/or salivary glands [31].
This prompts the question of whether TTV has a broad or specific host range. Given its presence in various samples, it might either recognize a common receptor and be pantropic or primarily infect leukocytes, leading to its widespread distribution [32]. Data obtained by Kosulin et al. [33] demonstrate that virus replication occurs in CD15+ cells, the most abundant fraction of granulocytes, suggesting that sufficiently high granulocyte numbers may be a prerequisite for efficient TTV expansion. The same study demonstrated a significant correlation between TTV copy numbers and neutrophil count. Conversely, the effect of anti-T lymphocyte drugs on TTV levels provided the basis for the suggestion that T lymphocytes are the host cells for TTV replication [34].
Interestingly, many studies have shown that certain TTV types may persist in PBMC after serum clearance [35,36], suggesting a type-dependent infection of PBMC. It is plausible that certain TTV types may have a predilection for hematopoietic cells, while others have an affinity for hepatocytes [37]. It is noteworthy that, in healthy individuals, TTV is predominantly present in granulocytes compared to other peripheral blood cell types [38].
Also, the high prevalence and the viral load of the virus in saliva suggested that it could also replicate in salivary glands and/or oropharyngeal tissues [31]; on the contrary, the almost-leak of virus load in cerebrospinal fluid hints that the central nervous system is less likely to be the site of viral replication [11,38].
In the search for a carrier of TTV, several options should be considered: T cells cannot be excluded as a site of persistence [34], but another option has been considered as TTV has been identified in plasma-depleted platelet concentrates, raising the possibility that TTV may be taken up by or adhered to platelets [33].
However, there is another factor to consider: viral persistence necessitates infecting long-lived cells, whereas viral replication, typically linked with cell destruction, can take place in short-lived cells that offer a suitable setting for the virus to reproduce.
For this reason, it is possible that peripheral granulocytes, although an important site of TTV replication, may not serve as host cells for the persistence of the virus because of their very short life span.
Until a cellular model for the persistence and replication of TTV in vitro can be established, its interaction with the human host will remain in the shadows.
Soon after its discovery, several attempts were made to isolate TTV by replication in cell culture. On the other hand, this has always been the classical virological approach to learning the molecular details of viral replication and interaction with the host.
It is rather unexpected that TTV still resists all efforts for in vitro replication and isolation. This situation could be considered paradoxical, considering the extensive clinical research on the virus and the relatively scant basic knowledge we have obtained about it. Surprisingly, TTV continues to elude all attempts at in vitro replication and isolation.
In 2001, Maggi and coworkers demonstrated reproductive infection in stimulated PBMC cultures [4,33]. The same observation was made in 2005 in PHA-stimulated PBMC cultures and the Raji lymphoblast cell line [29]. The same authors also showed that the Chang liver cell line, derived from non-malignant liver tissue, supports the growth of TTV, also showing a marked cytopathic effect, but with low levels of viral particles secreted in the supernatant fluid. On the contrary, the virus failed to establish a productive infection in the two HCC cell lines HepG2 and MH1C1.
Other attempts to replicate AVs in vitro have been unsuccessful. Human cell lines, such as the Chang liver and Raji cell lines [36], along with HEK293TT, lymphoma, and T-cell leukemia, have shown evidence of TTV infection in initial passages. However, the virus failed to replicate in subsequent passages [26,39,40].
One constant in vitro replication attempt of TTV is the lack of a true viral titer elevation indicative of its replication and cellular release. The other feature is the failure of any attempt to establish persistent infection. Unlike in vivo infection, in vitro infection by TTV is lost approximately two weeks after infection.
This inability to establish a persistent viral infection makes it extremely difficult to discover its receptor and, thus, its tropism, as well as to understand the intracellular factors that modulate its replication and, ultimately, the nature of the close relationship between TTV and the immune system.
3. The Origins of TTV: Where Does It Come from?
Following the discovery of TTV, numerous types of AV have been identified [12,41,42] in both humans and various mammals [14,43,44,45,46,47,48]. As of September 2023, the International Committee on Taxonomy of Viruses (ICTV) ratified 173 species classified in 34 genera and, in the same year, Butkovic and colleagues [49] proposed the placement of AVs in a new phylum, designated “Commensaviricota”, within the kingdom Shotokuvirae.
The same authors studied the evolution of AVs through a comparative structural analysis of their signature ORF1 proteins, observing considerable variability in the overall size of this protein among different AV genera. Notably, a systematic comparison of their structures suggests that the AV ORF1 protein has evolved from an ancestral virus through incremental increases in size and structural complexity. Profile–profile comparison has shown that AV ORF1 proteins are homologous to the capsid proteins of circoviruses, the only other small, circular, and ssDNA viruses known to infect mammals [50], suggesting that AVs evolved from a circovirus-like ancestor. It is still not clear whether this virus was a member of the Circoviridae family or whether another virus was the ancestor of both circoviruses and AVs.
Previously classified in the family Circoviridae [14,43], the novel family Anelloviridae was introduced in 2009. This classification was derived from the Latin word “anellus”, meaning “ring”, and was chosen to signify the circular genome of all members of the family. Although the taxonomy of AVs is constantly being revised, it appears that only nine genera have been identified as capable of infecting humans: Alphatorquevirus (TTV), Betatorquevirus (TT mini viruses—TTMV), Gammatorquevirus (TT midi viruses—TTMDV), Hetorquevirus [43], Gyrovirus, and, more recently, Lamedtorquevirus, Memtorquevirus, Samektorquevirus, and Yodtorquevirus [45] (Figure 2). The prevailing view is that alpha, beta, and gamma viruses are commensal components of the human virome [7] and are predominantly acquired during early childhood [51]. In addition, research has shown that TTV genomic diversity increases with age [52], but it is unclear whether this pattern persists into adulthood or how long it lasts. Co-infection with different AV species and genotypes within the same genus is also possible [27]. The discovery of viral diversity and the identification and characterization of non-human AVs, which are particularly important for understanding viral evolutionary dynamics, have been profoundly impacted by the advent of high-throughput sequencing (HTS) technologies and metagenomic protocols.
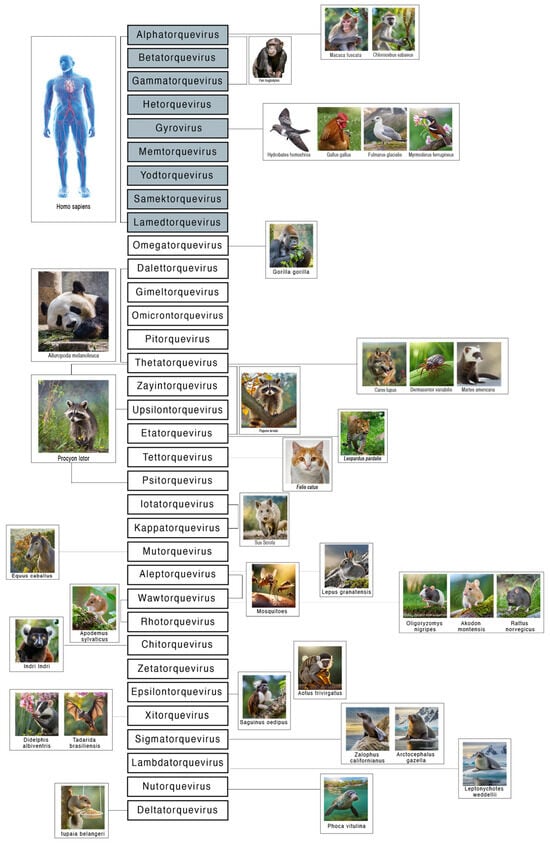
Figure 2.
Anelloviridae genera and their hosts. The 34 genera of the family Anelloviridae are shown. The nine genera identified as capable of infecting humans are shown in grey: Alphatorquevirus, Betatorquevirus, Gammatorquevirus, Hetorquevirus, Gyrovirus, Lamedtorquevirus, Memtorquevirus, Samektorquevirus, and Yodtorquevirus. For the other genera, mammalian and invertebrate hosts are indicated. As shown, some genera have a preferred host, while others have an extended host tropism.
Currently, AVs have been identified in several mammals (Hominidae, Canidae, Felidae, Suidae, Muridae, Ursidae, Molossidae, and Phocidae), but also in invertebrates such as Culicidae [46,47,48,53,54,55].
Interestingly, non-human AVs do not appear to be pathogenic, with the exception of Gyrovirus chicken anemia, which causes anemia, intramuscular hemorrhage, lymphoid atrophy, and bone marrow aplasia in chickens [56].
Characterizing of the diversity of AVs and their natural hosts is central to understanding their host range and the dynamics of virus transmission in nature. It has been hypothesized that AVs are mainly spread by fecal–oral transmission [57] and saliva [58,59], but it is possible that other ways of transmission, both intra- and inter-species, are possible.
An analysis of the distribution of the different genera of AVs shows that some have a preferred host (such as Lambdatorquevirus, which, according to current knowledge, is only found in Phocidae and Otariidae), while others have a wide host tropism, such as Thetatorquevirus, which infects mammals such as Ursidae, but also Canidae, Mustelidae, and even Ixodidae. The latter, also known as the American dog tick or wood tick, is a species of tick known to transmit the bacteria responsible for several human diseases, including Rocky Mountain spotted fever and tularemia (Francisella tularensis). It is speculative whether the presence of anellovirus in the wood tick is the result of a blood meal from an individual infected with thetatorquevirus, e.g., Canis lupus [52].
4. TTV: Not a Causative Factor but a Marker
A PubMed search using the keywords (Anellovirus) OR (Torque Teno Virus) shows that 1338 articles have been published since the discovery of the virus in 1997. It is noteworthy that, from 1998 to 2003, and, to a lesser extent, until 2010, many articles focused on the potential role of TTV as an etiologic agent of liver disease. Subsequently, the focus gradually shifted to the concept of TTV as a “commensal virus”, defined as a component of the human virome that is not known to cause pathology in humans.
The earliest known discussion of the virome can be traced back to 2002. About a decade later, the first paper linking TTV to the virome was published. Since 2012, numerous papers have been published discussing the potential of TTV as a marker for the immunosuppression status in the post-transplant period.
It is now known that TTV is highly prevalent, accounting for 97% of all anelloviruses, and represents a significant proportion (68%) of the blood virome in many solid organ transplant recipients [27]. As its replication is under the control of a functioning immune system, it is hypothesized that the quantification of the viral load in the blood may serve as a potential indicator of its functionality [27]. Results to date suggest that, in the presence of a competent immune system, the circulating levels of TTV are approximately 102–103 copies/mL [60], which is considered to be within the normal range. Most studies investigating TTV have shown an association between unfavorable outcomes or disease progression and TTV loads that are either too low or too high [61,62,63].
Its presence has been extensively studied in transplant patients, with the aim of ensuring proper post-transplant management and avoiding the two most feared complications: infection and organ rejection [27]. A search using the keywords (Anellovirus) OR (Torque Teno Virus) AND (transplant) yielded 225 results, with a gradual increase from 2014 to the present. Most of these papers focus on the correct management of immunosuppression, which is important for long-term care, to prevent the development of both opportunistic infections [61] and cancer [64]. The correlation between TTV, induced immunosuppression, and the infection risks directly associated with this condition showed that individuals with severely compromised immune systems have a higher TTV load. In contrast, patients receiving inadequate doses of immunosuppressive drugs have a low TTV load [65]. Opportunistic infections, including Cytomegalovirus (CMV), BK virus (BKV), and Epstein–Barr virus (EBV), have the potential to cause virus-specific disease or even graft loss, or post-transplant lymphoproliferative disease [64]. Research has shown a significant association between BKV, CMV, and TTV. In particular, increased immunosuppression correlates with increased viral loads of both pathogenic viruses and TTV [61,63,66]. Some studies suggest that an elevated TTV plasma load may occur in the pre-infection period, suggesting its potential use as a predictive marker. However, other studies have not found a consistent relationship between TTV levels and viral infections [67,68]. The current focus is on determining the optimal TTV load for predicting infection in order to prevent adverse events [69].
Conversely, TTV appears to be a promising indicator for the detection of graft rejection. Studies have shown that TTV plasma levels are significantly lower in the presence of rejection at only 25% of the levels observed in the absence of rejection for both kidney and lung transplantation [70,71,72]. There is also a lack of agreement on this issue. Most studies suggest an inverse association between rejection and TTV burden; one found no association between TTV burden and rejection [66], and this study could not replicate its earlier findings. Some studies reported reduced odds or hazards associated with increased TTV burden [62,72,73,74,75,76], or found a lower TTV load in patients who experienced rejection compared to those who did not [76,77].
Given the complex interactions between the immune system and a variety of disease processes, the applicability of TTV may be extended to broader immunodeficiencies, inflammatory processes, and cancers [78,79,80].
Indeed, another factor that has been investigated over the years is the association between TTV and tumors. A PubMed search using the terms (Anellovirus) OR (Torque Teno Virus) AND (tumor) yielded 101 papers published from 1998 to the present.
Some viruses are known to have oncogenic properties, and for many, the mechanisms by which they increase cell proliferation and/or inhibit apoptosis are known. EBV, HBV, HCV, HPV, HHV-8, and HTLV-1 and 2 are among the viruses associated with cancer pathology [81], in line with the 2.2 million cancer cases attributed to infection in 2018 [8]. In the first period after the discovery of TTV, in the years between 1998 and the early 2000s, scientific activity focused on the presence of TTV in the serum of patients with non-B, non-C hepatitis, and its possible role in non-B non-C hepatocellular carcinoma (HCC). Possible transmission by transfusion was emphasized, but it soon became apparent that TTV was present in different biological samples, suggesting different modes of transmission. The assessment of the presence of TTV over time also shows that the virus can cause persistent infection, strengthening the hypothesis of its possible oncogenic role [82].
Several studies have attempted to correlate TTV with various cancer pathologies, from hepatocarcinoma to HPV-related cancers, breast cancer, non-Hodgkin’s lymphoma, and colorectal cancer, but have yielded inconsistent results [83,84,85].
The turning point came in 2007, when scientists began to question whether TTV, which is widespread and found in over 90 percent of the world’s adult population without any known pathology, might be associated with various inflammatory conditions [86]. In 2007, Zheng H. and coworkers [15] correlated the activity of the ORF2 protein in the regulation of innate and adaptive immune response, but were still searching for a pathogenetic mechanism that could correlate TTV with a pathological state.
In 2016, nine years later, Hettmann A. investigated the presence of TTV in saliva and biopsy samples from patients with head and neck cancer (HNCC), oral cancer, and controls. The study concluded that “our data are compatible with the idea that TTV may act as a cocarcinogen in certain cases of HNCC. Alternatively, HNCC may facilitate either TTV replication or TTV entry into saliva” [81].
This started an ongoing debate: is TTV a cause or just a sentinel? When TTV was classified as part of the virome [87], it went from being a potentially oncogenic virus to an enigmatic one, lacking pathology but possibly indicative of immune system function, even in cancer patients [78,88,89,90] (Table 1).

Table 1.
Papers published from 1998 to the present regarding (Anellovirus) OR (Torque Teno Virus) associated with various clinical conditions.
5. TTV: A Longtime Human Passenger
Human infections have been shown to occur at an early age [65] and have been identified in almost all human tissue [32,87], consistent with the lymphocytes being the primary site of AV replication [34]. It has been suggested that AVs positively influence human health by shaping immunity during early development [32], and are now considered part of the ‘healthy human virome’ [12,282], likely due to the extensive co-evolution of AVs with their mammalian hosts [283,284,285,286].
Numerous studies have suggested horizontal and vertical routes of TTV transmission. Horizontal transmission includes parenteral, fecal–oral, and sexual routes. Vertical transmission includes the possible passage of virus from mother to fetus during pregnancy and breastfeeding [287]. However, while the potential for vertical transmission has been suggested, alphatorquevirus DNA has not been consistently detected in umbilical cord blood. This finding supports the conclusion that transplacental transmission of AVs cannot be efficient [287]. It can be hypothesized that AVs may be transmitted by breastfeeding, as the presence of AV DNA has been identified in breast milk [288]. However, no association was observed between the infant’s breastfeeding status and AV richness [289].
It is noteworthy that a study of interest evidenced the prevalence of TTV in the vaginal ecosystem of pregnant women, thus representing a possible predictor of local immune status. Indeed, the presence and load of the virus vary according to local vaginal conditions, being more prevalent in cases of higher levels of leukocytes, higher levels of microbes related to bacterial vaginosis, and a lack of Lactobacillus crispatus [290].
It is noteworthy that some studies have identified alphatorquevirus DNA in blood as early as the second month of life, and also in stool samples in the first months of life, suggesting replication in the gut at a very young age [51]. The peak of AV abundance in the gut was found between the sixth and twelfth months of life, after which the abundance decreased [291]. The same is probably true for the AV virome in the blood (anellome), and the early-life dynamics of the anellome may contribute to the maturation of children’s immune systems.
6. TTV and the Immune System: How Are They Linked?
The interaction between TTV and the immune system represents another challenging area of research, with the mechanisms underlying this close relationship remaining poorly understood.
Due to the lack of an efficient culture system to support TTV replication, the transcription profile of TTV has been largely gained from human cell lines (COS1, HEK293, and L428) transfected with TTV plasmids [26]. Three spliced mRNAs of TTV that produce at least six proteins by alternative translation initiation have been reported [292]. At present, the functional role of ORF2 protein is well characterized. The overexpression of the TTV ORF2-encoded protein has been shown to suppress NF-κB activation elicited by TNFα in various human cancer cell lines, including HeLa and HepG2, and in the mouse macrophage line RAW 264.7 [15]. Further analyses revealed that the TTV ORF2 protein can suppress NF-κB activity in vitro in a dose-dependent manner, affecting translocation of NF-κB p65 and p50 subunits to the cell nucleus, thus inhibiting the transcription of downstream genes such as interleukin IL-6, IL-8, and cyclooxygenase-2. Together, these findings indicate that the TTV ORF2 protein may be involved in the negative regulation of host cell inflammatory responses.
There is growing evidence that AVs interact with cells and soluble substances known to support the equilibrium of innate and adaptive immunity, and this interaction can significantly affect an infected host [293,294]. Human AVs, especially TTV, have established a suitable interaction with the host’s immune system, and it has been shown that the replication rate of these viruses can be an appropriate measure to monitor the overall function of the host immune system. Research has also demonstrated the interaction between TTVs and immune molecules known as “pathogen-associated molecular patterns” (PAMP), recognized by pathogen recognition receptors (PRRs), which cause inflammatory and immunological reactions [13,295]. The adaptive immune responses of infected hosts play a critical role in determining the resolution of primary AV infections and the circulation of AVs, such as TTV, in the peripheral blood. As shown, of two chimpanzees inoculated with the same volume of human serum containing TTV, one developed IgM and IgG antibodies to the virus, while the other, which developed no detectable antibodies, became persistently infected [13,294,296,297]. The information that is now available concerning humoral responses suggests that TTV induces antiviral antibodies that, at least in most cases, fail to eradicate the virus.
Toll-like receptors (TLRs) belong to a group of cell-surface proteins that are crucial for identifying a wide range of pathogens and initiating an innate immune response. TLR9 recognizes intracellular unmethylated heterodimers of guanosine and cytosine (CpGs), which are abundant in the genomes of DNA viruses. Depending on the number of nucleotides flanking CpGs, this may stimulate the production of either pro- or anti-inflammatory cytokines [270]. It has been reported that the genome as well as the replicative intermediates of anellovirus are unusually rich in CpG sequences [295]. The DNA of genogroup 1 of anellovirus (ViPiSAL strain) was found to provoke the robust activation of TLR9 and the production of proinflammatory cytokines in ex vivo mouse spleen cells [270]. Nevertheless, the genomes of other anellovirus strains failed to promote inflammatory responses. These findings may indicate that the effects of anelloviruses on the host’s inflammatory status vary depending on genogroups.
Evidence suggests that TTV encodes microRNAs (miRNA) that cooperate with viral proteins to regulate the expression of viral genes involved in replication, pathogenesis, inflammation, and immune evasion. The functional relevance of proteins translated from other TTV ORFs and TTV-encoded miRNAs warrants further study.
Another fundamental aspect that remains to be elucidated is the location of TTV persistence. However, its ubiquitous presence suggests that granulocytes could be the reservoir for TTV.
In a study by Kosulin et al. [33], the potential site of TTV replication in different leukocyte subsets was assessed by flow-sorting separation. This study evidenced granulocytes as a site of TTV persistence without any correlation between TTV and T lymphocyte count. Conversely, other studies have utilized molecular methods to demonstrate the presence of TTV in the bloodstream and have shown that the highest levels of TTV are found in patients living with HIV who have a low CD4 T lymphocyte count [298]. Honorato et al. studied the possible correlation between the presence of TTV in saliva and circulating CD4+ T lymphocytes in HIV patients. In this case too, the levels of TTV in saliva had an inverse correlation with CD4+ T lymphocytes, but a direct correlation with HIV viremia [255].
It is evident that these results once again serve to demonstrate the utility of TTV in the monitoring of immunosuppression. However, it must be noted that this utility is predicated on an absence of an understanding of the underlying mechanism.
7. TTV Detection: From Traditional to Innovative Screening Methods
TTV detection and quantification have been important for understanding its epidemiology, potential clinical relevance, and role as a marker of immune competence. Various methodologies have been employed for TTV screening over the years, ranging from traditional PCR-based techniques to innovative molecular approaches that enhance sensitivity, specificity, and reproducibility. Despite these advancements, standardization remains a significant challenge affecting the comparability of results across different studies and laboratories.
The most widely used method for TTV detection is quantitative PCR (qPCR), which provides a sensitive and specific approach for measuring viral loads in clinical and research settings. qPCR assays typically target conserved regions of the TTV genome, such as the untranslated region (UTR), which is less affected by the virus’s extensive genetic diversity. Several commercial and in-house qPCR assays have been developed, including those by Maggi et al. [58] and Okamoto et al. [44], which are often used in studies assessing TTV viremia as a potential biomarker for immune function. Among commercial assays, the bioMérieux ARGENE® TTV R-GENE® kit (bioMérieux S.A., Marcy l’Etoile, France)is commonly used in clinical laboratories for standardized TTV quantification. This assay provides reproducible viral load measurements and has been applied in monitoring TTV viremia in transplant recipients to assess immune suppression levels. qPCR is extensively applied in monitoring TTV loads in organ transplant patients, where high viremia has been correlated with immunosuppression levels, offering a non-invasive tool for optimizing therapy [27,106].
Nested PCR was one of the first molecular techniques used to detect TTV, and remains valuable for research applications requiring high sensitivity. This method involves two rounds of PCR amplification, enhancing the ability to detect low viral loads, which is particularly useful for identifying TTV in samples with minimal viral DNA. Early studies on TTV prevalence relied on nested PCR, with Nishizawa et al. [1] using this approach to characterize TTV sequences in human serum. However, nested PCR has significant limitations, including a high risk of cross-contamination due to multiple amplification steps and the lack of quantitative capabilities, making it unsuitable for clinical monitoring.
More recently, digital droplet PCR (ddPCR) has emerged as a tool for the absolute quantification of TTV DNA. Unlike qPCR, which relies on standard curves for quantification, ddPCR partitions the sample into thousands of nanoliter-sized droplets, allowing for a more precise and absolute measurement of viral DNA copies. This technique has been applied in research investigating the role of TTV as an immune monitoring biomarker in organ transplantation, showing improved reproducibility over qPCR. For example, Schmitz et al. [212] demonstrated that ddPCR provided a more accurate quantification of TTV loads in transplant recipients, minimizing variability due to primer mismatches. Additionally, a ddPCR assay developed by Maggi et al. [299] at the University of Pisa has been successfully applied for TTV quantification in different clinical settings, including transplant patients and immunocompromised individuals. Their work demonstrated that ddPCR offers improved sensitivity and reproducibility compared to qPCR, particularly in cases where precise viral load monitoring is required. Despite its advantages, ddPCR remains costly and technically complex, limiting its widespread adoption in routine diagnostics
Although molecular methods dominate TTV diagnostics, serological approaches have been explored to assess immune responses against the virus. However, the identification of reliable TTV antigenic targets has been challenging due to the virus’s genetic variability and unclear interaction with the immune system. Some studies have attempted to develop ELISA-based assays for detecting TTV-specific antibodies, but these have not gained widespread use due to the poor correlation between seropositivity and viral load [300].
Next-generation sequencing (NGS) and metagenomic approaches have also advanced TTV research, enabling the discovery of novel TTV species and comprehensive virome analyses. These techniques have been particularly useful in epidemiological studies exploring the diversity of TTV strains across different populations and environments. For example, the metagenomic sequencing of blood and respiratory samples has revealed the co-presence of multiple TTV species, suggesting a dynamic and complex viral population structure. Furthermore, wastewater-based surveillance using NGS has been employed to monitor TTV circulation at the population level, highlighting its potential as a public health marker. However, these approaches require significant bioinformatics expertise, have high associated costs, and are not yet suitable for routine clinical applications [164].
Another emerging technology for TTV detection is CRISPR-based diagnostics, leveraging the sequence-specific recognition capabilities of CRISPR-Cas systems. CRISPR-Cas12 and Cas13 enzymes can be programmed to detect specific TTV DNA sequences with high specificity and sensitivity. This technology has been explored for the rapid detection of viral infections, with potential applications in point-of-care diagnostics [301]. While CRISPR-based assays for TTV are still in the early stages of development, they hold promise for future clinical use, particularly in settings requiring rapid and accurate viral detection.
A major challenge in TTV screening is the lack of standardization, which affects the comparability of viral load measurements across different laboratories and clinical studies. Variability in primer design, detection platforms, and analytical workflows has led to inconsistent results, making it difficult to establish universal clinical cutoffs for TTV quantification. To address this issue, the European TTXGUIDE project has been launched with the goal of developing harmonized protocols for TTV detection and quantification. This initiative is part of a broader effort to optimize immunosuppressive therapy in transplant patients by using TTV levels as a biomarker of immune function. Within TTXGUIDE, transplant recipients are monitored for TTV viremia, and viral load measurements are used to adjust immunosuppressive treatment to prevent both rejection and opportunistic infections. As part of this project, efforts are being made to establish standardized qPCR assays, reference materials for viral load calibration, and clinically relevant thresholds to ensure reliable and reproducible TTV quantification. By creating a unified framework for TTV-based immune monitoring, TTXGUIDE aims to facilitate the integration of TTV measurements into routine clinical practice, enhancing its reliability as a diagnostic and prognostic tool in transplant medicine.
In conclusion, TTV detection has evolved from early PCR-based methods to sophisticated molecular techniques, each with distinct advantages and limitations. While qPCR remains the gold standard for clinical applications, newer approaches like ddPCR, NGS, and CRISPR-based diagnostics offer promising avenues for improving sensitivity, specificity, and reproducibility. Addressing the challenges of standardization will be essential for enhancing the clinical utility of TTV screening, particularly in areas such as transplant medicine, immune monitoring, and virome research.
8. A Step Forward: The Future of TTV
AVs have evolved over millions of years and have developed several distinctive traits that make them ideal candidates for use as viral therapeutics.
As a ubiquitous component of the human virome, AVs are characterized by their lack of pathogenicity. These properties render them optimal candidates for use as vectors in the development of next-generation genetic medicines.
In addition, AVs are among the most genetically diverse and pantropic components, suggesting that they could be used as a gene therapy vector platform with broad tropism for multiple disease sites. Additionally, the extensively documented ability of AVs to persist and replicate in humans without triggering humoral immune responses could help minimize or avoid the problem of immunogenicity [300].
In recent decades, virologists have dedicated their research to understanding the vast and diverse world of human-associated viruses, leading to the discovery of what has been termed ’viral dark matter’. Remarkably, some of these viruses are harmless and can persist in the human body for long periods without causing any adverse effects. This is a defining characteristic of commensalism, a symbiotic relationship in which one organism benefits while the other remains unaffected. We believe that AVs, which are present in virtually every human being and a wide range of tissues, provide an opportunity to create a programmable platform for the development of viral therapeutics capable of safely treating a wide range of diseases with greater precision and dose adjustability.
In addition, TTV does not elicit an immune response, allowing patients to be treated with multiple doses, thus overcoming the major obstacle to viral-based therapies, namely the inability to administer repeated doses due to the generation of a strong immune response to each subsequent exposure [292].
Finally, because TTV is a ubiquitous virus, its ability to target inaccessible tissues has the potential to open up solutions for a wide range of diseases by using multiple routes of administration and ensuring the sustained expression of therapeutic proteins, but without the potentially harmful integration into the human genome, as AVs remain as episomes in the nucleus [301].
Author Contributions
All authors participated in reviewing and discussing the published data and in manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Italian Ministry of Health “Ricerca Corrente–Linea 1–INMI L. Spallanzani I.R.C.C.S.” funding and by the European Union’s Horizon 2020 research and innovation program under grant agreement number 896932 (TTVguideTX project).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
Michele Giordano is gratefully acknowledged for the graphic support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nishizawa, T.; Okamoto, H.; Konishi, K.; Yoshizawa, H.; Miyakawa, Y.; Mayumi, M. A Novel DNA Virus (TTV) Associated with Elevated Transaminase Levels in Posttransfusion Hepatitis of Unknown Etiology. Biochem. Biophys. Res. Commun. 1997, 241, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Biagini, P. Classification of TTV and Related Viruses (Anelloviruses). Curr. Top. Microbiol. Immunol. 2009, 331, 21–33. [Google Scholar] [PubMed]
- Sekawi, Z.; Othman, Z.; Syazwani Jarkasi, N.; Yoke Kqueen, C.; Putra Malaysia, U.; Serdang, U. A Review on The Global Widespread of TTV Infection Among Humans Population. Pertanika J. Sch. Res. Rev. 2018, 4, 10–24. [Google Scholar]
- Mariscal, L.F.; López-Alcorocho, J.M.; Rodríguez-Iñigo, E.; Ortiz-Movilla, N.; De Lucas, S.; Bartolomé, J.; Carreño, V. TT Virus Replicates in Stimulated but Not in Nonstimulated Peripheral Blood Mononuclear Cells. Virology 2002, 301, 121–129. [Google Scholar] [CrossRef]
- Cebriá-Mendoza, M.; Beamud, B.; Andreu-Moreno, I.; Arbona, C.; Larrea, L.; Díaz, W.; Sanjuán, R.; Cuevas, J.M. Human Anelloviruses: Influence of Demographic Factors, Recombination, and Worldwide Diversity. Microbiol. Spectr. 2023, 11, e04928-22. [Google Scholar] [CrossRef]
- Young, J.C.; Chehoud, C.; Bittinger, K.; Bailey, A.; Diamond, J.M.; Cantu, E.; Haas, A.R.; Abbas, A.; Frye, L.; Christie, J.D.; et al. Viral Metagenomics Reveal Blooms of Anelloviruses in the Respiratory Tract of Lung Transplant Recipients. Am. J. Transplant. 2015, 15, 200–209. [Google Scholar] [CrossRef]
- Moustafa, A.; Xie, C.; Kirkness, E.; Biggs, W.; Wong, E.; Turpaz, Y.; Bloom, K.; Delwart, E.; Nelson, K.E.; Venter, J.C.; et al. The Blood DNA Virome in 8,000 Humans. PLoS Pathog. 2017, 13, e1006292. [Google Scholar] [CrossRef]
- Rani, A.; Ranjan, R.; McGee, H.S.; Metwally, A.; Hajjiri, Z.; Brennan, D.C.; Finn, P.W.; Perkins, D.L. A Diverse Virome in Kidney Transplant Patients Contains Multiple Viral Subtypes with Distinct Polymorphisms. Sci. Rep. 2016, 6, 33327. [Google Scholar] [CrossRef]
- Bal, A.; Destras, G.; Sabatier, M.; Pichon, M.; Regue, H.; Oriol, G.; Gillet, Y.; Lina, B.; Brengel-Pesce, K.; Josset, L.; et al. Metagenomic Analysis Reveals High Abundance of Torque Teno Mini Virus in the Respiratory Tract of Children with Acute Respiratory Illness. Viruses 2022, 14, 955. [Google Scholar] [CrossRef]
- Liang, G.; Bushman, F.D. The Human Virome: Assembly, Composition and Host Interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Gore, E.J.; Gard, L.; Niesters, H.G.M.; Van Leer Buter, C.C. Understanding Torquetenovirus (TTV) as an Immune Marker. Front. Med. 2023, 10, 1168400. [Google Scholar] [CrossRef] [PubMed]
- Arze, C.A.; Springer, S.; Dudas, G.; Patel, S.; Bhattacharyya, A.; Swaminathan, H.; Brugnara, C.; Delagrave, S.; Ong, T.; Kahvejian, A.; et al. Global Genome Analysis Reveals a Vast and Dynamic Anellovirus Landscape within the Human Virome. Cell Host Microbe 2021, 29, 1305–1315.e6. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghian, M.; Gheitasi, H.; Shekarchi, A.A.; Tavakoli, A.; Poortahmasebi, V. The Mysterious Anelloviruses: Investigating Its Role in Human Diseases. BMC Microbiol. 2024, 24, 40. [Google Scholar] [CrossRef]
- Mushahwar, I.K.; Erker, J.C.; Muerhoff, A.S.; Leary, T.P.; Simons, J.N.; Birkenmeyer, L.G.; Chalmers, M.L.; Pilot-Matias, T.J.; Dexai, S.M. Molecular and Biophysical Characterization of TT Virus: Evidence for a New Virus Family Infecting Humans. Proc. Natl. Acad. Sci. 1999, 96, 3177–3182. [Google Scholar] [CrossRef]
- Zheng, H.; Ye, L.; Fang, X.; Li, B.; Wang, Y.; Xiang, X.; Kong, L.; Wang, W.; Zeng, Y.; Ye, L.; et al. Torque Teno Virus (SANBAN Isolate) ORF2 Protein Suppresses NF-ΚB Pathways via Interaction with IκB Kinases. J. Virol. 2007, 81, 11917–11924. [Google Scholar] [CrossRef]
- Okamoto, H.; Nishizawa, T.; Kato, N.; Ukita, M.; Ikeda, H.; Iizuka, H.; Miyakawa, Y.; Mayumi, M. Molecular Cloning and Characterization of a Novel DNA Virus (TTV) Associated with Posttransfusion Hepatitis of Unknown Etiology. Hepatol. Res. 1998, 10, 1–16. [Google Scholar] [CrossRef]
- Kakkola, L.; Bondén, H.; Hedman, L.; Kivi, N.; Moisala, S.; Julin, J.; Ylä-Liedenpohja, J.; Miettinen, S.; Kantola, K.; Hedman, K.; et al. Expression of All Six Human Torque Teno Virus (TTV) Proteins in Bacteria and in Insect Cells, and Analysis of Their IgG Responses. Virology 2008, 382, 182–189. [Google Scholar] [CrossRef]
- Ukita, M.; Okamoto, H.; Kato, N.; Miyakawa, Y.; Mayumi, M. Excretion into Bile of a Novel Unenveloped DNA Virus (TT Virus) Associated with Acute and Chronic Non-A-G Hepatitis. J. Infect. Dis. 1999, 179, 1245–1248. [Google Scholar] [CrossRef]
- Jelcic, I.; Hotz-Wagenblatt, A.; Hunziker, A.; zur Hausen, H.; de Villiers, E.-M. Isolation of Multiple TT Virus Genotypes from Spleen Biopsy Tissue from a Hodgkin’s Disease Patient: Genome Reorganization and Diversity in the Hypervariable Region. J. Virol. 2004, 78, 7498–7507. [Google Scholar] [CrossRef]
- Nishizawa, T.; Okamoto, H.; Tsuda, F.; Aikawa, T.; Sugai, Y.; Konishi, K.; Akahane, Y.; Ukita, M.; Tanaka, T.; Miyakawa, Y.; et al. Quasispecies of TT Virus (TTV) with Sequence Divergence in Hypervariable Regions of the Capsid Protein in Chronic TTV Infection. J. Virol. 1999, 73, 9604–9608. [Google Scholar] [CrossRef]
- De Smit, M.H.; Noteborn, M.H.M. Apoptosis-Inducing Proteins in Chicken Anemia Virus and TT Virus. Curr. Top. Microbiol. Immunol. 2009, 331, 131–149. [Google Scholar] [PubMed]
- Kanda, Y.; Tanaka, Y.; Kami, M.; Saito, T.; Asai, T.; Izutsu, K.; Yuji, K.; Ogawa, S.; Honda, H.; Mitani, K.; et al. CLINICAL OBSERVATIONS, INTERVENTIONS, AND THERAPEUTIC TRIALS. TT Virus in Bone Marrow Transplant Recipients. Blood J. Am. Soc. Hematol. 1999, 93, 2485–2490. [Google Scholar]
- Okamoto, H.; Takahashi, M.; Nishizawa, T.; Tawara, A.; Sugai, Y.; Sai, T.; Tanaka, T.; Tsuda, F. Replicative Forms of TT Virus DNA in Bone Marrow Cells. Biochem. Biophys. Res. Commun. 2000, 270, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Fornai, C.; Zaccaro, L.; Morrica, A.; Vatteroni, M.L.; Isola, P.; Marchi, S.; Ricchiuti, A.; Pistello, M.; Bendinelli, M. TT Virus (TTV) Loads Associated with Different Peripheral Blood Cell Types and Evidence for TTV Replication in Activated Mononuclear Cells. J. Med. Virol. 2001, 64, 190–194. [Google Scholar] [CrossRef]
- Takahashi, M.; Asabe, S.; Okamoto, H.; Gotanda, Y.; Kishimoto, J.; Tsuda, F. TT Virus Is Distributed in Various Leukocyte Subpopulations at Distinct Levels, with the Highest Viral Load in Granulocytes. Biochem. Biophys. Res. Commun. 2002, 290, 242–248. [Google Scholar] [CrossRef]
- Kyathanahalli, C.; Snedden, M.; Hirsch, E. Human Anelloviruses: Prevalence and Clinical Significance During Pregnancy. Front. Virol. 2021, 1, 782886. [Google Scholar] [CrossRef]
- Dal Lago, S.; Brani, P.; Ietto, G.; Dalla Gasperina, D.; Gianfagna, F.; Bosi, A.; Drago Ferrante, F.; Genoni, A.; Zahira Manzoor, H.; Ambrosini, A.; et al. Torque Teno Virus: A Promising Biomarker in Kidney Trans Plant Recipients. Int. J. Mol. Sci. 2024, 25, 7744. [Google Scholar] [CrossRef]
- Kikuchi, K.; Miyakawa, H.; Abe, K.; Kako, M.; Katayama, K.; Fukushi, S.; Mishiro, S. Indirect Evidence of TTV Replication in Bone Marrow Cells, But Not in Hepatocytes, of a Subacute Hepatitis/Aplastic Anemia Patient. J. Med. Virol. 2000, 61, 165–170. [Google Scholar] [CrossRef]
- Desai, M.; Pal, R.; Deshmukh, R.; Banker, D. Replication of TT Virus in Hepatocyte and Leucocyte Cell Lines. J. Med. Virol. 2005, 77, 136–143. [Google Scholar] [CrossRef]
- Okamoto, H.; Ukita, M.; Nishizawa, T.; Kishimoto, J.; Hoshi, Y.; Mizuo, H.; Tanaka, T.; Miyakawa, Y.; Mayumi, M. Circular Double-Stranded Forms of TT Virus DNA in the Liver. J. Virol. 2000, 74, 5161–5167. [Google Scholar] [CrossRef]
- Deng, X.; Terunuma, H.; Handema, R.; Sakamoto, M.; Kitamura, T.; Ito, M.; Akahane, Y. Higher Prevalence and Viral Load of TT Virus in Saliva Than in the Corresponding Serum: Another Possible Transmission Route and Replication Site of TT Virus. J. Med. Virol. 2000, 62, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, J.; Van Der Hoek, L. Human Anelloviruses: Diverse, Omnipresent and Commensal Members of the Virome. FEMS Microbiol. Rev. 2020, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K.; Kernbichler, S.; Pichler, H.; Lawitschka, A.; Geyeregger, R.; Witt, V.; Lion, T. Post-Transplant Replication of Torque Teno Virus in Granulocytes. Front. Microbiol. 2018, 9, 2956. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Boggi, U.; Nelli, L.C.; Maggi, F. Short-Term Kinetics of Torque Teno Virus Viraemia after Induction Immunosuppression Confirm T Lymphocytes as the Main Replication-Competent Cells. J. Gen. Virol. 2015, 96, 115–117. [Google Scholar] [CrossRef]
- Okamoto, H.; Takahashi, M.; Kato, N.; Fukuda, M.; Tawara, A.; Fukuda, S.; Tanaka, T.; Miyakawa, Y.; Mayumi, M. Sequestration of TT Virus of Restricted Genotypes in Peripheral Blood Mononuclear Cells. J. Virol. 2000, 74, 10236–10239. [Google Scholar] [CrossRef]
- Okamura, A.; Yoshioka, M.; Kubota, M.; Kikuta, H.; Ishiko, H.; Kobayashi, K. Detection of a Novel DNA Virus (TTV) Sequence in Peripheral Blood Mononuclear Cells. J. Med. Virol. 1999, 58, 174–177. [Google Scholar] [CrossRef]
- Verine Vincent, S.; Gerlier, D.; Manié, S.N. Measles Virus Assembly within Membrane Rafts. J. Virol. 2000, 74, 9911–9915. [Google Scholar] [CrossRef]
- Maggi, F.; Fornai, C.; Vatteroni, M.L.; Siciliano, G.; Menichetti, F.; Tascini, C.; Specter, S.; Pistello, M.; Bendinelli, M. Low Prevalence of TT Virus in the Cerebrospinal Fluid of Viremic Patients with Central Nervous System Disorders. J. Med. Virol. 2001, 65, 418–422. [Google Scholar] [CrossRef]
- Kakkola, L.; Hedman, K.; Qiu, J.; Pintel, D.; Söderlund-Venermo, M. Replication of and Protein Synthesis by TT Viruses. Curr. Top. Microbiol. Immunol. 2009, 331, 53–64. [Google Scholar]
- Kamahora, T.; Hino, S.; Miyata, H. Three Spliced MRNAs of TT Virus Transcribed from a Plasmid Containing the Entire Genome in COS1 Cells. J. Virol. 2000, 74, 9980–9986. [Google Scholar] [CrossRef]
- Cebriá-Mendoza, M.; Bracho, M.A.; Arbona, C.; Larrea, L.; Díaz, W.; Sanjuán, R.; Cuevas, J.M. Exploring the Diversity of the Human Blood Virome. Viruses 2021, 13, 2322. [Google Scholar] [CrossRef] [PubMed]
- Tisza, M.J.; Pastrana, D.V.; Welch, N.L.; Stewart, B.; Peretti, A.; Starrett, G.J.; Pang, Y.Y.S.; Krishnamurthy, S.R.; Pesavento, P.A.; McDermott, D.H.; et al. Discovery of Several Thousand Highly Diverse Circular DNA Viruses. Elife 2020, 9, e51971. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomström, A.L.; Cadar, D.; Harrach, B.; Biagini, P.; Kraberger, S. Taxonomic Update for Mammalian Anelloviruses (Family Anelloviridae). Arch. Virol. 2021, 166, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Tsunoda, H.; Kazi, A.; Yamada, A.; Khan, M.A.; Murakami, J.; Kamahora, T.; Shiraki, K.; Hino, S. Identification of a Novel GC-Rich 113-Nucleotide Region to Complete the Circular, Single-Stranded DNA Genome of TT Virus, the First Human Circovirus. J. Virol. 1999, 73, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E.; et al. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Nishiyama, S.; Dutia, B.M.; Stewart, J.P.; Meredith, A.L.; Shaw, D.J.; Simmonds, P.; Sharp, C.P. Identification of Novel Anelloviruses with Broad Diversity in UK Rodents. J. Gen. Virol. 2014, 95, 1544–1553. [Google Scholar] [CrossRef]
- Okamoto, H. TT Viruses in Animals. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 35–52. [Google Scholar]
- Cibulski, S.P.; Teixeira, T.F.; de Sales Lima, F.E.; do Santos, H.F.; Franco, A.C.; Roehe, P.M. A Novel Anelloviridae Species Detected in Tadarida Brasiliensis Bats: First Sequence of a Chiropteran Anellovirus. Genome Announc. 2014, 2, e01028-14. [Google Scholar] [CrossRef]
- Butkovic, A.; Kraberger, S.; Smeele, Z.; Martin, D.P.; Schmidlin, K.; Fontenele, R.S.; Shero, M.R.; Beltran, R.S.; Kirkham, A.L.; Aleamotu’, M.; et al. Evolution of Anelloviruses from a Circovirus-like Ancestor through Gradual Augmentation of the Jelly-Roll Capsid Protein. Virus Evol. 2023, 9, vead035. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global Organization and Proposed Megataxonomy of the Virus World. Microbiol. Mol. Biol. Rev. 2020, 84, 10–128. [Google Scholar] [CrossRef]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early Life Dynamics of the Human Gut Virome and Bacterial Microbiome in Infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef]
- Waits, K.; Edwards, M.J.; Cobb, I.N.; Fontenele, R.S.; Varsani, A. Identification of an Anellovirus and Genomoviruses in Ixodid Ticks. Virus Genes. 2018, 54, 155–159. [Google Scholar] [CrossRef]
- Hrazdilová, K.; Slaninková, E.; Brožová, K.; Modrý, D.; Vodička, R.; Celer, V. New Species of Torque Teno Miniviruses Infecting Gorillas and Chimpanzees. Virology 2016, 487, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.F.F.; Wheeler, E.; Greig, D.; Waltzek, T.B.; Gulland, F.; Breitbart, M. Metagenomic Identification of a Novel Anellovirus in Pacific Harbor Seal (Phoca vitulina richardsii) Lung Samples and Its Detection in Samples from Multiple Years. J. Gen. Virol. 2011, 92, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, Y.; Hu, X.; Xiong, J.; Zhang, B.; Yuan, Z. A Metagenomic Survey of Viral Abundance and Diversity in Mosquitoes from Hubei Province. PLoS ONE 2015, 10, e0129845. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Bárcena, J.; Montoya, M. Virus-Like Particle-Based Vaccines for Animal Viral Infections. Inmunologia 2013, 32, 102–116. [Google Scholar] [CrossRef]
- Okamoto, H.; Akahane, Y.; Ukita, M.; Fukuda, M.; Tsuda, F.; Miyakawa, Y.; Mayumi, M. Fecal Excretion of a Nonenveloped DNA Virus (TTV) Associated with Posttransfusion Non-A-G Hepatitis. J. Med. Virol. 1998, 56, 128–132. [Google Scholar] [CrossRef]
- Maggi, F.; Pifferi, M.; Fornai, C.; Andreoli, E.; Tempestini, E.; Vatteroni, M.; Presciuttini, S.; Marchi, S.; Pietrobelli, A.; Boner, A.; et al. TT Virus in the Nasal Secretions of Children with Acute Respiratory Diseases: Relations to Viremia and Disease Severity. J. Virol. 2003, 77, 2418–2425. [Google Scholar] [CrossRef]
- Ali, S.; Fevery, J.; Peerlinck, K.; Verslype, C.; Schelstraete, R.; Gyselinck, F.; Emonds, M.P.; Vermylen, J.; Yap, S.H. TTV Infection and Its Relation to Serum Transaminases in Apparently Healthy Blood Donors and in Patients with Clotting Disorders Who Have Been Investigated Previously for Hepatitis C Virus and GBV-C/HGV Infection in Belgium. J. Med. Virol. 2002, 66, 561–566. [Google Scholar] [CrossRef]
- Bendinelli, M.; Pistello, M.; Maggi, F.; Fornai, C.; Freer, G.; Vatteroni, M.L. Molecular Properties, Biology, and Clinical Implications of TT Virus, a Recently Identified Widespread Infectious Agent of Humans. Clin. Microbiol. Rev. 2001, 14, 98–113. [Google Scholar] [CrossRef]
- van Rijn, A.L.; Wunderink, H.F.; Sidorov, I.A.; de Brouwer, C.S.; Kroes, A.C.; Putter, H.; de Vries, A.P.; Rotmans, J.I.; Feltkamp, M.C. Torque Teno Virus Loads after Kidney Transplantation Predict Allograft Rejection but Not Viral Infection. J. Clin. Virol. 2021, 140, 104871. [Google Scholar] [CrossRef]
- Blatter, J.A.; Sweet, S.C.; Conrad, C.; Danziger-Isakov, L.A.; Faro, A.; Goldfarb, S.B.; Hayes, D.; Melicoff, E.; Schecter, M.; Storch, G.; et al. Anellovirus Loads Are Associated with Outcomes in Pediatric Lung Transplantation. Pediatr. Transplant. 2018, 22, e13069. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Heilos, A.; Bond, G.; Meyer, E.; Böhm, M.; Puchhammer-Stöckl, E.; Arbeiter, K.; Müller-Sacherer, T.; Csaicsich, D.; Aufricht, C.; et al. Torque Teno Viral Load Reflects Immunosuppression in Paediatric Kidney-Transplanted Patients—A Pilot Study. Pediatr. Nephrol. 2021, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Shi, B.; Kelly, P.J.; Pilmore, H.; Clayton, P.A.; Chadban, S.J. Death after Kidney Transplantation: An Analysis by Era and Time Post-Transplant. J. Am. Soc. Nephrol. 2020, 31, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, J.; Cicilionytė, A.; Timmerman, A.L.; Deijs, M.; Jebbink, M.F.; van Goudoever, J.B.; van Keulen, B.J.; Bakker, M.; van der Hoek, L. Early-Life Colonization by Anelloviruses in Infants. Viruses 2022, 14, 865. [Google Scholar] [CrossRef]
- Nordén, R.; Magnusson, J.; Lundin, A.; Tang, K.W.; Nilsson, S.; Lindh, M.; Andersson, L.M.; Riise, G.C.; Westin, J. Quantification of Torque Teno Virus and Epstein-Barr Virus Is of Limited Value for Predicting the Net State of Immunosuppression after Lung Transplantation. Open Forum Infect. Dis. 2018, 5, ofy050. [Google Scholar] [CrossRef]
- Herrmann, A.; Sandmann, L.; Adams, O.; Herrmann, D.; Dirks, M.; Widera, M.; Westhaus, S.; Kaiser, R.; Di Cristanziano, V.; Manns, M.P.; et al. Role of BK Polyomavirus (BKV) and Torque Teno Virus (TTV) in Liver Transplant Recipients with Renal Impairment. J. Med. Microbiol. 2018, 67, 1496–1508. [Google Scholar] [CrossRef]
- Ruiz, P.; Martínez-Picola, M.; Santana, M.; Muñoz, J.; Pérez-del-Pulgar, S.; Koutsoudakis, G.; Sastre, L.; Colmenero, J.; Crespo, G.; Navasa, M. Torque Teno Virus Is Associated with the State of Immune Suppression Early After Liver Transplantation. Liver Transplant. 2019, 25, 302–310. [Google Scholar] [CrossRef]
- Agrawal, A.; Ison, M.G.; Danziger-Isakov, L. Long-Term Infectious Complications of Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2022, 17, 286–295. [Google Scholar] [CrossRef]
- Kuczaj, A.; Przybyłowski, P.; Hrapkowicz, T. Torque Teno Virus (TTV)—A Potential Marker of Immunocompetence in Solid Organ Recipients. Viruses 2024, 16, 17. [Google Scholar] [CrossRef]
- Querido, S.; Martins, C.; Gomes, P.; Pessanha, M.A.; Arroz, M.J.; Adragão, T.; Casqueiro, A.; Oliveira, R.; Costa, I.; Azinheira, J.; et al. Kinetics of Torque Teno Virus Viral Load Is Associated with Infection and De Novo Donor Specific Antibodies in the First Year after Kidney Transplantation: A Prospective Cohort Study. Viruses 2023, 15, 1464. [Google Scholar] [CrossRef]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torquetenovirus Viremia for Early Prediction of Graft Rejection after Kidney Transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef]
- Görzer, I.; Jaksch, P.; Strassl, R.; Klepetko, W.; Puchhammer-Stöckl, E. Association between Plasma Torque Teno Virus Level and Chronic Lung Allograft Dysfunction after Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, M.; Puchhammer-Stöckl, E.; Eskandary, F.; Kohlbeck, P.; Rasoul-Rockenschaub, S.; Heilos, A.; Kozakowski, N.; Görzer, I.; Kikić, Ž.; Herkner, H.; et al. Torque Teno Virus Load-Inverse Association with Antibody-Mediated Rejection after Kidney Transplantation. Transplantation 2017, 101, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Strassl, R.; Doberer, K.; Rasoul-Rockenschaub, S.; Herkner, H.; Görzer, I.; Kläger, J.P.; Schmidt, R.; Haslacher, H.; Schiemann, M.; Eskandary, F.A.; et al. Torque Teno Virus for Risk Stratification of Acute Biopsyproven Alloreactivity in Kidney Transplant Recipients. J. Infect. Dis. 2019, 219, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; San Juan, R.; Polanco, N.; Andrés, A.; Navarro, D.; et al. Monitoring of Alphatorquevirus DNA Levels for the Prediction of Immunosuppression-Related Complications after Kidney Transplantation. Am. J. Transplant. 2019, 19, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Frye, B.C.; Bierbaum, S.; Falcone, V.; Köhler, T.C.; Gasplmayr, M.; Hettich, I.; Dürk, T.; Idzko, M.; Zissel, G.; Hengel, H.; et al. Kinetics of Torque Teno Virus-DNA Plasma Load Predict Rejection in Lung Transplant Recipients. Transplantation 2019, 103, 815–822. [Google Scholar] [CrossRef]
- Pescarmona, R.; Mouton, W.; Walzer, T.; Dalle, S.; Eberhardt, A.; Brengel-Pesce, K.; Villard, M.; Lombard, C.; Trouillet-Assant, S.; Viel, S. Evaluation of TTV Replication as a Biomarker of Immune Checkpoint Inhibitors Efficacy in Melanoma Patients. PLoS ONE 2021, 16, e0255972. [Google Scholar] [CrossRef]
- Abbate, I.; Rozera, G.; Cimini, E.; Carletti, F.; Tartaglia, E.; Rubino, M.; Pittalis, S.; Esvan, R.; Gagliardini, R.; Mondi, A.; et al. Kinetics of TTV Loads in Peripheral Blood Mononuclear Cells of Early Treated Acute HIV Infections. Viruses 2023, 15, 1931. [Google Scholar] [CrossRef]
- Gergely, P.; Blazsek, A.; Dankó, K.; Ponyi, A.; Poór, G. Detection of TT Virus in Patients with Idiopathic Inflammatory Myopathies. Proc. Ann. N. Y. Acad. Sci. 2005, 1050, 304–313. [Google Scholar] [CrossRef]
- Hettmann, A.; Demcsak, A.; Bach, A.; Decsi, G.; Dencs, A.; Palinko, D.; Rovo, L.; Nagy, K.; Minarovits, J.; Takacs, M. Detection and Phylogenetic Analysis of Torque Teno Virus in Salivary and Tumor Biopsy Samples from Head and Neck Carcinoma Patients. Intervirology 2016, 59, 123–129. [Google Scholar] [CrossRef]
- Tangkijvanich, P.; Hirsch, P.; Theamboonlers, A.; Nuchprayoon, I.; Poovorawan, Y. Association of Hepatitis Viruses with Hepatocellular Carcinoma in Thailand. J. Gastroenterol. 1999, 34, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yeo, W.; Tang, M.W.; Lin, X.-R.; Mo, F.; Ho, W.M.; Johnson, P.J. Gross Elevation of TT Virus Genome Load in the Peripheral Blood Mononuclear Cells of Cancer Patients. Ann. N. Y. Acad. Sci. 2001, 945, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tomasiewicz, K.; Modrzewska, R.; Lyczak, A.; Krawczuk, G. TT Virus Infection and Pancreatic Cancer: Relationship or Accidental Coexistence. World J. Gastroenterol. 2005, 11, 2847–2849. [Google Scholar] [CrossRef]
- Szládek, G.; Juhász, A.; Kardos, K.; Szöke, K.; Major, T.; Sziklai, I.; Tor, I.; Márton, I.; Kónya, J.; Gergely, L.; et al. High Co-Prevalence of Genogroup 1 TT Virus and Human Papillomavirus Is Associated with Poor Clinical Outcome of Laryngeal Carcinoma. J. Clin. Pathol. 2005, 58, 402–405. [Google Scholar] [CrossRef]
- Hino, S.; Miyata, H. Torque Teno Virus (TTV): Current Status. Rev. Med. Virol. 2007, 17, 45–57. [Google Scholar] [CrossRef]
- Focosi, D.; Antonelli, G.; Pistello, M.; Maggi, F. Torquetenovirus: The Human Virome from Bench to Bedside. Clin. Microbiol. Infect. 2016, 22, 589–593. [Google Scholar] [CrossRef]
- Ullah Khan, N.; Sadiq, A.; Khan, J.; Basharat, N.; Hassan, Z.U.; Ali, I.; Shah, T.A.; Bourhia, M.; Bin Jardan, Y.A.; Wondmie, G.F. Molecular Characterization of Plasma Virome of Hepatocellular Carcinoma (HCC) Patients. AMB Express 2024, 14, 46. [Google Scholar] [CrossRef]
- Stefani, D.; Hegedues, B.; Collaud, S.; Zaatar, M.; Ploenes, T.; Valdivia, D.; Elsner, C.; Bleekmann, B.; Widera, M.; Dittmer, U.; et al. Torque Teno Virus Load in Lung Cancer Patients Correlates with Age but Not with Tumor Stage. PLoS ONE 2021, 16, e0252304. [Google Scholar] [CrossRef]
- de la Asunción, C.S.; Giménez, E.; Hernández-Boluda, J.C.; Terol, M.J.; Albert, E.; López, J.; García-Gutiérrez, V.; Andreu, R.; Malo, M.D.G.; Fox, M.L.; et al. Assessment of the Potential Value of Plasma Torque Teno Virus DNA Load Monitoring to Predict Cytomegalovirus DNAemia in Patients with Hematological Malignancies Treated with Small Molecule Inhibitors: A Proof-of-Concept Study. J. Med. Virol. 2023, 95, e28933. [Google Scholar] [CrossRef]
- Engel, B.; Görzer, I.; Campos-Murguia, A.; Hartleben, B.; Puchhammer-Stöckl, E.; Jaeckel, E.; Taubert, R. Association of Torque Teno Virus Viremia with Liver Fibrosis in the First Year after Liver Transplantation. Front. Immunol. 2023, 14, 1215868. [Google Scholar] [CrossRef]
- Kazemi, M.J.; Yaghobi, R.; Saadi, M.I.; Geramizadeh, B.; Moayedi, J. Association between TT Virus Infection and Cirrhosis in Liver Transplant Patients. Hepat. Mon. 2015, 15, e28370. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, F.; Pradier, A.; Masouridi-Levrat, S.; Van Delden, C.; Giostra, E.; Morard, I.; Mueller, N.; Muellhaupt, B.; Valli, P.V.; Semmo, N.; et al. Torque Teno Virus Load and Acute Rejection after Orthotopic Liver Transplantation. Transplantation 2017, 101, e219–e221. [Google Scholar] [CrossRef] [PubMed]
- Béland, K.; Dore-Nguyen, M.; Gagné, M.J.; Patey, N.; Brassard, J.; Alvarez, F.; Halac, U. Torque Teno Virus in Children Who Underwent Orthotopic Liver Transplantation: New Insights about a Common Pathogen. J. Infect. Dis. 2014, 209, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Masier, A.; Boldrin, C.; Calistri, A.; Andreoli, E.; Senzolo, M.; Zorzi, M.; Sgarabotto, D.; Guido, M.; Cillo, U.; et al. Torque Teno Virus: Any Pathological Role in Liver Transplanted Patients? Transpl. Int. 2008, 21, 972–979. [Google Scholar] [CrossRef]
- Zhang, X.; Park, W.D.; Thijssen, M.; Xu, Y.; Tse, L.P.V.; Pourkarim, M.R.; Aurora, R.; Fan, X. Expansion of Betatorquevirus and/or Gammatorquevirus in Patients with Severe Clinical Outcomes of the Liver Diseases. Viruses 2023, 15, 1635. [Google Scholar] [CrossRef]
- Piaggio, F.; Dodi, F.; Bottino, G.; Andorno, E.; Gentile, R.; Ferrari, C.; Barabino, G.; Giannone, A.; Immordino, G.; Miggino, M.; et al. Torque Teno Virus-Cause of Viral Liver Disease Following Liver Transplantation: A Case Report. Transplant. Proc. 2009, 41, 1378–1379. [Google Scholar] [CrossRef]
- Kanda, Y.; Hirai, H. TT Virus in Hematological Disorders and Bone Marrow Transplant Recipients. Leuk. Lymphoma 2001, 40, 483–489. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawada, J.I.; Okuno, Y.; Hayano, S.; Horiba, K.; Torii, Y.; Takahashi, Y.; Umetsu, S.; Sogo, T.; Inui, A.; et al. Comprehensive Detection of Viruses in Pediatric Patients with Acute Liver Failure Using Next-Generation Sequencing. J. Clin. Virol. 2017, 96, 67–72. [Google Scholar] [CrossRef]
- Usta, M.; Dilek, K.; Ersoy, A.; Ozdemir, B.; Mistik, R.; Vuruskan, H.; Gullulu, M.; Yavuz, M.; Oktay, B.; Yurtkuran, M. Prevalence of Transfusion Transmitted Virus Infection and Its Effect on Renal Graft Survival in Renal Transplant Recipients. Scand. J. Urol. Nephrol. 2002, 36, 473–477. [Google Scholar] [CrossRef]
- Yang Zhou, J.; Eder, D.; Weber, F.; Heumann, P.; Kronenberg, K.; Werner, J.M.; Geissler, E.K.; Schlitt, H.J.; Hutchinson, J.A.; Bitterer, F. Case Report: Predictability of Clinical Response and Rejection Risk after Immune Checkpoint Inhibition in Liver Transplantation. Front. Transplant. 2023, 2, 1211916. [Google Scholar] [CrossRef]
- Kheradpezhouh, M.; Taremi, M.; Gachkar, L.; Aghabozorgi, S.; Khoshbaten, M. Presence and Significance of Transfusion-Transmitted Virus Infection in Iranian Patients on Maintenance Hemodialysis. J. Microbiol. Immunol. Infect. 2007, 40, 106–111. [Google Scholar] [PubMed]
- Okumura, T.; Horiba, K.; Kamei, H.; Takeuchi, S.; Suzuki, T.; Torii, Y.; Kawada, J.I.; Takahashi, Y.; Ogura, Y.; Ogi, T.; et al. Temporal Dynamics of the Plasma Microbiome in Recipients at Early Post-Liver Transplantation: A Retrospective Study. BMC Microbiol. 2021, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, N.; Maximova, N.; Campisciano, G.; Del Savio, R.; Pizzol, A.; Casalicchio, G.; Berton, E.; Comar, M. Up-Regulation of the Monocyte Chemotactic Protein-3 in Sera from Bone Marrow Transplanted Children with Torquetenovirus Infection. J. Clin. Virol. 2015, 63, 6–11. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Forque, L.; Albert, E.; Redondo, N.; Giménez, E.; López-Medrano, F.; González, E.; Polanco, N.; Ruiz-Merlo, T.; Parra, P.; et al. Human Pegivirus Type 1 Infection in Kidney Transplant Recipients: Replication Kinetics and Clinical Correlates. Transpl. Infect. Dis. 2022, 24, e13771. [Google Scholar] [CrossRef]
- Maggi, F.; Focosi, D.; Statzu, M.; Bianco, G.; Costa, C.; Macera, L.; Spezia, P.G.; Medici, C.; Albert, E.; Navarro, D.; et al. Early Post-Transplant Torquetenovirus Viremia Predicts Cytomegalovirus Reactivations In Solid Organ Transplant Recipients. Sci. Rep. 2018, 8, 15490. [Google Scholar] [CrossRef]
- Focosi, D.; Macera, L.; Pistello, M.; Maggi, F. Torque Teno Virus Viremia Correlates with Intensity of Maintenance Immunosuppression in Adult Orthotopic Liver Transplant. J. Infect. Dis. 2014, 210, 667–668. [Google Scholar] [CrossRef]
- Béland, K.; Dore-Nguyen, M.; Gagné, M.J.; Patey, N.; Brassard, J.; Alvarez, F.; Halac, U. Torque Teno Virus Load as a Biomarker of Immunosuppression? New Hopes and Insights. J. Infect. Dis. 2014, 210, 668–670. [Google Scholar] [CrossRef][Green Version]
- Reshetnyak, V.I.; Maev, I.V.; Burmistrov, A.I.; Chekmazov, I.A.; Karlovich, T.I. Torque Teno Virus in Liver Diseases: On the Way towards Unity of View. World J. Gastroenterol. 2020, 26, 1691–1707. [Google Scholar] [CrossRef]
- Mrzljak, A.; Vilibic-Cavlek, T. Torque Teno Virus in Liver Diseases and after Liver Transplantation. World J. Transplant. 2020, 10, 291–296. [Google Scholar] [CrossRef]
- Mushahwar, I.K.; Erker, J.E.; Dille, B.J.; Desai, S.M. Recently Discovered Blood-Borne Viruses. Forum-Trends Exp. Clin. Med. 2001, 11, 98–122. [Google Scholar]
- Dharnidharka, V.R.; Ruzinova, M.B.; Chen, C.C.; Parameswaran, P.; O’Gorman, H.; Goss, C.W.; Gu, H.; Storch, G.A.; Wylie, K. Metagenomic Analysis of DNA Viruses from Posttransplant Lymphoproliferative Disorders. Cancer Med. 2019, 8, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, M.; Tacke, F.; Beller, L.; Deboutte, W.; Yinda, K.C.; Nevens, F.; Laleman, W.; Van Ranst, M.; Pourkarim, M.R. Clinical Relevance of Plasma Virome Dynamics in Liver Transplant Recipients. EBioMedicine 2020, 60, 103009. [Google Scholar] [CrossRef] [PubMed]
- Masia, G.; Ingianni, A.; Demelia, L.; Faa, G.; Manconi, P.E.; Pilleri, G.; Ciancio, A.; Rizzetto, M.; Coppola, R.C. TT Virus Infection in Italy: Prevalence and Genotypes in Healthy Subjects, Viral Liver Diseases and Asymptomatic Infections by Parenterally Transmitted Viruses. J. Viral Hepat. 2001, 8, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Genovese, D.; Dettori, S.; Argentini, C.; Kondili, L.A.; La Sorsa, V.; Tisone, G.; Angelico, M.; Rapicetta, M. Molecular Characterisation of SENV and TTV Infections in Hepatopathic Liver-Transplant Patients. Arch. Virol. 2004, 149, 1423–1433. [Google Scholar] [CrossRef]
- Ohto, H.; Ujiie, N.; Takeuchi, C.; Sato, A.; Hayashi, A.; Ishiko, H.; Nishizawa, T. TT Virus Infection during Childhood. Transfusion 2002, 42, 892–898. [Google Scholar] [CrossRef]
- Horváth, R.; Studeník, P.; Benedík, J.; Dendis, M.; Cerný, J. TT Virus Infection in Liver Transplant Recipients with Cryptogennic Cirrhosis. Vnitr. Lek. 2002, 48, 177–181. [Google Scholar]
- Yokosuka, O.; Ikeuchi, T.; Kanda, T.; Kawai, S.; Imazeki, F.; Saisho, H.; Mazzalli, M.; Alves Filho, G.; Nishimura, N.F.; Soares, E.C. The Prevalence of TT Virus Infection in Renal Transplant Recipients in Brazil. Transplantation 2000, 70, 1194–1197. [Google Scholar] [CrossRef]
- Doorenbos, C.S.E.; Jonker, J.; Hao, J.; Gore, E.J.; Kremer, D.; Knobbe, T.J.; de Joode, A.A.E.; Sanders, J.S.F.; Thaunat, O.; Niesters, H.G.M.; et al. Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients. Viruses 2023, 15, 2387. [Google Scholar] [CrossRef]
- Solis, M.; Benotmane, I.; Gallais, F.; Caillard, S.; Fafi-Kremer, S. Torque Teno Virus Viral Load Predicts SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients. J. Med. Virol. 2023, 95, e28936. [Google Scholar] [CrossRef]
- Redondo, N.; Rodríguez-Goncer, I.; Parra, P.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; López-Medrano, F.; San Juan, R.; González, E.; Sevillano, Á.; et al. Impact of Polymorphisms in Genes Orchestrating Innate Immune Responses on Replication Kinetics of Torque Teno Virus after Kidney Transplantation. Front. Genet. 2022, 13, 1069890. [Google Scholar] [CrossRef]
- Haupenthal, F.; Rahn, J.; Maggi, F.; Gelas, F.; Bourgeois, P.; Hugo, C.; Jilma, B.; Böhmig, G.A.; Herkner, H.; Wolzt, M.; et al. A Multicentre, Patient- and Assessor-Blinded, Non-Inferiority, Randomised and Controlled Phase II Trial to Compare Standard and Torque Teno Virus-Guided Immunosuppression in Kidney Transplant Recipients in the First Year after Transplantation: TTVguideIT. Trials 2023, 24, 213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tang, Y.; Lin, T.; Song, T. Torque-Teno Virus for the Prediction of Graft Rejection and Infection Disease after Kidney Transplantation: A Systematic Review and Meta-Analysis. J. Med. Virol. 2023, 95, e28677. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Reineke, M.; Bundschuh, C.; Klein, J.A.F.; Kühn, T.; Zeier, M.; Bartenschlager, R.; Schnitzler, P.; Morath, C.; Speer, C. Quantification of Torque Teno Virus Load to Monitor Short-Term Changes in Immunosuppressive Therapy in Kidney Transplant Recipients. Transplantation 2023, 107, E363–E369. [Google Scholar] [CrossRef]
- Mafi, S.; Essig, M.; Rerolle, J.P.; Lagathu, G.; Crochette, R.; Brodard, V.; Schvartz, B.; Gouarin, S.; Bouvier, N.; Engelmann, I.; et al. Torque Teno Virus Viremia and QuantiFERON®-CMV Assay in Prediction of Cytomegalovirus Reactivation in R+ Kidney Transplant Recipients. Front. Med. 2023, 10, 1180769. [Google Scholar] [CrossRef]
- Cañamero, L.; Benito-Hernández, A.; González, E.; Escagedo, C.; Rodríguez-Vidriales, M.; García-Saiz, M.D.; Valero, R.; Belmar, L.; de Cos, M.A.; Francia, M.V.; et al. Torque Teno Virus Load Predicts Opportunistic Infections after Kidney Transplantation but Is Not Associated with Maintenance Immunosuppression Exposure. Biomedicines 2023, 11, 1410. [Google Scholar] [CrossRef]
- Jaksch, P.; Görzer, I.; Puchhammer-Stöckl, E.; Bond, G. Integrated Immunologic Monitoring in Solid Organ Transplantation: The Road Toward Torque Teno Virus-Guided Immunosuppression. Transplantation 2022, 106, 1940–1951. [Google Scholar] [CrossRef]
- Kelly, E.; Awan, A.; Sweeney, C.; Wildes, D.; De Gascun, C.; Hassan, J.; Riordan, M. Torque Teno Virus Loads as a Marker of Immunosuppression in Pediatric Kidney Transplant Recipients. Pediatr. Transplant. 2024, 28, e14857. [Google Scholar] [CrossRef]
- Jonker, J.; Doorenbos, C.S.E.; Kremer, D.; Gore, E.J.; Niesters, H.G.M.; van Leer-Buter, C.; Bourgeois, P.; Connelly, M.A.; Dullaart, R.P.F.; Berger, S.P.; et al. High-Density Lipoprotein Particles and Torque Teno Virus in Stable Outpatient Kidney Transplant Recipients. Viruses 2024, 16, 143. [Google Scholar] [CrossRef]
- Taher, N.M.; Hussein, M.R.; Kadhim, H.S. The Predicting Role of Torque Teno Virus Infection after Renal Transplantation. Saudi J. Kidney Dis. Transplant. 2021, 32, 1054–1064. [Google Scholar] [CrossRef]
- Grenda, R. Torque Teno (TTV) Viral Load as a Biomarker of Immunosuppressive Strength after Kidney Transplantation in Children. Pediatr. Nephrol. 2021, 36, 1–3. [Google Scholar] [CrossRef]
- Batista, A.M.; Caetano, M.W.; Stincarelli, M.A.; Mamana, A.C.; Zerbinati, R.M.; Sarmento, D.J.S.; Gallottini, M.; Caixeta, R.A.V.; Medina-Pestana, J.; Hasséus, B.; et al. Quantification of Torque Teno Virus (TTV) DNA in Saliva and Plasma Samples in Patients at Short Time before and after Kidney Transplantation. J. Oral. Microbiol. 2022, 14, 2008140. [Google Scholar] [CrossRef] [PubMed]
- Handala, L.; Descamps, V.; Morel, V.; Castelain, S.; François, C.; Duverlie, G.; Helle, F.; Brochot, E. No Correlation between Torque Teno Virus Viral Load and BK Virus Replication after Kidney Transplantation. J. Clin. Virol. 2019, 116, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Eibensteiner, F.; Messner, I.; Uhl, P.; Bond, G.; Puchhammer-Stoeckl, E.; Mueller-Sacherer, T.; Aufricht, C.; Rusai, K. The Association of Torque Teno Viral Load with CMV and BKV Infection in Pediatric and Adolescent Kidney Transplant Patients. J. Clin. Virol. 2024, 172, 105673. [Google Scholar] [CrossRef] [PubMed]
- Reineke, M.; Morath, C.; Speer, C.; Rudek, M.; Bundschuh, C.; Klein, J.A.F.; Mahler, C.F.; Kälble, F.; Nusshag, C.; Beimler, J.; et al. Dynamics of Torque Teno Virus Load in Kidney Transplant Recipients with Indication Biopsy and Therapeutic Modifications of Immunosuppression. Front. Med. 2024, 11, 1337367. [Google Scholar] [CrossRef]
- Davis, J.S.; Chu, G.; Pathinayake, P.; Jones, D.; Giffard, P.; Macera, L.; Choi, P.; Bartlett, N.W. Seroprevalence of Torque Teno Virus in Hemodialysis and Renal Transplant Patients in Australia: A Cross-Sectional Study. Transpl. Infect. Dis. 2020, 22, e13400. [Google Scholar] [CrossRef]
- Reyes, N.S.; Laham, G.; Boccia, N.; García, G.; Jara, R.; Hermida, E.; Ricarte, C.; Diaz, C.; Soler Pujol, G.; Poletta, F.A.; et al. Prospective Cohort Study of Torque Teno Virus (TTV) Viral Load Kinetics and the Association with Graft Rejection in Renal Transplant Patients. J. Clin. Virol. 2023, 165, 105501. [Google Scholar] [CrossRef]
- Doberer, K.; Schiemann, M.; Strassl, R.; Haupenthal, F.; Dermuth, F.; Görzer, I.; Eskandary, F.; Reindl-Schwaighofer, R.; Kikić, Ž.; Puchhammer-Stöckl, E.; et al. Torque Teno Virus for Risk Stratification of Graft Rejection and Infection in Kidney Transplant Recipients—A Prospective Observational Trial. Am. J. Transplant. 2020, 20, 2081–2090. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F. Torque Teno Virus Monitoring in Transplantation: The Quest for Standardization. Am. J. Transplant. 2019, 19, 1599–1601. [Google Scholar] [CrossRef]
- Chauvelot, L.; Barba, T.; Saison, C.; Siska, E.; Kulifaj, D.; Bakker, S.J.L.; Koenig, A.; Rabeyrin, M.; Buron, F.; Picard, C.; et al. Longitudinal Monitoring of Torque Teno Virus DNAemia in Kidney Transplant Recipients Correlates with Long-Term Complications of Inadequate Immunosuppression. J. Med. Virol. 2024, 96, e29806. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Rodríguez-Goncer, I.; Andrés, A.; Navarro, D.; Aguado, J.M. Early Kinetics of Torque Teno Virus DNA Load and BK Polyomavirus Viremia after Kidney Transplantation. Transpl. Infect. Dis. 2020, 22, e13240. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M. Torque Teno Virus Load as a Surrogate Marker for the Net State of Immunosuppression: The Beneficial Side of the Virome. Am. J. Transplant. 2020, 20, 1963–1964. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, L.; Truffot, A.; Germi, R.; Bugnazet, M.; Malvezzi, P.; Gnesotto, M.; Rostaing, L.; Jouve, T.; Noble, J. Evaluation of Torque Teno Virus DNA Load as a Predictive Biomarker in Kidney Transplant Recipients Converted from Calcineurin Inhibitors to Belatacept. Kidney Int. Rep. 2024, 9, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Roberto, P.; Cinti, L.; Napoli, A.; Paesani, D.; Riveros Cabral, R.J.; Maggi, F.; Garofalo, M.; Pretagostini, R.; Centofanti, A.; Carillo, C.; et al. Torque Teno Virus (TTV): A Gentle Spy Virus of Immune Status, Predictive Marker of Seroconversion to COVID-19 Vaccine in Kidney and Lung Transplant Recipients. J. Med. Virol. 2023, 95, e28512. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schrag, T.A.; Havel, E.F.; Kainz, A.; Omic, H.; Doberer, K.; Kozakowski, N.; Körmöczi, G.F.; Schönbacher, M.; Fischer, G.; et al. Polyomavirus Nephropathy in ABO Blood Group-Incompatible Kidney Transplantation: Torque Teno Virus and Immunosuppressive Burden as an Approximation to the Problem. Kidney Int. Rep. 2024, 9, 1730–1741. [Google Scholar] [CrossRef]
- Regele, F.; Haupenthal, F.; Doberer, K.; Görzer, I.; Kapps, S.; Strassl, R.; Bond, G. The Kinetics of Torque Teno Virus Plasma Load Following Calcineurin Inhibitor Dose Change in Kidney Transplant Recipients. J. Med. Virol. 2024, 96, e29554. [Google Scholar] [CrossRef]
- Imhof, C.; Messchendorp, L.; van Baarle, D.; Gansevoort, R.T.; Van Leer-Buter, C.; Sanders, J.S.F. The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients. Viruses 2023, 15, 2189. [Google Scholar] [CrossRef]
- Strassl, R.; Schiemann, M.; Doberer, K.; Görzer, I.; Puchhammer-Stöckl, E.; Eskandary, F.; Kikić, Ž.; Gualdoni, G.A.; Vossen, M.G.; Rasoul-Rockenschaub, S.; et al. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J. Infect. Dis. 2018, 218, 1191–1199. [Google Scholar] [CrossRef]
- Doberer, K.; Haupenthal, F.; Nackenhorst, M.; Bauernfeind, F.; Dermuth, F.; Eigenschink, M.; Schiemann, M.; Kläger, J.; Görzer, I.; Eskandary, F.; et al. Torque Teno Virus Load Is Associated with Subclinical Alloreactivity in Kidney Transplant Recipients: A Prospective Observational Trial. Transplantation 2021, 105, 2112–2118. [Google Scholar] [CrossRef]
- Forqué, L.; Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Monzó, C.; Chaves, J.; Redondo, N.; Rodríguez-Goncer, I.; Ruiz-Merlo, T.; Parra, P.; et al. Dynamics of Human Anelloviruses in Plasma and Clinical Outcomes Following Kidney Transplantation. Transplantation 2023, 107, 511–520. [Google Scholar] [CrossRef]
- Ahlenstiel-Grunow, T.; Pape, L. Novel Ways to Monitor Immunosuppression in Pediatric Kidney Transplant Recipients—Underlying Concepts and Emerging Data. Mol. Cell. Pediatr. 2021, 8, 8. [Google Scholar] [CrossRef]
- Graninger, M.; Stumpf, J.; Bond, G.; Görzer, I.; Springer, D.N.; Kessel, F.; Kröger, H.; Frank, K.; Tonn, T.; Hugo, C.; et al. Prediction of Humoral and Cellular Immune Response to COVID-19 MRNA Vaccination by TTV Load in Kidney Transplant Recipients and Hemodialysis Patients. J. Clin. Virol. 2023, 162, 105428. [Google Scholar] [CrossRef] [PubMed]
- Kapps, S.; Mühlbacher, J.; Kulifaj, D.; Courjal, S.; Eskandary, F.; Schiemann, M.; Jilma, B.; Böhmig, G.A.; Bond, G.; Wahrmann, M. Torque Teno Virus Control by the Classical Pathway of Complement Activation—A Retrospective Analysis From a First-in-Human Trial Utilizing Sutimlimab. J. Med. Virol. 2024, 96, e70039. [Google Scholar] [CrossRef] [PubMed]
- Kulifaj, D.; Durgueil-Lariviere, B.; Meynier, F.; Munteanu, E.; Pichon, N.; Dubé, M.; Joannes, M.; Essig, M.; Hantz, S.; Barranger, C.; et al. Development of a Standardized Real Time PCR for Torque Teno Viruses (TTV) Viral Load Detection and Quantification: A New Tool for Immune Monitoring. J. Clin. Virol. 2018, 105, 118–127. [Google Scholar] [CrossRef]
- Tian, X.; Duan, W.; Zhang, X.; Wu, X.; Zhang, C.; Wang, Z.; Cao, G.; Gu, Y.; Shao, F.; Yan, T. Metagenomic Next-Generation Sequencing Reveals the Profile of Viral Infections in Kidney Transplant Recipients During the COVID-19 Pandemic. Front. Public Health 2022, 10, 888064. [Google Scholar] [CrossRef]
- Doberer, K.; Bond, G. An Author’s Reply to the Editorial “Torque Teno Virus Load as a Surrogate Marker for the Net State of Immunosuppression: The Beneficial Side of the Virome”. Am. J. Transplant. 2020, 20, 2280. [Google Scholar] [CrossRef]
- Sinha, R.; Zhu, Z.; Park, S.; Rebello, C.; Kinsella, B.; Friedewald, J.; Kleiboeker, S. Combined Metagenomic Viral Detection and Donor-Derived Cell-Free DNA Quantification in Plasma From Kidney Transplant Recipients. Transplant. Proc. 2024, 56, 1522–1530. [Google Scholar] [CrossRef]
- Moen, E.M.; Sagedal, S.; Bjøro, K.; Degré, M.; Opstad, P.K.; Grinde, B. Effect of Immune Modulation on TT Virus (TTV) and TTV-like-Mini-Virus (TLMV) Viremia. J. Med. Virol. 2003, 70, 177–182. [Google Scholar] [CrossRef]
- Martelli, F.; Macera, L.; Spezia, P.G.; Medici, C.; Pistello, M.; Guasti, D.; Romagnoli, P.; Maggi, F.; Giannecchini, S. Torquetenovirus Detection in Exosomes Enriched Vesicles Circulating in Human Plasma Samples. Virol. J. 2018, 15, 145. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Li, J.; Feng, M.; Wang, Z.; Huang, F.; Xu, H.; Liu, L.; Shang, W. Metagenomic versus Targeted Next-Generation Sequencing for Detection of Microorganisms in Bronchoalveolar Lavage Fluid among Renal Transplantation Recipients. Front. Immunol. 2024, 15, 1443057. [Google Scholar] [CrossRef]
- Querido, S.; Calça, R.; Weigert, A.; Francisco, D.; Adragão, T.; Ana Pessanha, M.; Gomes, P.; Rodrigues, L.; Figueira, J.M.; Cardoso, C.; et al. Kinetics of Torquetenovirus DNA Load in a Recent Kidney Transplant Recipient with Mild SARS-CoV-2 Infection and a Failed Antibody Response. Transpl. Infect. Dis. 2021, 23, e13524. [Google Scholar] [CrossRef]
- Eskandary, F.; Bond, G.; Mohan, K. Torque Teno Virus-Guided Immunosuppression in Kidney Transplantation: Expanding the Application. Kidney Int. Rep. 2024, 9, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.S.; Spezia, P.G.; Jara, R.; Filippini, F.; Boccia, N.; García, G.; Hermida, E.; Poletta, F.A.; Pistello, M.; Laham, G.; et al. Torque Teno Virus (TTV) in Renal Transplant Recipients: Species Diversity and Variability. Viruses 2024, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Kulifaj, D.; Tilloy, V.; Scaon, E.; Guerin, E.; Essig, M.; Pichon, N.; Hantz, S.; De Bernardi, A.; Joannes, M.; Barranger, C.; et al. Viral Metagenomics Analysis of Kidney Donors and Recipients: Torque Teno Virus Genotyping and Prevalence. J. Med. Virol. 2020, 92, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Szládek, G.; Juhász, A.; Asztalos, L.; Szöke, K.; Murvai, M.; Szarka, K.; Veress, G.; Gergely, L.; Kónya, J. Persisting TT Virus (TTV) Genogroup 1 Variants in Renal Transplant Recipients. Arch. Virol. 2003, 148, 841–851. [Google Scholar] [CrossRef]
- El-Taher, S.M.; Fouad, N.A.; Fouad, M.A.; Mahedy, A.W.; Elnazi, A.K. Transfusion-Transmitted Virus Infection in Hemodialysis Patients in Arar, Saudi Arabia: Prevalence, Predictors and Genotyping. Saudi J. Kidney Dis. Transpl. 2015, 26, 1215–1222. [Google Scholar] [CrossRef]
- Pan, L.; Wu, F.; Cai, Q.; Xu, Z.; Hu, H.; Tang, T.; Yue, R.; Hou, Y.; Zhang, X.; Fang, Y.; et al. Whole Genome Profiling of Lung Microbiome in Solid Organ Transplant Recipients Reveals Virus Involved Microecology May Worsen Prognosis. Front. Cell. Infect. Microbiol. 2022, 12, 863399. [Google Scholar] [CrossRef]
- Moneke, I.; Ogutur, E.D.; Kornyeva, A.; Fähndrich, S.; Schibilsky, D.; Bierbaum, S.; Czerny, M.; Stolz, D.; Passlick, B.; Jungraithmayr, W.; et al. Donor Age over 55 Is Associated with Worse Outcome in Lung Transplant Recipients with Idiopathic Pulmonary Fibrosis. BMC Pulm. Med. 2024, 24, 499. [Google Scholar] [CrossRef]
- Görzer, I.; Jaksch, P.; Kundi, M.; Seitz, T.; Klepetko, W.; Puchhammer-Stöckl, E. Pre-Transplant Plasma Torque Teno Virus Load and Increase Dynamics after Lung Transplantation. PLoS ONE 2015, 10, e0122975. [Google Scholar] [CrossRef]
- Gallais, F.; Renaud-Picard, B.; Solis, M.; Laugel, E.; Soulier, E.; Caillard, S.; Kessler, R.; Fafi-Kremer, S. Torque Teno Virus DNA Load as a Predictive Marker of Antibody Response to a Three-Dose Regimen of COVID-19 MRNA-Based Vaccine in Lung Transplant Recipients. J. Heart Lung Transplant. 2022, 41, 1429–1439. [Google Scholar] [CrossRef]
- Jaksch, P.; Kundi, M.; Görzer, I.; Muraközy, G.; Lambers, C.; Benazzo, A.; Hoetzenecker, K.; Klepetko, W.; Puchhammer-Stöckl, E. Torque Teno Virus as a Novel Biomarker Targeting the Efficacy of Immunosuppression after Lung Transplantation. J. Infect. Dis. 2018, 218, 1922–1928. [Google Scholar] [CrossRef]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA Levels of Torque Teno Virus and Immunosuppression after Lung Transplantation. J. Heart Lung Transplant. 2014, 33, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Blatter, J.A.; Takahashi, T.; Mittler, B.; Nava, R.G.; Puri, V.; Kreisel, D.; Wang, D. Anellovirus Dynamics Are Associated with Primary Graft Dysfunction in Lung Transplantation. Transplant. Direct 2020, 6, E521. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Diamond, J.M.; Chehoud, C.; Chang, B.; Kotzin, J.J.; Young, J.C.; Imai, I.; Haas, A.R.; Cantu, E.; Lederer, D.J.; et al. The Perioperative Lung Transplant Virome: Torque Teno Viruses Are Elevated in Donor Lungs and Show Divergent Dynamics in Primary Graft Dysfunction. Am. J. Transplant. 2017, 17, 1313–1324. [Google Scholar] [CrossRef]
- Widder, S.; Görzer, I.; Friedel, B.; Rahimi, N.; Schwarz, S.; Jaksch, P.; Knapp, S.; Puchhammer-Stöckl, E. Metagenomic Sequencing Reveals Time, Host, and Body Compartment-Specific Viral Dynamics after Lung Transplantation. Microbiome 2022, 10, 66. [Google Scholar] [CrossRef]
- Segura-Wang, M.; Görzer, I.; Jaksch, P.; Puchhammer-Stöckl, E. Temporal Dynamics of the Lung and Plasma Viromes in Lung Transplant Recipients. PLoS ONE 2018, 13, e0200428. [Google Scholar] [CrossRef]
- Gottlieb, J.; Reuss, A.; Mayer, K.; Weide, K.; Schade-Brittinger, C.; Hoyer, S.; Jaksch, P. Viral Load-Guided Immunosuppression after Lung Transplantation (VIGILung)—Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 48. [Google Scholar] [CrossRef]
- Focosi, D.; Baj, A.; Azzi, L.; Novazzi, F.; Maggi, F. TTV Viral Load as a Predictor of Antibody Response to SARS COV-2 Vaccination. J. Heart Lung Transplant. 2023, 42, 143–144. [Google Scholar] [CrossRef]
- Hoek, R.A.; Verschuuren, E.A.; de Vries, R.D.; Vonk, J.M.; van Baarle, D.; van der Heiden, M.; van Gemert, J.P.; Gore, E.J.; Niesters, H.G.; Erasmus, M.; et al. High Torque Tenovirus (TTV) Load before First Vaccine Dose Is Associated with Poor Serological Response to COVID-19 Vaccination in Lung Transplant Recipients. J. Heart Lung Transplant. 2022, 41, 765–772. [Google Scholar] [CrossRef]
- Lewandowska, D.W.; Schreiber, P.W.; Schuurmans, M.M.; Ruehe, B.; Zagordi, O.; Bayard, C.; Greiner, M.; Geissberger, F.D.; Capaul, R.; Zbinden, A.; et al. Metagenomic Sequencing Complements Routine Diagnostics in Identifying Viral Pathogens in Lung Transplant Recipients with Unknown Etiology of Respiratory Infection. PLoS ONE 2017, 12, e0177340. [Google Scholar] [CrossRef]
- Mitchell, A.B.; Glanville, A.R. Kinetics of Ttv-DNA Plasma Load: A Global Measure of Immune Suppression? Transplantation 2019, 103, 660–661. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Drabe, C.H.; Sørensen, S.S.; Rasmussen, A.; Perch, M.; Ostrowski, S.R.; Nielsen, S.D. Torque-Teno Virus Viral Load as a Potential Endogenous Marker of Immune Function in Solid Organ Transplantation. Transplant. Rev. 2019, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Young, J.C.; Clarke, E.L.; Diamond, J.M.; Imai, I.; Haas, A.R.; Cantu, E.; Lederer, D.J.; Meyer, K.; Milewski, R.K.; et al. Bidirectional Transfer of Anelloviridae Lineages between Graft and Host during Lung Transplantation. Am. J. Transplant. 2019, 19, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Microbiome: Anelloviridae Go Viral. Nat. Rev. Microbiol. 2014, 12, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Clemmensen, T.S.; Petersen, M.S.; Mogensen, L.J.H.; Christiansen, M.; Rolid, K.; Nytrøen, K.; Møller, B.K.; Gullestad, L.; Eiskjær, H.; et al. Kinetics of Torque Teno Virus in Heart Transplant Patients. Hum. Immunol. 2023, 84, 110720. [Google Scholar] [CrossRef]
- Huang, A.L.; Hendren, N.; Carter, S.; Larsen, C.; Garg, S.; La Hoz, R.; Farr, M. Biomarker-Based Assessment for Infectious Risk Before and After Heart Transplantation. Curr. Heart Fail. Rep. 2022, 19, 236–246. [Google Scholar] [CrossRef]
- Rauff, B.; Idrees, M.; Shah, S.A.R.; Butt, S.; Butt, A.M.; Ali, L.; Hussain, A.; Irshad-Ur-Rehman; Ali, M. Hepatitis Associated Aplastic Anemia: A Review. Virol. J. 2011, 8, 87. [Google Scholar] [CrossRef]
- Lin, C.; Huang, Y.; Chen, W. Analysis of TTV Infection in Patients of Hemodialysis and Bone Marrow Transplantation. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999, 13, 194–196. [Google Scholar]
- Bueno, F.; Albert, E.; Piñana, J.L.; Pérez, A.; Úbeda, C.; Gómez, M.D.; Hernández-Boluda, J.C.; Gonzalez-Barberá, E.M.; Montoro, J.; Giménez, E.; et al. Kinetics of Torque Teno Virus DNA in Stools May Predict Occurrence of Acute Intestinal Graft versus Host Disease Early after Allogeneic Hematopoietic Stem Cell Transplantation. Transpl. Infect. Dis. 2021, 23, e13507. [Google Scholar] [CrossRef]
- Masouridi-Levrat, S.; Pradier, A.; Simonetta, F.; Kaiser, L.; Chalandon, Y.; Roosnek, E. Torque Teno Virus in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Malignancies. Bone Marrow Transplant. 2016, 51, 440–442. [Google Scholar] [CrossRef]
- Forqué, L.; Albert, E.; Piñana, J.L.; Pérez, A.; Hernani, R.; Solano, C.; Navarro, D.; Giménez, E. Monitoring of Plasma Torque Teno Virus, Total Anelloviridae and Human Pegivirus 1 Viral Load for the Prediction of Infectious Events and Acute Graft versus Host Disease in the Allogeneic Hematopoietic Stem Cell Transplantation Setting. J. Med. Virol. 2023, 95, e29107. [Google Scholar] [CrossRef]
- Spiertz, A.; Tsakmaklis, A.; Farowski, F.; Knops, E.; Heger, E.; Wirtz, M.; Kaiser, R.; Holtick, U.; Vehreschild, M.J.G.T.; Di Cristanziano, V. Torque Teno Virus-DNA Load as Individual Cytomegalovirus Risk Assessment Parameter upon Allogeneic Hematopoietic Stem Cell Transplantation. Eur. J. Haematol. 2023, 111, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Grenier, C.; Magro, L.; Hober, D.; Yakoub-Agha, I.; Engelmann, I. High Torque Teno Virus Load and Outcome of Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. J. Med. Virol. 2024, 96, e29458. [Google Scholar] [CrossRef]
- Ramzi, M.; Saadi, M.I.; Zarei, T.; Yaghobi, R.; Arandi, N. Association between Cytotoxic T-Lymphocyte Antigen 4 Gene Polymorphisms and Torque Teno Virus Infection after Hematopoietic Stem Cell Transplantation. Exp. Clin. Transplant. 2021, 19, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Pradier, A.; Masouridi-Levrat, S.; Bosshard, C.; Dantin, C.; Vu, D.L.; Zanella, M.C.; Boely, E.; Tapparel, C.; Kaiser, L.; Chalandon, Y.; et al. Torque Teno Virus as a Potential Biomarker for Complications and Survival After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Giménez, E.; Monzó, C.; Albert, E.; Fuentes-Trillo, A.; Seda, E.; Piñana, J.L.; Hernández Boluda, J.C.; Solano, C.; Chaves, J.; Navarro, D. Diversity and Dynamic Changes of Anelloviruses in Plasma Following Allogeneic Hematopoietic Stem Cell Transplantation. J. Med. Virol. 2021, 93, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Solano, C.; Pascual, T.; Torres, I.; Macera, L.; Focosi, D.; Maggi, F.; Giménez, E.; Amat, P.; Navarro, D. Dynamics of Torque Teno Virus Plasma DNAemia in Allogeneic Stem Cell Transplant Recipients. J. Clin. Virol. 2017, 94, 22–28. [Google Scholar] [CrossRef]
- Albert, E.; Torres, I.; Talaya, A.; Giménez, E.; Piñana, J.L.; Hernández-Boluda, J.C.; Focosi, D.; Macera, L.; Maggi, F.; Solano, C.; et al. Kinetics of Torque Teno Virus DNA Load in Saliva and Plasma Following Allogeneic Hematopoietic Stem Cell Transplantation. J. Med. Virol. 2018, 90, 1438–1443. [Google Scholar] [CrossRef]
- Peker, B.O.; Daloğlu, A.E.; Görzer, I.; Puchhammer-Stöckl, E.; Parkan, Ö.M.; Akbaş, H.; Kintrup, G.T.; Mutlu, D.; Küpesiz, O.A.; Çolak, D. Investigation of Torque Teno Virus (TTV) DNA as an Immunological and Virological Marker in Pediatric Hematopoietic Stem Cell Transplantation (HSCT) Patients. Microb. Pathog. 2020, 149, 104397. [Google Scholar] [CrossRef]
- Zanella, M.C.; Cordey, S.; Kaiser, L. Beyond Cytomegalovirus and Epstein-Barr Virus: A Review of Viruses Composing the Blood Virome of Solid Organ Transplant and Hematopoietic Stem Cell Transplant Recipients. Clin. Microbiol. Rev. 2020, 33, e00027-20. [Google Scholar] [CrossRef]
- Redondo, N.; Navarro, D.; Aguado, J.M.; Fernández-Ruiz, M. Viruses, Friends, and Foes: The Case of Torque Teno Virus and the Net State of Immunosuppression. Transpl. Infect. Dis. 2022, 24, e13778. [Google Scholar] [CrossRef]
- Maximova, N.; Pizzol, A.; Ferrara, G.; Maestro, A.; Tamaro, P. Does Teno Torque Virus Induce Autoimmunity after Hematopoietic Stem Cell Transplantation? A Case Report. J. Pediatr. Hematol. Oncol. 2015, 37, e194–e197. [Google Scholar] [CrossRef] [PubMed]
- Gilles, R.; Herling, M.; Holtick, U.; Heger, E.; Awerkiew, S.; Fish, I.; Höller, K.; Sierra, S.; Knops, E.; Kaiser, R.; et al. Dynamics of Torque Teno Virus Viremia Could Predict Risk of Complications after Allogeneic Hematopoietic Stem Cell Transplantation. Med. Microbiol. Immunol. 2017, 206, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mouton, W.; Conrad, A.; Bal, A.; Boccard, M.; Malcus, C.; Ducastelle-Lepretre, S.; Balsat, M.; Barraco, F.; Larcher, M.V.; Fossard, G.; et al. Torque Teno Virus Viral Load as a Marker of Immune Function in Allogeneic Haematopoietic Stem Cell Transplantation Recipients. Viruses 2020, 12, 1292. [Google Scholar] [CrossRef]
- Wohlfarth, P.; Leiner, M.; Schoergenhofer, C.; Hopfinger, G.; Goerzer, I.; Puchhammer-Stoeckl, E.; Rabitsch, W. Torquetenovirus Dynamics and Immune Marker Properties in Patients Following Allogeneic Hematopoietic Stem Cell Transplantation: A Prospective Longitudinal Study. Biol. Blood Marrow Transplant. 2018, 24, 194–199. [Google Scholar] [CrossRef]
- Albert, E.; Giménez, E.; Hernani, R.; Piñana, J.L.; Solano, C.; Navarro, D. Torque Teno Virus DNA Load in Blood as an Immune Status Biomarker in Adult Hematological Patients: The State of the Art and Future Prospects. Viruses 2024, 16, 459. [Google Scholar] [CrossRef]
- Albert, E.; Solano, C.; Giménez, E.; Focosi, D.; Pérez, A.; Macera, L.; Piñana, J.L.; Boluda, J.C.H.; Maggi, F.; Navarro, D. The Kinetics of Torque Teno Virus Plasma DNA Load Shortly after Engraftment Predicts the Risk of High-Level CMV DNAemia in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Bone Marrow Transplant. 2018, 53, 180–187. [Google Scholar] [CrossRef]
- Albert, E.; Solano, C.; Giménez, E.; Focosi, D.; Pérez, A.; Macera, L.; Piñana, J.L.; Mateo, E.M.; Boluda, J.C.H.; Maggi, F.; et al. Kinetics of Alphatorquevirus Plasma DNAemia at Late Times after Allogeneic Hematopoietic Stem Cell Transplantation. Med. Microbiol. Immunol. 2019, 208, 253–258. [Google Scholar] [CrossRef]
- Iovino, L.; Mazziotta, F.; Carulli, G.; Guerrini, F.; Morganti, R.; Mazzotti, V.; Maggi, F.; Macera, L.; Orciuolo, E.; Buda, G.; et al. High-Dose Zinc Oral Supplementation after Stem Cell Transplantation Causes an Increase of TRECs and CD4+ Naïve Lymphocytes and Prevents TTV Reactivation. Leuk. Res. 2018, 70, 20–24. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F.; Albani, M.; Macera, L.; Ricci, V.; Gragnani, S.; Di Beo, S.; Ghimenti, M.; Antonelli, G.; Bendinelli, M.; et al. Torquetenovirus Viremia Kinetics after Autologous Stem Cell Transplantation Are Predictable and May Serve as a Surrogate Marker of Functional Immune Reconstitution. J. Clin. Virol. 2010, 47, 189–192. [Google Scholar] [CrossRef]
- Zhang, B.; Gui, R.; Wang, Q.; Jiao, X.; Li, Z.; Wang, J.; Han, L.; Zhou, L.; Wang, H.; Wang, X.; et al. Comparing the Application of MNGS after Combined Pneumonia in Hematologic Patients Receiving Hematopoietic Stem Cell Transplantation and Chemotherapy: A Retrospective Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 969126. [Google Scholar] [CrossRef]
- Schmitz, J.; Kobbe, G.; Kondakci, M.; Schuler, E.; Magorsch, M.; Adams, O. The Value of Torque Teno Virus (TTV) as a Marker for the Degree of Immunosuppression in Adult Patients after Hematopoietic Stem Cell Transplantation (HSCT): TTV as a Biomarker in Adult Patients after HSCT. Biol. Blood Marrow Transplant. 2020, 26, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, M.; Arandi, N.; Zarei, T.; Iravani Saadi, M.; Yaghobi, R.; Moghadam, M.; Geramizadeh, B. Genetic Variation of TNF-α and IL-10, IL-12, IL-17 Genes and Association with Torque Teno Virus Infection Post Hematopoietic Stem Cell Transplantation. Acta Virol. 2019, 63, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pou, C.; Barrientos-Somarribas, M.; Marin-Juan, S.; Bogdanovic, G.; Bjerkner, A.; Allander, T.; Gustafsson, B.; Andersson, B. Virome Definition in Cerebrospinal Fluid of Patients with Neurological Complications after Hematopoietic Stem Cell Transplantation. J. Clin. Virol. 2018, 108, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.F.F.; Dill, J.A.; Camus, A.C.; Delwart, E.; Van Meir, E.G. Two New Species of Betatorqueviruses Identified in a Human Melanoma That Metastasized to the Brain. Oncotarget 2017, 8, 105800–105808. [Google Scholar] [CrossRef]
- Li, G.; Jin, Y.; Chen, B.; Lin, A.; Wang, E.; Xu, F.; Hu, G.; Xiao, C.; Liu, H.; Hou, X.; et al. Exploring the Relationship between the Gut Mucosal Virome and Colorectal Cancer: Characteristics and Correlations. Cancers 2023, 15, 3555. [Google Scholar] [CrossRef]
- De Villiers, E.M.; Bulajic, M.; Nitsch, C.; Kecmanovic, D.; Pavlov, M.; Kopp-Schneider, A.; Löhr, M. TTV Infection in Colorectal Cancer Tissues and Normal Mucosa. Int. J. Cancer 2007, 121, 2109–2112. [Google Scholar] [CrossRef]
- Rosa, A.S.; Araujo, O.C.; Savassi-Ribas, F.; Fernandes, C.A.; Coelho, H.S.; Niel, C.; Villela-Nogueira, C.A.; Araujo, N.M. Prevalence of Occult Hepatitis B Virus Infection and Torque Teno Virus Infection and Their Association with Hepatocellular Carcinoma in Chronic Hepatitis C Patients. Virus Res. 2017, 242, 166–172. [Google Scholar] [CrossRef]
- Zhong, S.; Yeo, W.; Tang, M.; Liu, C.; Lin, X.R.; Ho, W.M.; Hui, P.; Johnson, P.J. Frequent Detection of the Replicative Form of TT Virus DNA in Peripheral Blood Mononuclear Cells and Bone Marrow Cells in Cancer Patients. J. Med. Virol. 2002, 66, 428–434. [Google Scholar] [CrossRef]
- De Villiers, E.M.; Schmidt, R.; Delius, H.; Zur Hausen, H. Heterogeneity of TT Virus Related Sequences Isolated from Human Tumour Biopsy Specimens. J. Mol. Med. 2002, 80, 44–50. [Google Scholar] [CrossRef]
- Tokita, H.; Murai, S.; Kamitsukasa, H.; Yagura, M.; Harada, H.; Takahashi, M.; Okamoto, H. High TT Virus Load as an Independent Factor Associated with the Occurrence of Hepatocellular Carcinoma among Patients with Hepatitis C Virus-Related Chronic Liver Disease. J. Med. Virol. 2002, 67, 501–509. [Google Scholar] [CrossRef]
- Comar, M.; Dal Molin, G.; D’Agaro, P.; Crocè, S.L.; Tiribelli, C.; Campello, C. HBV, HCV, and TTV Detection by in Situ Polymerase Chain Reaction Could Reveal Occult Infection in Hepatocellular Carcinoma: Comparison with Blood Markers. J. Clin. Pathol. 2006, 59, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Hepatitis Viruses (Other than Hepatitis B and C Viruses) as Causes of Hepatocellular Carcinoma: An Update. J. Viral Hepat. 2013, 20, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Xu, Y.; Aurora, R.; Di Bisceglie, A.M.; Fan, X. Within-Host Quantitation of Anellovirus Genome Complexity from Clinical Samples. J. Virol. Methods 2022, 302, 114493. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kato, N.; Shiratori, Y.; Lan, K.H.; Ono-Nita, S.K.; Feng, Z.; Shiina, S.; Omata, M. Poor Association of TT Virus Viremia with Hepatocellular Carcinoma. Liver 2000, 20, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qian, J.M.; Yang, X.O.; Sun, G.; Lu, C.M. Relationship between TT Virus Infection and Hepatocellular Carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2001, 23, 627–630. [Google Scholar]
- Hassoba, H.; Mahmoud, M.; Fahmy, H.; Leheta, O.; Sayed, A.; Fathy, A.; Serwah, A.; Abbas, A.; Nooman, Z.; Attia, F.; et al. TT Virus Infection among Egyptian Patients with Hepatocellular Carcinoma. Egypt. J. Immunol. Egypt. Assoc. Immunol. 2003, 10, 9–16. [Google Scholar]
- Michitaka, K.; Onji, M. Causes of Non-B, Non-C Hepatocellular Carcinoma: Is TTV a Causative Agent? Intern. Med. 2003, 42, 1157–1158. [Google Scholar] [CrossRef]
- Toshikuni, N.; Kayano, K.; Nishioka, S.; Mitsunaga, M.; Omoto, A.; Nagao, T.; Kojo, M. TT Virus-Positive Hepatocellular Carcinoma Arising from Non-Cirrhotic Liver in an Elderly Man. Intern. Med. 2003, 42, 1172–1177. [Google Scholar] [CrossRef]
- Hafez, M.M.; Shaarawy, S.M.; Hassan, A.A.; Salim, R.F.; Abd El Salam, F.M.; Ali, A.E. Prevalence of Transfusion Transmitted Virus (TTV) Genotypes among HCC Patients in Qaluobia Governorate. Virol. J. 2007, 4, 135. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Dong, J.F. Detection of TT Virus DNA in Peripheral Blood Mononuclear Cells in Patients with Chronic Hepatitis B and Hepatocellular Carcinoma Using PCR and in Situ Hybridization. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999, 13, 332–334. [Google Scholar]
- Shimizu, T.; Moriyama, M.; Tanaka, N.; Arakawa, Y. Persistent TT Virus Infection Does Not Contribute to the Development of Non-A to -G Hepatocellular Carcinoma: A Case-Control Study of 19 Patients in Japan. Intervirology 2000, 43, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Liweń, I. TT Virus--Incidence and Role in Pathogenesis of Liver Diseases. Postpy Hig. I Med. Doświadczalnej 2000, 54, 767–776. [Google Scholar]
- Li, Y.; Cao, L.; Han, X.; Ma, Y.; Liu, Y.; Gao, S.; Zhang, C. Altered Vaginal Eukaryotic Virome Is Associated with Different Cervical Disease Status. Virol. Sin. 2023, 38, 184–197. [Google Scholar] [CrossRef]
- Sasivimolrattana, T.; Chantratita, W.; Sensorn, I.; Chaiwongkot, A.; Oranratanaphan, S.; Bhattarakosol, P. Human Virome in Cervix Controlled by the Domination of Human Papillomavirus. Viruses 2022, 14, 2066. [Google Scholar] [CrossRef]
- Kaelin, E.A.; Skidmore, P.T.; Łaniewski, P.; Holland, L.A.; Chase, D.M.; Herbst-Kralovetz, M.M.; Lim, E.S. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. mSystems 2022, 7, e0006422. [Google Scholar] [CrossRef]
- Britto, A.M.A.; Siqueira, J.D.; Curty, G.; Goes, L.R.; Policarpo, C.; Meyrelles, A.R.; Furtado, Y.; Almeida, G.; Giannini, A.L.M.; Machado, E.S.; et al. Microbiome Analysis of Brazilian Women Cervix Reveals Specific Bacterial Abundance Correlation to RIG-like Receptor Gene Expression. Front. Immunol. 2023, 14, 1147950. [Google Scholar] [CrossRef]
- Siqueira, J.D.; Curty, G.; Xutao, D.; Hofer, C.B.; Machado, E.S.; Seuánez, H.N.; Soares, M.A.; Delwart, E.; Soares, E.A. Composite Analysis of the Virome and Bacteriome of HIV/HPV Co-Infected Women Reveals Proxies for Immunodeficiency. Viruses 2019, 11, 422. [Google Scholar] [CrossRef]
- Bando, M.; Takahashi, M.; Ohno, S.; Hosono, T.; Hironaka, M.; Okamoto, H.; Sugiyama, Y. Torque Teno Virus DNA Titre Elevated in Idiopathic Pulmonary Fibrosis with Primary Lung Cancer. Respirology 2008, 13, 263–269. [Google Scholar] [CrossRef]
- Hettmann, A.; Demcsák, A.; Decsi, G.; Bach, A.; Pálinkó, D.; Rovó, L.; Nagy, K.; Takács, M.; Minarovits, J. Infectious Agents Associated with Head and Neck Carcinomas. Adv. Exp. Med. Biol. 2016, 897, 63–80. [Google Scholar]
- Jauhiainen, M.K.; Mohanraj, U.; Lehecka, M.; Niemelä, M.; Hirvonen, T.P.; Pratas, D.; Perdomo, M.F.; Söderlund-Venermo, M.; Mäkitie, A.A.; Sinkkonen, S.T. Herpesviruses, Polyomaviruses, Parvoviruses, Papillomaviruses, and Anelloviruses in Vestibular Schwannoma. J. Neurovirol. 2023, 29, 226–231. [Google Scholar] [CrossRef]
- Fehér, E.; Kardos, G.; Gáll, T.; Kis, A.; Gergely, L.; Szarka, K. Comparison of Diversity of Torque Teno Virus 1 in Different Mucosal Tissues and Disorders. Acta Microbiol. Immunol. Hung. 2011, 58, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Leijonhufvud, G.; Bajalan, A.; Teixeira Soratto, T.A.; Gustafsson, B.; Bogdanovic, G.; Bjerkner, A.; Allander, T.; Ljungman, G.; Andersson, B. Better Detection of Torque Teno Virus in Children with Leukemia by Metagenomic Sequencing than by Quantitative PCR. J. Med. Virol. 2022, 94, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Zhang, L.; Dhayalan, A.; Agagnina, B.M.; Magli, A.R.; Fraher, G.; Didier, S.; Johnson, L.P.; Kennedy, W.J.; Damle, R.N.; et al. Torque Teno Virus 10 Isolated by Genome Amplification Techniques from a Patient with Concomitant Chronic Lymphocytic Leukemia and Polycythemia Vera. Mol. Med. 2011, 17, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Leijonhufvud, G.; Soratto, T.A.T.; Matos, G.M.; Bajalan, A.; Eichler-Jonsson, C.; Gustafsson, B.; Bogdanovic, G.; Allander, T.; Ljungman, G.; Andersson, B. Metagenomic Characterization of Viruses in the Serum of Children with Newly Diagnosed Cancer. J. Clin. Virol. 2024, 175, 105736. [Google Scholar] [CrossRef] [PubMed]
- Benzaquén, A.; Giménez, E.; Iacoboni, G.; Guerreiro, M.; Hernani, R.; Albert, E.; Carpio, C.; Balaguer, A.; Pérez, A.; S de la Asunción, C.; et al. Torque Teno Virus Plasma DNA Load: A Novel Prognostic Biomarker in CAR-T Therapy. Bone Marrow Transplant. 2024, 59, 93–100. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Franz-Vasconcelos, H.C.F.; di Giunta, G.; Mazzuco, T.L.; Caon, T.; Fernandes, A.L.M.; Simões, C.M.O.; Antunes, V.L.S.; Niel, C.; Barardi, C.R.M. Detection of Torque Teno Virus in Epstein-Barr Virus Positive and Negative Lymph Nodes of Patients with Hodgkin Lymphoma. Leuk. Lymphoma 2007, 48, 731–735. [Google Scholar] [CrossRef]
- Leppik, L.; Gunst, K.; Lehtinen, M.; Dillner, J.; Streker, K.; de Villiers, E.-M. In Vivo and In Vitro Intragenomic Rearrangement of TT Viruses. J. Virol. 2007, 81, 9346–9356. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Iezzi, T.; Capobianchi, M.R.; Pignoloni, P.; Pulsoni, A.; Sourdis, J.; Pescarmona, E.; Vitolo, D.; Mandelli, F. Detection of TT Virus in Lymph Node Biopsies of B-Cell Lymphoma and Hodgkin’s Disease, and Its Association with EBV Infection. Int. J. Immunopathol. Pharmacol. 2003, 16, 109–118. [Google Scholar] [CrossRef]
- Katano, H.; Sato, S.; Sekizuka, T.; Kinumaki, A.; Fukumoto, H.; Sato, Y.; Hasegawa, H.; Morikawa, S.; Saijo, M.; Mizutani, T.; et al. Pathogenic Characterization of a Cervical Lymph Node Derived from a Patient with Kawasaki Disease. Int. J. Clin. Exp. Pathol. 2012, 5, 814–823. [Google Scholar]
- Cacoub, P.; Rosenthal, E.; Gerolami, V.; Hausfater, P.; Ghillani, P.; Sterkers, Y.; Thibault, V.; Khiri, H.; Piette, J.C.; Halfon, P. Transfusion-Associated TT Virus Co-Infection in Patients with Hepatitis C Virus Is Associated with Type II Mixed Cryoglobulinemia but Not with B-Cell Non-Hodgkin Lymphoma. Clin. Microbiol. Infect. 2003, 9, 39–44. [Google Scholar] [CrossRef][Green Version]
- Shiramizu, B.; Yu, Q.; Hu, N.; Yanagihara, R.; Nerurkar, V.R. Investigation of TT Virus in the Etiology of Pediatric Acute Lymphoblastic Leukemia. Pediatr. Hematol. Oncol. 2002, 19, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Wang, H.; Tan, S.D.; Yang, S.X.; Shi, X.F.; Zhang, W. Viral Metagenomics Reveals Diverse Anelloviruses in Bone Marrow Specimens from Hematologic Patients. J. Clin. Virol. 2020, 132, 104643. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Ottomani, L.; Ducos, J.; Dereure, O.; Carles, M.J.; Guillot, B. High Prevalence of Torque Teno (TT) Virus in Classical Kaposi’s Sarcoma. Acta Derm. Venereol. 2007, 87, 14–17. [Google Scholar] [CrossRef]
- Honorato, L.; Witkin, S.S.; Mendes-Correa, M.C.; Conde Toscano, A.L.C.; Linhares, I.M.; de Paula, A.V.; Paião, H.G.O.; de Paula, V.S.; Lopes, A.D.O.; Lima, S.H.; et al. The Torque Teno Virus Titer in Saliva Reflects the Level of Circulating CD4+ T Lymphocytes and HIV in Individuals Undergoing Antiretroviral Maintenance Therapy. Front. Med. 2022, 8, 809312. [Google Scholar] [CrossRef]
- Giacconi, R.; Maggi, F.; Macera, L.; Spezia, P.G.; Pistello, M.; Provinciali, M.; Piacenza, F.; Basso, A.; Bürkle, A.; Moreno-Villanueva, M.; et al. Prevalence and Loads of Torquetenovirus in the European Mark-Age Study Population. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2020, 75, 1838–1845. [Google Scholar] [CrossRef]
- Giacconi, R.; Laffon, B.; Costa, S.; Teixeira-Gomes, A.; Maggi, F.; MacEra, L.; Spezia, P.G.; Piacenza, F.; Bürkle, A.; Moreno-Villanueva, M.; et al. Association of Torquetenovirus Viremia with Physical Frailty and Cognitive Impairment in Three Independent European Cohorts. Gerontology 2023, 69, 684–693. [Google Scholar] [CrossRef]
- Haloschan, M.; Bettesch, R.; Görzer, I.; Weseslindtner, L.; Kundi, M.; Puchhammer-Stöckl, E. TTV DNA Plasma Load and Its Association with Age, Gender, and HCMV IgG Serostatus in Healthy Adults. Age 2014, 36, 9716. [Google Scholar] [CrossRef]
- Autio, A.; Kettunen, J.; Nevalainen, T.; Kimura, B.; Hurme, M. Herpesviruses and Their Genetic Diversity in the Blood Virome of Healthy Individuals: Effect of Aging. Immun. Ageing 2022, 19, 15. [Google Scholar] [CrossRef]
- Dodi, G.; Attanasi, M.; Di Filippo, P.; Di Pillo, S.; Chiarelli, F. Virome in the Lungs: The Role of Anelloviruses in Childhood Respiratory Diseases. Microorganisms 2021, 9, 1357. [Google Scholar] [CrossRef]
- Giacconi, R.; Maggi, F.; Macera, L.; Pistello, M.; Provinciali, M.; Giannecchini, S.; Martelli, F.; Spezia, P.G.; Mariani, E.; Galeazzi, R.; et al. Torquetenovirus (TTV) Load Is Associated with Mortality in Italian Elderly Subjects. Exp. Gerontol. 2018, 112, 103–111. [Google Scholar] [CrossRef]
- Freer, G.; Maggi, F.; Pifferi, M.; Di Cicco, M.E.; Peroni, D.G.; Pistello, M. The Virome and Its Major Component, Anellovirus, a Convoluted System Molding Human Immune Defenses and Possibly Affecting the Development of Asthma and Respiratory Diseases in Childhood. Front. Microbiol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Forqué, L.; Albert, E.; Giménez, E.; Torres, I.; Carbonell, N.; Ferreres, J.; Blasco, M.L.; Navarro, D. Monitoring of Torque Teno Virus DNAemia in Critically Ill COVID-19 Patients: May It Help to Predict Clinical Outcomes? J. Clin. Virol. 2022, 148, 105082. [Google Scholar] [CrossRef] [PubMed]
- Feyzioğlu, B.; Teke, T.; Özdemir, M.; Karaibrahimoğlu, A.; Doğan, M.; Yavşan, M. The Presence of Torque Teno Virus in Chronic Obstructive Pulmonary Disease. Int. J. Clin. Exp. Med. 2014, 7, 3461–3466. [Google Scholar] [PubMed]
- Freer, G.; Maggi, F.; Pistello, M. Virome and Inflammasomes, a Finely Tuned Balance with Important Consequences for the Host Health. Curr. Med. Chem. 2019, 26, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Piacenza, F.; Maggi, F.; Bürkle, A.; Moreno-Villanueva, M.; Mancinelli, L.; Spezia, P.G.; Novazzi, F.; Drago Ferrante, F.; Minosse, C.; et al. Association Between TTV Viremia, Chronic Inflammation, and Ischemic Heart Disease Risk: Insights From MARK-AGE and Report-Age Projects. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae228. [Google Scholar] [CrossRef]
- Stincarelli, M.A.; Baj, A.; Guidotti, B.; Spezia, P.G.; Novazzi, F.; Lucenteforte, E.; Tillati, S.; Focosi, D.; Maggi, F.; Giannecchini, S. Plasma Torquetenovirus (TTV) MicroRNAs and Severity of COVID-19. Virol. J. 2022, 19, 79. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kawada, J.I.; Okuno, Y.; Horiba, K.; Suzuki, T.; Torii, Y.; Yasuda, K.; Numaguchi, A.; Kato, T.; Takahashi, Y.; et al. Identification of Potential Pathogenic Viruses in Patients with Acute Myocarditis Using Next-Generation Sequencing. J. Med. Virol. 2018, 90, 1814–1821. [Google Scholar] [CrossRef]
- Liang, G.; Conrad, M.A.; Kelsen, J.R.; Kessler, L.R.; Breton, J.; Albenberg, L.G.; Marakos, S.; Galgano, A.; Devas, N.; Erlichman, J.; et al. Dynamics of the Stool Virome in Very Early- Onset Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1600–1610. [Google Scholar] [CrossRef]
- Caixeta, R.A.V.; Batista, A.M.; Caetano, M.W.; Palmieri, M.; Schwab, G.; Zerbinati, R.M.; Victor, A.S.P.; Gallo, C.D.; Tozetto-Mendoza, T.R.; Junges, R.; et al. Investigation of Oral Shedding of Torquetenovirus (TTV) in Moderate-to-Severe COVID-19 Hospitalised Patients. Viruses 2024, 16, 831. [Google Scholar] [CrossRef]
- Rocchi, J.; Ricci, V.; Albani, M.; Lanini, L.; Andreoli, E.; Macera, L.; Pistello, M.; Ceccherini-Nelli, L.; Bendinelli, M.; Maggi, F. Torquetenovirus DNA Drives Proinflammatory Cytokines Production and Secretion by Immune Cells via Toll-like Receptor 9. Virology 2009, 394, 235–242. [Google Scholar] [CrossRef]
- Vietzen, H.; Simonitsch, C.; Friedel, B.; Berger, S.M.; Kühner, L.M.; Furlano, P.L.; Florian, D.M.; Görzer, I.; Koblischke, M.; Aberle, J.H.; et al. Torque Teno Viruses Exhaust and Imprint the Human Immune System via the HLA-E/NKG2A Axis. Front. Immunol. 2024, 15, 1447980. [Google Scholar] [CrossRef] [PubMed]
- Blazsek, A.; Sillo, P.; Ishii, N.; Gergely, P.; Poor, G.; Preisz, K.; Hashimoto, T.; Medvecz, M.; Kárpáti, S. Searching for Foreign Antigens as Possible Triggering Factors of Autoimmunity: Torque Teno Virus DNA Prevalence Is Elevated in Sera of Patients with Bullous Pemphigoid. Exp. Dermatol. 2008, 17, 446–454. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.R.; da Costa, I.P.; Devalle, S.; de Castro, A.R.C.M.; Freitas, S.Z. Prevalence and Genetic Diversity of Torque Teno Virus in Patients with Systemic Lupus Erythematosus in a Reference Service in Mato Grosso Do Sul. Rev. Bras. Reumatol. 2012, 52, 49–54. [Google Scholar] [CrossRef]
- Gan, L.; Miller, F.W. State of the Art: What We Know about Infectious Agents and Myositis. Curr. Opin. Rheumatol. 2011, 23, 585–594. [Google Scholar] [CrossRef]
- Kakalacheva, K.; Münz, C.; Lünemann, J.D. Viral Triggers of Multiple Sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 132–140. [Google Scholar] [CrossRef]
- Gergely, P.; Perl, A.; Poór, G. Possible Pathogenic Nature of the Recently Discovered TT Virus: Does It Play a Role in Autoimmune Rheumatic Diseases? Autoimmun. Rev. 2006, 6, 5–9. [Google Scholar] [CrossRef]
- Perlejewski, K.; Pawełczyk, A.; Bukowska-Ośko, I.; Rydzanicz, M.; Dzieciątkowski, T.; Paciorek, M.; Makowiecki, M.; Cortés, K.C.; Grochowska, M.; Radkowski, M.; et al. Search for Viral Infections in Cerebrospinal Fluid from Patients with Autoimmune Encephalitis. Open Forum Infect. Dis. 2020, 7, ofaa468. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Enomoto, M.; Shiomi, S.; Obata, N.; Tanaka, M.; Fukuda, K.; Tamori, A.; Habu, D.; Takeda, T.; Tanaka, T.; et al. GB Virus C and TT Virus Infections in Japanese Patients with Autoimmune Hepatitis. J. Med. Virol. 2002, 66, 258–262. [Google Scholar] [CrossRef]
- Hekmatdoost, A.; Ghaziani, T.; Esteghamathanzaie, F.; Alavian, S.; Mohaghegh, H.; Zali, M. Transfusion Transmitted Virus and Autoimmune Hepatitis. Is There Any Association? Saudi Med. J. 2004, 25, 1760–1761. [Google Scholar]
- Sospedra, M.; Zhao, Y.; Hausen, H.Z.; Muraro, P.A.; Hamashin, C.; De Villiers, E.M.; Pinilla, C.; Martin, R. Recognition of Conserved Amino Acid Motifs of Common Viruses and Its Role in Autoimmunity. PLoS Pathog. 2005, 1, e41. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The Healthy Human Virome: From Virus–Host Symbiosis to Disease. Curr. Opin. Virol. 2021, 47, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fahsbender, E.; Burns, J.M.; Kim, S.; Kraberger, S.; Frankfurter, G.; Eilers, A.A.; Shero, M.R.; Beltran, R.; Kirkham, A.; McCorkell, R.; et al. Diverse and Highly Recombinant Anelloviruses Associated with Weddell Seals in Antarctica. Virus Evol. 2017, 3, vex017. [Google Scholar] [CrossRef] [PubMed]
- Crane, A.; Goebel, M.E.; Kraberger, S.; Stone, A.C.; Varsani, A. Novel Anelloviruses Identified in Buccal Swabs of Antarctic Fur Seals. Virus Genes. 2018, 54, 719–723. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.M.; Fumagalli, M.J.; de Araujo, J.; Sabino-Santos, G.; Maia, F.G.M.; Romeiro, M.F.; Modha, S.; Nardi, M.S.; Queiroz, L.H.; Durigon, E.L.; et al. Discovery of Novel Anelloviruses in Small Mammals Expands the Host Range and Diversity of the Anelloviridae. Virology 2018, 514, 9–17. [Google Scholar] [CrossRef]
- Webb, B.; Rakibuzzaman, A.G.M.; Ramamoorthy, S. Torque Teno Viruses in Health and Disease. Virus Res. 2020, 285, 198013. [Google Scholar] [CrossRef]
- Tyschik, E.A.; Shcherbakova, S.M.; Ibragimov, R.R.; Rebrikov, D.V. Transplacental Transmission of Torque Teno Virus. Virol. J. 2017, 14, 92. [Google Scholar] [CrossRef]
- Schro¨ter, M.; Schro¨ter, S.; Polywka, S.; Zo¨llner, B.; Zo¨llner, Z.; Scha¨fer, P.; Scha¨fer, S.; Laufs, R.; Feucht, H.-H. Detection of TT Virus DNA and GB Virus Type C/Hepatitis G Virus RNA in Serum and Breast Milk: Determination of Mother-to-Child Transmission. J. Clin. Microbiol. 2000, 38, 745–747. [Google Scholar] [CrossRef]
- Beller, L.; Deboutte, W.; Vieira-Silva, S.; Falony, G.; Tito, R.Y.; Rymenans, L.; Kwe Yinda, C.; Vanmechelen, B.; Van Espen, L.; Jansen, D.; et al. The Virota and Its Transkingdom Interactions in the Healthy Infant Gut. Proc. Natl. Acad. Sci. USA 2020, 117, 11220–11222. [Google Scholar] [CrossRef]
- Morselli, S.; Foschi, C.; Laghi, L.; Zagonari, S.; Patuelli, G.; Camboni, T.; Ceccarani, C.; Consolandi, C.; Djusse, M.E.; Pedna, M.F.; et al. Torquetenovirus in Pregnancy: Correlation with Vaginal Microbiome, Metabolome and pro-Inflammatory Cytokines. Front. Microbiol. 2022, 13, 998849. [Google Scholar] [CrossRef]
- Taboada, B.; Morán, P.; Serrano-Vázquez, A.; Iša, P.; Rojas- Velázquez, L.; Pérez-Juárez, H.; López, S.; Torres, J.; Ximenez, C.; Arias, C.F. The Gut Virome of Healthy Children during the First Year of Life Is Diverse and Dynamic. PLoS ONE 2021, 16, e0240958. [Google Scholar] [CrossRef]
- Liou, S.H.; Boggavarapu, R.; Cohen, N.R.; Zhang, Y.; Sharma, I.; Zeheb, L.; Mukund Acharekar, N.; Rodgers, H.D.; Islam, S.; Pitts, J.; et al. Structure of Anellovirus-like Particles Reveal a Mechanism for Immune Evasion. Nat. Commun. 2024, 15, 7219. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Pistello, M.; Vatteroni, M.; Presciuttini, S.; Marchi, S.; Isola, P.; Fornai, C.; Fagnani, S.; Andreoli, E.; Antonelli, G.; et al. Dynamics of Persistent TT Virus Infection, as Determined in Patients Treated with Alpha Interferon for Concomitant Hepatitis C Virus Infection. J. Virol. 2001, 75, 11999–12004. [Google Scholar] [CrossRef]
- Maggi, F.; Bendinelli, M. Immunobiology of the Torque Teno Viruses and Other Anelloviruses. Curr. Top. Microbiol. Immunol. 2009, 331, 65–90. [Google Scholar] [PubMed]
- Krieg, A.M. CpG Motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Tawara, A.; Akahane, Y.; Takahashi, M.; Nishizawa, T.; Ishikawa, T.; Okamoto, H. Transmission of Human TT Virus of Genotype 1a to Chimpanzees with Fecal Supernatant or Serum from Patients with Acute TTV Infection. Biochem. Biophys. Res. Commun. 2000, 278, 470–476. [Google Scholar] [CrossRef]
- Lefrè, J.-J.; Roudot-Thoraval, F.; Lefrè, F.; Kanfer, A.; Mariotti, M.; Lerable, J.; Thauvin, M.; Lefè, G.; Rouger, P.; Girot, R. Natural History of the TT Virus Infection through Follow-up of TTV DNA-Positive Multiple-Transfused Patients. Blood J. Am. Soc. Hematol. 2000, 95, 347–351. [Google Scholar]
- Esser, P.L.; Rubio Quintanares, G.H.; Langhans, B.; Heger, E.; Böhm, M.; Jensen, B.E.O.L.E.; Esser, S.; Lübke, N.; Fätkenheuer, G.; Lengauer, T.; et al. Torque Teno Virus Load Is Associated with Centers for Disease Control and Prevention Stage and CD4+ Cell Count in People Living with Human Immunodeficiency Virus but Seems Unrelated to AIDS-Defining Events and Human Pegivirus Load. J. Infect. Dis. 2024, 230, e437–e446. [Google Scholar] [CrossRef]
- Macera, L.; Spezia, P.G.; Medici, C.; Rofi, E.; Del Re, M.; Focosi, D.; Mazzetti, P.; Navarro, D.; Antonelli, G.; Danesi, R.; et al. Comparative Evaluation of Molecular Methods for the Quantitative Measure of Torquetenovirus Viremia, the New Surrogate Marker of Immune Competence. J. Med. Virol. 2022, 94, 491–498. [Google Scholar] [CrossRef]
- Timmerman, A.L.; Schönert, A.L.M.; van der Hoek, L. Anelloviruses versus Human Immunity: How Do We Control These Viruses? FEMS Microbiol. Rev. 2024, 48, fuae005. [Google Scholar] [CrossRef]
- Prince, C.; Bounoutas, G.; Zhou, B.; Raja, W.; Gold, I.; Thakker, P.; Boisvert, N.; Reardon, C.; Ozturk, E.; Boggavarapu, R.; et al. A Novel Functional Gene Delivery Platform Based on a Commensal Human 1 Anellovirus Demonstrates Transduction in Multiple Tissue Types 2. BioRxiv 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).