Abstract
Despite significant advancements in early diagnosis and treatment, breast cancer (BC) remains a major global health challenge. Ongoing research is essential to identify novel risk factors, implement innovative screening programs, and develop personalized treatment approaches. Among the various risk factors, infection with certain oncogenic viruses has emerged as a potential contributor to BC development. Increasing evidence suggests that bovine leukemia virus (BLV) may contribute to zoonotic infections in humans, with a potential role in BC initiation and progression. This review evaluates clinical and experimental data on BLV presence in both malignant and non-malignant breast tissues, exploring potential mechanisms through which BLV may access human breast tissue and contribute to carcinogenesis. Current data reveal a higher prevalence of BLV infection in BC tissues compared to non-tumor tissues, correlating with an increased risk of BC development. In this context, dairy and meat products from BLV-infected animals have been proposed as potential transmission sources. BLV-encoded proteins disrupt key oncogenic pathways, which support their possible role in breast carcinogenesis. However, the interpretation of these findings is limited by potential confounding factors such as genetic predisposition, environmental exposures, and dietary influences. Further research, including well-controlled epidemiological studies, longitudinal cohorts, and mechanistic investigations into BLV proteins in human breast cells, is necessary to determine its role in BC development.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy among women globally and the leading cause of cancer-related death in this population [1]. In the United States alone, an estimated 310,720 new cases and 42,250 deaths from BC among women were reported for 2024 [2]. Notably, the highest BC incidence rates occur in regions such as Australia/New Zealand, Northern America, Northern Europe, and Western Europe, where the rates are nearly three times higher compared to those in Eastern Africa, South-Central Asia, and Middle Africa [1,3]. In general, the differences in BC incidence rates among countries and ethnicities are associated with differences in the exposure level to risk factors and the implementation and/or access to primary prevention programs [3].
A variety of reproductive and lifestyle risk factors have been associated with BC development, including, but not limited to, age at menarche and menopause, number of children, breastfeeding, use of hormonal therapy, obesity, genetic mutations, family history, and unhealthy food habits [4]. Furthermore, the potential relation of some viruses like mouse mammary tumor virus (MMTV), Epstein-Barr virus (EBV), human papillomavirus (HPV), and human cytomegalovirus (HCMV) to BC development has also been proposed [5,6,7,8,9]. In addition, a potential association between viral co-infections and BC development was previously suggested [10]. However, it is well accepted that breast carcinogenesis is most related to a combination of environmental, genetic, and lifestyle risk factors [11].
Particularly, the potential association of BC development and the intake of animal products have been addressed in numerous studies [12,13,14]. For instance, bovine meat and dairy products could contain high levels of saturated fats [15,16], xenoestrogens, growth factors [17], and endocrine disruptors such as heterocyclic amines found in processed or cooked red meat [18,19], all of which may contribute to BC development and progression [20]. Despite these findings, the causal relationship between dietary factors and BC remains unclear.
Interestingly, the presence of the bovine leukemia virus (BLV) DNA in bovine milk and meat for human consumption has been reported [21,22,23]. BLV is a deltaretrovirus that naturally infects cattle, zebu, and buffalo [24,25]. BLV is the causative agent of enzootic bovine leucosis (EBL), characterized by persistent B-cell lymphocytosis and lymphoma development [26]. In addition, BLV is able to infect a broad variety of cells, including bovine mammary epithelial cells [27,28]. Recently, a meta-analysis conducted by Bushi et al. (2024), including 48 studies and 101,120 cattle samples, reported a global prevalence of BLV at 26.8% (95% CI: 20.0–33.0) [29].
The occurrence of anti-BLV antibodies was demonstrated in human females [21,30,31], which supports previous exposure to the virus. Moreover, BLV DNA was detected in blood samples from human females [30,32], which was also genetically related to the virus-infected cattle in the same geographical area and time period [33]. Furthermore, an association between the increased consumption of dairy products by females and the risk of BLV infection was reported [34], suggesting that bovine products could be a route for zoonotic BLV infection in humans.
Given the high consumption of dairy products in certain regions, BLV exposure may have public health implications. While its role in human disease remains uncertain, potential transmission through dairy consumption warrants further investigation to assess associated risks and preventive measures. In this regard, the presence of BLV has been frequently detected in breast tissue samples, and it has also been related to an increased risk of BC development [35,36,37]. Notably, the presence of BLV was demonstrated in initial benign breast tissues and in the posterior BC specimens from the same patients [38]. However, the relationship between BLV and BC remains highly controversial due to inconsistencies in the available data. While some studies report a strong association, others fail to find significant differences in BLV prevalence between BC cases and controls. These discrepancies may be attributed to variations in detection methodologies, sample sizes, and study designs. Differences in BLV DNA target regions, geographic variations in viral strains, and potential confounding factors further complicate the interpretation of findings.
This review provides a comprehensive analysis of the current clinical evidence linking BLV infection to human BC development. Additionally, it explores the potential mechanisms by which BLV enters breast epithelial cells and examines its contribution to BC initiation and progression.
2. BLV Structure and Lifecycle
BLV is a diploid single-stranded RNA that belongs to the Retroviridae family and Deltaretrovirus genus [39]. This virus is closely related to the human T-cell leukemia virus type 1 (HTLV-1), both of which are characterized by their ability to infect lymphocytes and integrate their genetic material into the host genome—a process known as establishing the proviral state—which is an essential hallmark of retroviral replication [40]. The BLV genome comprises an 8714-nucleotide sequence, enclosed by an icosahedral nucleocapsid surrounded by a double-layered lipid membrane structure derived from the host cell during viral budding [41]. This genome encodes structural proteins, viral enzymes, regulatory proteins, and accessory proteins. It is flanked by two identical long terminal repeat (LTR) sequences, the 5′ LTR and 3′ LTR, which regulate viral gene expression and replication [42] (Figure 1).
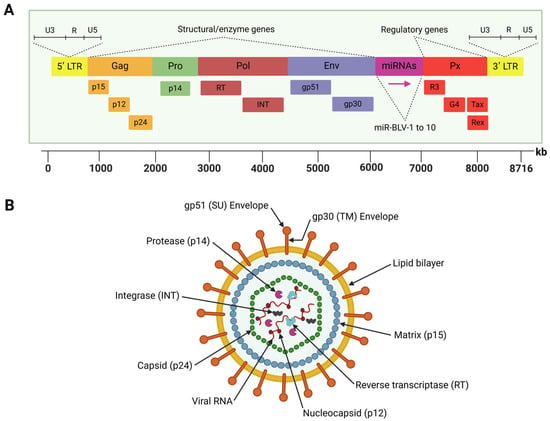
Figure 1.
Schematic representation of the BLV genome structure (A) and viral particle (B). (A) The BLV genome encodes structural proteins and viral enzymes, regulatory proteins, and accessory proteins. It is flanked by two identical long terminal repeat (LTR) sequences. The region located between the env and R3 genes encodes ten viral microRNAs (miRNAs). (B) The BLV particle has a spherical shape with the lipid bilayer envelope surrounding the icosahedral nucleocapsid that contains the viral genome inside. Created by BioRender.com.
The 5′ LTR contains promoter elements that drive the activity of RNA polymerase II (RNAPII), which transcribes BLV protein-coding genes. These include the structural genes (gag, pro, pol, and env), regulatory genes (tax and rex), and accessory genes (R3 and G4), all of which are essential for the virus lifecycle [43].
The gag gene encodes three non-glycosylated and structural proteins: the nucleocapsid protein (p12), the matrix protein (p15), and the other nucleocapsid protein (p24), which form the viral core [44]. The pro gene encodes the viral protease (p14), which cleaves gag and gag-pol polyproteins during virion maturation, a process critical for the release of infectious particles [45]. However, the pol gene encodes two essential enzymes: reverse transcriptase (RT), which converts viral RNA into double-stranded proviral DNA, and integrase (INT), which facilitates the integration of this DNA into the host genome [46]. The env gene encodes two glycosylated envelope proteins, glycoprotein (gp)51 (surface) and gp30 (transmembrane). The gp51 protein mediates virus–host cell fusion, while gp30 is involved in signal transduction pathways and stabilizing gp51 [45]. Notably, the env gene exhibits genetic polymorphism, with eleven distinct BLV genotypes identified through gp51 sequencing and phylogenetic studies. These genotypes are distributed differently across the globe, reflecting the evolutionary adaptation of BLV and geographical spread [47,48,49,50,51].
Located between the env gene and the 3′ LTR, the pX region encodes key regulatory proteins (Tax and Rex) and accessory proteins (R3 and G4) [52]. Tax functions as a transcriptional activator, stimulating LTR activity and driving viral gene expression while playing a critical role in BLV-induced tumorigenesis and lymphoproliferative disorders [53,54,55]. Likewise, Rex is involved in post-transcriptional regulation, ensuring the efficient export of unspliced viral RNA from the nucleus and supporting the synthesis of structural proteins [56]. Meanwhile, the accessory proteins R3 and G4 play critical roles in maintaining high viral loads during persistent infections, with G4 also associated with oncogenic activity, further highlighting its role in BLV pathogenesis [57,58].
Another unique feature of the BLV genome is a region located between the env and R3 genes, which encode ten viral microRNAs (miRNAs) under the control of RNA polymerase III [59]. These miRNAs are involved in various biological processes, including immune modulation, cell signaling, apoptosis, and tumorigenesis [60]. These small RNA molecules contribute to immune evasion and viral persistence by downregulating host antiviral responses.
Some of BLV-encoded miRNAs share sequence identity with human miRNAs, suggesting a potential overlap in the target molecules of both viral and human miRNAs. For instance, it was found that miR-B2-5p displays a common seed overlap with human miR-943 [61]. The overexpression of miR-943 was evidenced in the stem population of primary human mammalian epithelial cells (HMECs), and it was also upregulated in cells when the tumor suppressor p53 was knocked down [62]. Moreover, miR-943 is negatively correlated with the gene expression of Ataxia-telangiectasia mutated (ATM) and breast cancer gene 1 (BRCA1) in BC, which suggests its potential involvement in the repair of DNA double-strand breaks [63]. Furthermore, it was found that BLV-miR-B4–3p also has a matching seed sequence with the human miR-29a [61]. The miR-29a targets SUV420H2 and downregulates the trimethylation of the histone H4K20, inducing the epithelial-to-mesenchymal transition (EMT), migration, and invasion of BC cells [64]. These facts together may suggest a possible oncogenic role of BLV miRNAs in human breast cells.
In the BLV lifecycle, a defining step is the integration of the virus as a provirus into the genomic DNA of peripheral blood cells, establishing a persistent infection in the host [65]. The retroviral lifecycle can be divided into two phases: the early phase and the late phase. The early phase includes viral entry, reverse transcription, and proviral integration. Viral envelope proteins mediate entry by interacting with specific host cell receptors. Inside the cell, viral RNA is reverse transcribed into DNA, which is then integrated into the host genome by the viral integrase enzyme, while the late phase involves the transcription and translation of viral RNA, assembly of new virions, and their release through budding [66].
Both phases require coordinated interplay between viral and host factors [67,68]. For example, the processing of viral proteins relies not only on the viral protease but also on host proteases, highlighting the viral dependence on host cellular machinery [69]. Efficient transcription of the BLV proviral genome is driven by the viral transcriptional activator Tax, which works in tandem with host factors to regulate viral gene expression [70]. Additionally, the LTR interacts with host transcription factors to fine-tune viral replication, demonstrating the intricate relationship between the virus and its host. This complex lifecycle and genetic makeup allow BLV to evade immune responses, persist in host populations, and contribute to oncogenesis, making it a significant concern for animal health and a useful model for studying retroviral biology.
BLV can also infect and replicate in cells from non-bovine animal species (e.g., lamb, canine, feline, murine, and human cells) [70,71]. However, the ability of BLV to infect non-bovine cells depends on diverse factors, such as the competence and response of the host immune system, the presence of viral receptors on the cell surface, and the capacity of BLV to hijack and customize the host cell machinery for replication [27]. For example, the expression of BLV precursor and mature proteins was also reported in COS-1 cells (African green monkey kidney cells) and FLK cells (fetal lamb kidney cells) transfected with wild-type proviral DNA [71]. Notably, it was demonstrated that the expression of gPr72Env, Pr70Gag, and pr45Gag precursor proteins as well as gp51, gp30, and p24 mature proteins was detected in the lysates of 293T human cells transfected with an infectious BLV clone. Moreover, the production and secretion of BLV viral particles by 293T-transfected cells was verified [72]. These facts together may suggest similarities in the BLV lifecycle across cells from different animal species, including human cells.
3. Routes for BLV Infection in Human Breast Tissues
The BLV is an enzootic retrovirus that naturally infects cattle, zebu, and buffalo [24,25], causing B-cell lymphomas in 1–5% of infected cattle [73]. In addition, BLV can be experimentally transmitted to other species, including goats [74], sheep [75], chickens [76], rabbits [77], and rats [78], which provide evidence concerning the capacity of BLV to cross species barriers. Remarkably, in humans, the presence of BLV has been detected in breast [79] and lung [80] tissues, as well as in blood cells [30].
In cattle, BLV is detected in biological fluids such as blood, colostrum, and milk, which are considered potential sources for viral transmission [81,82]. BLV can be transmitted horizontally through iatrogenic procedures permitting blood or fluid transfer like rectal palpation using contaminated sleeves, injections with reused needles, or animal-to-animal contact through nasal excretions or saliva [83]. Vertical transmission occurs from BLV-infected dams to calves mainly through the consumption of infected colostrum or milk [84].
BLV DNA was detected in 38.9% of the milk samples and in 32.1% of the meat samples obtained from BLV-positive cows [21]. Similarly, the BLV proviral gag segment was found in 48.0% of the milk samples and in 50.0% of the meat samples for human consumption [22]. Moreover, the ex vivo infectivity of milk cells from BLV-infected cattle was previously demonstrated [85], which suggests that milk and meat consumption could be a route for BLV infection in humans.
On the other hand, BLV DNA was detected in 33/95 (34.7%) samples of leukocytes and platelets (buffy coat cells) from the blood from human females using PCR and DNA sequencing [30]. Similarly, BLV DNA was found in 16.5% (33/200) of blood samples from women without BC [32]. Interestingly, Canova et al. reported a genetic relationship between the BLV DNA sequences found in the blood from virus-infected cattle and in women’s breast tissue in the same geographical area and time period [33].
Additionally, Buehring et al. detected antibodies against BLV capsid protein (p24) in 191/257 (74.3%) samples of the analyzed human sera. The distribution for the three antibody isotypes tested was as follows: IgG = 101/257 (39.3%), IgM = 80/257 (31.1%), and IgA = 96/256 (37.5%) [31]. However, the same authors reported no significant correlation between the occurrence of anti-BLV p24 antibodies and the presence of BLV DNA in the blood samples [30]. However, de Quadros et al. reported the presence of anti-BLV antibodies in 4.1% of the tested human serum samples [21]. The presence of anti-BLV antibodies in human sera provides evidence of previous exposure to the virus, although others have reported the lack of BLV antibodies in human serum samples [86].
Overall, the evidence suggests that uncooked or partially cooked meat and unpasteurized milk derived from BLV-infected cattle could be a potential source of a zoonotic infection to humans. In this regard, a statistically significant association was reported between an increased consumption of dairy products by females and the risk of BLV infection (OR = 2.4, CI 95%: 1.063–5.527; p = 0.035), although no relation with meat consumption was obtained in this study [34]. Remarkably, it was suggested that another oncogenic virus in humans (high-risk HPV) could reach the breast tissue through circulation (blood or lymphatic systems) in patients with HPV-positive cervical cancer [87].
A hypothetical scheme regarding the potential routes for BLV infection in human breast tissues through infected milk and meat consumption is shown in Figure 2. Other hypotheses for BLV entry to humans, such as direct contact with infected animals or through vaccine production processes that involve the use of BLV-contaminated fetal bovine sera, have also been proposed [88]. As occurs in cattle-to-cattle transmission, the lack of protection when handling infected animals, blood/tissue exposure, or inadequate safety protocols could theoretically be alternative routes for the transmission of BLV to humans [81,83]. Despite these potential risks, there are no confirmed reports of BLV transmitting infection in humans in this way. However, the detection of BLV in human blood cells may suggest a potential route of transmission to other individuals via the blood [30]. Further investigations are required to elucidate the routes by which BLV reaches human tissues.
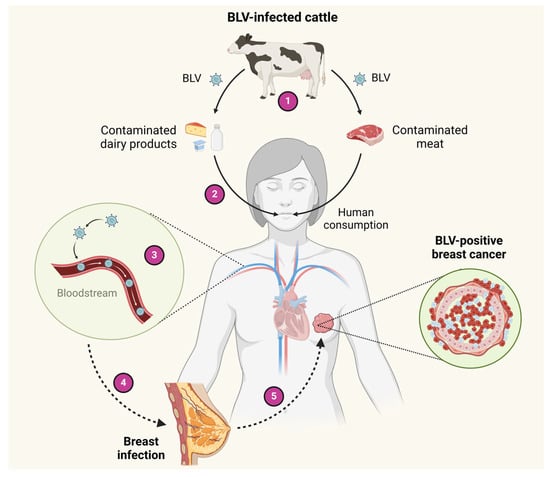
Figure 2.
Hypothetical schemes of BLV transmission to humans. 1—Cattle can be infected with BLV. 2—BLV DNA can be transmitted from virus-infected cattle to milk and meat for human consumption. 3—After the ingestion of dairy products and meat infected by BLV, the virus can pass into blood circulation. 4—BLV can reach the breast tissue through blood circulation. 5—In the BLV-infected breast epithelial cells, the virus could cooperate with other factors to induce breast carcinogenesis. Created by BioRender.com.
4. BLV Entry into Human Cells
BLV shows a preferential tropism for B lymphocytes, but it was demonstrated that BLV in vivo also targets other leukocytes such as monocytes, granulocytes, and CD8+ T lymphocytes [89]. Moreover, BLV is able to infect a broad variety of cells, such as canine, feline, and murine cells, and also bovine mammary epithelial cells [27,28]. Furthermore, the capacity of BLV to infect a variety of human cells of different origins, such as embryonic kidney (293T) [90], cervical carcinoma (HeLa) [90], glioblastoma (U-118 MG), ovarian teratocarcinoma (PA-1) [91], and breast adenocarcinoma (MCF-7) [92], was demonstrated. Particularly, evidence suggests the tropism of BLV towards human breast epithelial cells, based on the fact that these epithelial cells express viral proteins after BLV infection [36,79,93]. Overall evidence suggests that the cellular receptors involved in BLV entry could be broadly expressed rather than restricted to a specific cell type.
It was reported that BLV is able to infect the human WI-38 cells (lung fibroblast cells) in vitro. However, WI-38 cells released only small amounts of the viral particles despite remaining infected, as measured by the expression of BLV antigen and the syncytia formation assay [94]. This assay evaluates the capacity of retroviruses to induce fusion between infected and uninfected cells, leading to the creation of multinucleated cells, known as syncytia. The syncytia formation assay is commonly used to monitor BLV infection in vitro [90,92]. Also, it demonstrated the capacity of BLV to infect human glioblastoma, neuroblastoma, and ovarian teratocarcinoma cells [91]. Interestingly, some human cell lines were susceptible to BLV infection, including MCF-102A (non-malignant) and MCF-7 (adenocarcinoma) breast cells. But, only MCF-7 cells were able to sustain BLV infection and also showed syncytia formation and multinucleated cells [92].
The high-affinity cationic amino acid transporter 1 (CAT1), also known as solute carrier family 7 member 1 (SLC7A1), is a transmembrane protein primarily responsible for the uptake of cationic amino acids into cells. This protein plays a critical role in cellular metabolism, growth, and immune response by regulating the availability of essential amino acids required for protein synthesis and other metabolic pathways [95]. SLC7A1 is considered a potential entry receptor for BLV into human epithelial cells. In fact, HeLa (human cervical carcinoma cells) and 293T (human embryonic kidney cells) expressing SLC7A1 formed syncytia in culture after inoculation with BLV [90]. Moreover, the knockdown of SLC7A1 using specific siRNAs significantly decreased both the binding between SLC7A1 and BLV Env protein and the BLV infection of CC81-GREMG cells [90]. In addition, overexpression of SLC7A1 was able to enhance the susceptibility of the same cells to BLV infection [96]. These facts together permit the consideration of the potential role of SLC7A1 in BLV binding and infection. Interestingly, the levels of SLC7A1 mRNA were significantly increased in BC tissues compared to normal breast samples (p < 0.01) [97].
According to the TCGA database [98,99], SLC7A1 expression was also higher in BC tissues and metastasis compared to normal breast tissues (Figure 3). These facts together could suggest that increased levels of SLC7A1 make BC cells more susceptible to BLV infection (Figure 3). Moreover, it was demonstrated that the overexpression of SLC7A1 confers survival advantages and apoptosis resistance in MCF-7 and T47D BC cells [100].
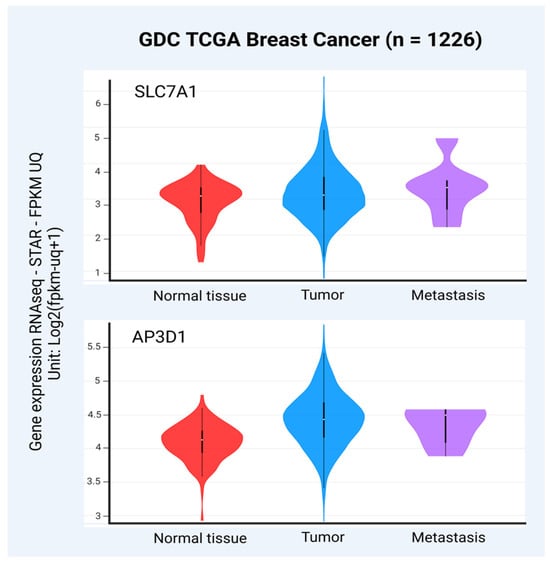
Figure 3.
Levels of SLC7A1 and AP3D1 transcripts in primary BC, metastasis, and normal breast tissues (GDC TCGA BC, n = 1226 after removing samples with nulls). The levels of both SLC7A1 and AP3D1 mRNA were significantly increased in primary tumors and metastasis compared to normal breast samples (p = 0.0023 and p = 1.110 × 10−16, respectively, one-way ANOVA). The University of California, Santa Cruz (UCSC) genome database was used to obtain the raw data (https://xena.ucsc.edu). For the correlation of SLC7A1 and AP3D1 expression levels and type of samples, the UCSC Xena platform was used (https://xenabrowser.net accessed on 8 and 13 December 2024, respectively). The graphs were obtained from the Xena platform, and the figure was created using BioRender.com.
The adaptor-related protein complex 3 subunit delta 1 (AP3D1) is a critical component of the adaptor protein complex-3 (AP-3), which is involved in intracellular protein trafficking [101]. This complex facilitates the sorting and transport of cargo proteins from the trans-Golgi network to lysosomes and other endosomal compartments [102]. The AP3D1 protein has also been proposed as a potential receptor for BLV in humans, sharing 88% of identity compared to the bovine receptor [103]. In fact, it was reported that HEp-2 human cancer cells ectopically expressing the bovine binding domain for BLV gp51 (clone BLVRcpl) had an increased susceptibility to BLV infection compared to untransfected control cells [104]. Furthermore, a significant increase in the sensitivity to BLV infection was evidenced in BLVRcpl-transfected cells after antibiotic selection, which highlights the potential role of this molecule in BLV infection [104]. Posteriorly, the BLV receptor (BLVR) is related to AP-3, which participates in intracellular protein transport [105]. According to The Cancer Genome Atlas (TCGA) database [98,99], AP3D1 expression was increased in primary BC and metastasis compared to normal breast tissues, suggesting again that BC cells with increased levels of AP3D1 could be more susceptible to BLV infection (Figure 3). Moreover, an increased AP3D1 gene expression was evidenced in invasive ductal carcinoma compared to invasive lobular carcinoma [106].
Taken together, these findings suggest that BLV entry into breast epithelial cells can be induced by overexpressing key receptors involved in this process. Nevertheless, further functional studies in breast epithelial cells are required to validate this hypothesis.
5. BLV Infection and the Risk of BC Development
Although the potential involvement of BLV in BC still remains controversial, the virus has been widely detected in BC samples and has been associated with an increased risk of tumor development [35,36,37]. For instance, a meta-analysis conducted by Khatami et al., which included nine case-control studies with a total of 826 BC cases and 898 individuals in the control groups, found an association between BLV infection and the risk of BC development (OR = 2.57; 95% CI: 1.45–4.56; p = 0.001) [107]. Another meta-analysis conducted by Saeedi-Moghaddam et al. including 11 studies, 3340 cases, and 635 controls also found an association of BLV infection and BC (OR = 3.92, 95% CI: 2.98–5.16; p < 0.00001) [108].
Particularly, BLV DNA was evidenced in 22/72 (30.5%) samples of BC tissues, which was statistically increased compared to samples from patients with healthy breast tissues (10/72; 13.9%) (OR = 2.73, 95% CI: 1.18–6.29; p = 0.027) [35]. Similarly, Olaya-Galán et al. found the occurrence of BLV DNA in 46/75 (61.3%) samples from BC cases and in 40/83 (48.2%) of the control tissues by nested PCR, linking the viral presence with an increased risk of BC development (OR = 2.45, 95% CI: 1.08–5.52; p = 0.031) [36]. While Buehring et al. (2015) demonstrated the presence of BLV DNA in 67/114 (59%) of BC samples using in situ PCR, and it was significantly increased compared to the 30/104 (29%) samples obtained from the controls (OR = 3.07, 95% CI: 1.66–5.69; p = 0.0004) [79].
Additionally, an increased presence of BLV DNA in premalignant breast tissues compared to healthy breast specimens was also demonstrated. In fact, Buehring et al. (2015) found an intermediate frequency of BLV DNA in premalignant breast tissues (8/21; 38%) between the BC and normal control groups (p for trend < 0.001) [79]. In the same way, Baltzell et al. (2018) obtained an increased frequency of BLV tax DNA by in situ PCR from normal breast specimens (20/103; 19.6%) compared to premalignant tissues (18/52; 34.0%) and BC plus ductal carcinoma in situ (DCIS) samples (49/89; 54.4%). In consequence, the presence of BLV tax DNA was related to an increased risk of BC or DCIS (OR = 5.25, 95% CI: 2.69–10.23; p < 0.0001) [37].
A study conducted by Lawson and Glenn (2017) analyzing the paired samples of benign breast tissue and subsequent BC specimens from the same patients revealed a higher frequency of BLV DNA in both benign breast tissues (18/23; 78.3%) and the later BC samples (20/22; 90.9%) compared to healthy breast specimens (6/17; 35.3%) [38]. Similarly, in a paired study, Buehring et al. (2017) observed an increased probability of BC development in women with BLV-positive results in both initial and subsequent samples, compared to those in whom BLV was either not detected or only positive in one of the paired specimens (p = 0.0484) [109].
Finally, Lendez et al. (2018) demonstrated that BLV DNA occurrence in BC tissues was associated with increased proliferation rates (p = 0.014) and HER-2 oncogene expression (p = 0.042) [110]. Similarly, Khan et al. reported a higher prevalence of BLV positivity in grade II invasive ductal carcinoma (IDC) (500/559; 89.4%) compared to grade I (59/559; 10.5%) and grade III tumors (0/559) [111]. However, other studies found no significant association between the presence of BLV and the tumor size, disease stage, estrogen receptor (ER) levels, progesterone receptor (PR) levels, human epidermal growth factor receptor 2 (HER-2) levels, proliferation index, or other clinicopathological features in BC patients [35,112,113]. Interestingly, it was suggested that the failure to efficiently eliminate BLV due to low binding affinity for HLA-II may contribute to BC development [114].
However, the presence of BLV DNA in BC tissues does not necessarily imply an active viral infection or causation. In this regard, the expression of the BLV p24 protein was detected in 10% of BC tissues [36], while Buehring et al. found the same protein in 12/236 (5.1%) of the BC specimens [79]. In addition, the presence of gp51 was evidenced in 7% of BC samples [93]. Furthermore, it was demonstrated that the MCF-7 cells were able to maintain a stable BLV infection over the 3-month follow-up, confirmed by both PCR and the immunohistochemical expression of the BLV p42 protein [92]. These facts together allow the consideration of a potential active replication of BLV in BC tissues.
Overall, the evidence indicates an increased frequency of BLV in BC tissues compared to non-tumor controls, suggesting a potential association between BLV infection and the risk of BC development. However, some studies have reported a decreased frequency of BLV infection in BC tissues [86,115,116], while others found no significant differences in BLV presence between BC cases and non-tumor controls [117]. The discrepancies regarding the potential association of BLV infection and BC may be attributed to differences in detection methods (PCR, in situ PCR, nested PCR) and also in the viral DNA sequence used as a target (tax, gag, env, LTR). Furthermore, differences in the amount of beef and dairy product consumption across the study populations (North American, South American, European, Australian, and Asian) could also explain the discrepancies between the frequencies of BLV DNA found in BC patients [86]. Additionally, it is important to consider that other risk factors, such as genetics and environmental exposure, may confound the observed association between BLV infection and BC development. Future studies should adjust for these variables to clarify the relationship. A summary of these studies is presented in Table 1.

Table 1.
Presence of BLV in BC tissues and controls.
6. Oncogenic Properties of the BLV Encoded Proteins
The oncogenic properties of BLV in animal and human cells have been demonstrated [54,120,121]. However, to the best of our knowledge, there is no information available regarding the direct role of BLV in human BC initiation or progression. This knowledge gap is critical given the potential implications of BLV as a zoonotic agent with oncogenic capabilities. This section focuses on the oncogenic potential of BLV, addressing three key areas: (1) BLV in bovine mammary cells, (2) the closely related HTVL-1 tax protein in human BC cells, and (3) BLV in human cells from different origins as potential models for a better understanding of BLV’s contribution to human BC.
BLV has been shown to enhance the proliferation rate of C72 bovine mammary cells, although these cells were unable to grow in soft agar, a hallmark of oncogenic transformation [120]. Infected bovine mammary epithelial cells (MAC-T) exhibited significantly higher expression levels of TNF-α mRNA compared to uninfected cells (p < 0.001) [122]. Conversely, another study reported reduced viability in BLV-infected MAC-T cells, accompanied by Bcl-2 downregulation and increased TLR9 mRNA expression [123]. Furthermore, the BLV Tax protein was observed to induce DNA damage and impair DNA repair mechanisms in mammalian cells, including bovine mammary epithelial cells [121]. BLV p34 was shown to cooperate with the Ha-ras oncogene in transforming rat embryo fibroblasts, which subsequently formed tumors when injected into nude mice [55].
On the other hand, some authors reported the potential role of the HTLV-1 tax protein in human BC development [124,125,126]. HTLV-1 is a closely related retrovirus to BLV that encodes a similar tax protein [127]. In this regard, the contribution of HTLV-1 tax protein to epithelial cell carcinogenesis could provide valuable information to elucidate the potential role of the BLV tax protein in BC development. For instance, it was reported that the HTLV-1 tax protein interacts with CREB-binding protein (CBP)/p300, inhibiting the activation of BRCA1 induced by ERα [124]. In addition, the HTLV-1 tax protein stimulated the E2–ERα-mediated expression of genes controlled by estrogen response elements (EREs) [125]. Moreover, the HTLV-1 tax protein has the capacity to inhibit BRCA1-mediated activation of p53 target promoters [126].
Finally, the capacity of BLV Tax to deregulate the expression of 122 genes (upregulated: 90, downregulated: 32) was demonstrated in HeLa cells ectopically expressing this molecule [54]. Among them, the overexpression of CYR61, FOS, JUN, RORA, NR4A2, RRAD, GEM, and TNFAIP6 was evidenced at both the transcript and protein levels. In contrast, BLV Tax induced the downregulation of ID2, TNFSF10, IFIT1, and IFIT3 [54]. The capacity of BLV Tax to induce the c-fos promoter activation in SW480 human carcinoma colon cells was demonstrated [128]. Interestingly, human glioblastoma cells with integrated BLV showed proliferation advantages compared to uninfected parental cells [91]. In non-small cell lung cancer (NSCLC), the presence of BLV was significantly associated with PSG4 and CPB2 downregulation [80]. Additionally, BLV Tax induced DNA damage and disrupted DNA repair mechanisms in H9 human T cells, consistent with observations in bovine mammary cells [121].
Collectively, these findings suggest that BLV may affect critical cellular functions, including DNA damage repair, cell proliferation, apoptosis resistance, and immune responses, thereby contributing to oncogenesis. The presence of BLV p24 and gp51 proteins in BC tissues, as detected by immunohistochemistry, supports the possibility of active viral infection [36,79,93]. However, despite the mounting evidence, the precise molecular mechanisms by which BLV proteins contribute to BC initiation and progression remain unclear. Therefore, further clinical and experimental studies are needed to establish the direct role of BLV proteins in BC initiation and progression.
7. Conclusions
The present study showed evidence to support the correlation between BLV infection and BC development in women. Although the exact molecular mechanisms by which BLV could act as a cofactor in breast carcinogenesis are not fully understood, the present review highlights some possibilities. Firstly, the detection of BLV in dairy products and meat for human consumption jointly with the occurrence of anti-BLV antibodies in female blood makes possible the potential transmission of BLV to humans through the diet. Second, some host receptors involved in BLV attachment and fusion to epithelial cells are overexpressed in BC, which make BLV entry into human mammary epithelial cells plausible. Thirdly, the detection of BLV proteins in BC tissues, joined with the capacity of some viral proteins to disrupt key cellular pathways, makes the potential contribution of BLV to human breast carcinogenesis reasonable. However, further clinical and experimental studies focusing on the potential contribution of BLV to BC initiation and progression are strongly necessary. For instance, longitudinal studies following BLV-infected women would be valuable in assessing the long-term effects of BLV infection on BC development. Furthermore, additional experiments are necessary to evaluate the function of BLV-encoded proteins, including Tax, in human breast epithelial cells to enhance understanding of their possible role in BC development and progression. In the future, if BLV is confirmed as a risk factor for BC, it will be important to strengthen strategies to reduce exposure and potential viral transmission to humans, such as the expansion of screening programs to test for BLV presence in dairy products and beef.
Author Contributions
Conceptualization, R.B. and J.P.M.; methodology, R.B.; software, R.B. and C.Q.-R.; validation, R.B. and J.P.M.; formal analysis, R.B.; investigation, R.B. and J.P.M.; resources, R.B.; data curation, R.B.; writing—original draft preparation, R.B.; writing—review and editing, J.P.M.; visualization, R.B. and J.P.M.; supervision, R.B. and J.P.M.; project administration, C.Q.-R. and J.P.M.; funding acquisition, C.Q.-R. and J.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) under the FONDECYT grant 11231072 (J.P.M) and by the Universidad de Tarapacá under the Programa Fortalecimiento Grupos de Investigación, UTA 4769-24 (J.P.M and C.Q.-R). The APC was funded by the Universidad de Tarapacá.
Acknowledgments
The authors express their gratitude to the Universidad de Tarapacá (Arica, Chile) for the financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AP-3 | Adaptor Protein Complex-3 |
| AP3D1 | Adaptor-related Protein Complex 3 Subunit Delta 1 |
| ATM | Ataxia-Telangiectasia Mutated |
| BC | Breast Cancer |
| BLV | Bovine Leukemia Virus |
| BLVR | Bovine Leukemia Virus Receptor |
| BRCA1 | Breast Cancer Gene 1 |
| CAT1 | High-affinity Cationic Amino acid Transporter 1 |
| CBP | CREB-binding Protein |
| DCIS | Ductal Carcinoma in situ |
| DNA | Deoxyribonucleic Acid |
| EBL | Enzootic Bovine Leukosis |
| EBV | Epstein-Barr Virus |
| EMT | Epithelial-to-Mesenchymal Transition |
| ER | Estrogen Receptor |
| ERE | Estrogen Response Elements |
| FFPE | Formalin-Fixed and Paraffin-Embedded Tissues |
| gp | Glycoprotein |
| HCMV | Human Cytomegalovirus |
| HER-2 | Human Epidermal Growth Factor Receptor 2 |
| HMECs | Human Mammalian Epithelial Cells |
| HPV | Human Papillomavirus |
| HTLV-1 | Human T-cell leukemia virus type 1 |
| IDC | Invasive Ductal Carcinoma |
| INT | Integrase |
| IS-PCR | In situ Polymerase Chain Reaction |
| LTR | Long Terminal Repeat |
| miRNA | Micro Ribonucleic Acid |
| MMTV | Mouse Mammary Tumor Virus |
| NSCLC | Non-Small Cell Lung Cancer |
| OR | Odds Ratio |
| PCR | Polymerase Chain Reaction |
| PR | Progesterone Receptor |
| RNA | Ribonucleic Acid |
| RNAPII | RNA Polymerase II |
| RT | Reverse transcriptase |
| SLC7A1 | Solute Carrier Family 7 Member 1 |
| TCGA | The Cancer Genome Atlas |
| TLR9 | Toll-Like Receptor 9 |
| TNF-α | Tumor Necrosis Factor Alpha |
| UCSC | University of California, Santa Cruz |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Obeagu, E.I.; Obeagu, G.U. Breast cancer: A review of risk factors and diagnosis. Medicine 2024, 103, e36905. [Google Scholar] [CrossRef]
- Lawson, J.S.; Salmons, B.; Glenn, W.K. Oncogenic Viruses and Breast Cancer: Mouse Mammary Tumor Virus (MMTV), Bovine Leukemia Virus (BLV), Human Papilloma Virus (HPV), and Epstein-Barr Virus (EBV). Front. Oncol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.P.; Valmary-Degano, S.; et al. The Human Cytomegalovirus Strain DB Activates Oncogenic Pathways in Mammary Epithelial Cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Arias-Calvachi, C.; Blanco, R.; Calaf, G.M.; Aguayo, F. Epstein-Barr Virus Association with Breast Cancer: Evidence and Perspectives. Biology 2022, 11, 799. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K. The viral origins of breast cancer. Infect. Agent Cancer 2024, 19, 39. [Google Scholar] [CrossRef]
- Brantley, K.D.; Tamimi, R.M. The association between infectious agents and breast cancer: A review of the epidemiologic evidence. Breast Cancer Res. Treat. 2024, 207, 235–252. [Google Scholar] [CrossRef]
- Khasawneh, A.I.; Himsawi, N.; Alorjani, M.; Al-Momani, H.; Shahin, U.; Sammour, A.; Saleh, T.; Al-Momani, H.; Khasawneh, R.; Al Shboul, S. Triple Viral Infections in Advanced Breast Cancer: Insights from a Three-Case Report and Literature Review. Diagnostics 2024, 15, 51. [Google Scholar] [CrossRef]
- Song, M.; Lee, K.M.; Kang, D. Breast cancer prevention based on gene-environment interaction. Mol. Carcinog. 2011, 50, 280–290. [Google Scholar] [CrossRef]
- Boyd, N.F.; Stone, J.; Vogt, K.N.; Connelly, B.S.; Martin, L.J.; Minkin, S. Dietary fat and breast cancer risk revisited: A meta-analysis of the published literature. Br. J. Cancer 2003, 89, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Barati-Boldaji, R.; Soltani, S.; Mohammadipoor, N.; Esmaeilinezhad, Z.; Clark, C.C.T.; Babajafari, S.; Akbarzadeh, M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2021, 12, 809–849. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, P.; Riboli, E.; Shore, R.E.; Pasternack, B.S. Consumption of meat, animal products, protein, and fat and risk of breast cancer: A prospective cohort study in New York. Epidemiology 1994, 5, 391–397. [Google Scholar] [CrossRef]
- Cho, E.; Spiegelman, D.; Hunter, D.J.; Chen, W.Y.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Premenopausal fat intake and risk of breast cancer. J. Natl. Cancer Inst. 2003, 95, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.B. A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am. J. Hum. Biol. 2011, 23, 601–608. [Google Scholar] [CrossRef]
- Ganmaa, D.; Sato, A. The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med. Hypotheses 2005, 65, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Lukas Jung, S.U.; Linseisen, J.; Pfau, W. Dietary intake of meat and meat-derived heterocyclic aromatic amines and their correlation with DNA adducts in female breast tissue. Mutagenesis 2009, 24, 127–132. [Google Scholar] [CrossRef]
- Lauber, S.N.; Gooderham, N.J. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine promotes invasive behaviour of breast cancer cells. Toxicology 2011, 279, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer. Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef]
- de Quadros, D.L.; Ribeiro, V.A.; Rezende, M.A.; Maté, Y.A.; Gomes, M.A.; Secchi, K.; Strottmann, D.M.; Frandoloso, R.; Kreutz, L.C. Oncogenic viral DNA related to human breast cancer found on cattle milk and meat. Comp. Immunol. Microbiol. Infect. Dis. 2023, 101, 102053. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Galán, N.N.; Corredor-Figueroa, A.P.; Guzmán-Garzón, T.C.; Ríos-Hernandez, K.S.; Salas-Cárdenas, S.P.; Patarroyo, M.A.; Gutierrez, M.F. Bovine leukaemia virus DNA in fresh milk and raw beef for human consumption. Epidemiol. Infect. 2017, 145, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, H.; Mirshahabi, H.; Motamed, N.; Yavarmanesh, M.; Mahdavi Poor, B.; Moaddab, S.R.; Asgharzadeh, M. Identification of bovine leukemia virus in raw milk samples in North-West of Iran. Vet. Res. Forum. 2021, 12, 223–227. [Google Scholar] [PubMed]
- Manaa, E.; Marawan, M.; Abdelhady, A.; Selim, A. Association between bovine leukemia virus infection, reproductive performance and milk production in water buffaloes and dairy cattle in Egypt. Adv. Anim. Vet. Sci. 2020, 8, 1109–1113. [Google Scholar] [CrossRef]
- Moe, K.K.; Polat, M.; Borjigin, L.; Matsuura, R.; Hein, S.T.; Moe, H.H.; Aida, Y. New evidence of bovine leukemia virus circulating in Myanmar cattle through epidemiological and molecular characterization. PLoS ONE 2020, 15, e0229126. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, L.H. Enzootic bovine leukosis. EFSA J. 2015, 13, 4188. [Google Scholar]
- Derse, D.; Martarano, L. Construction of a recombinant bovine leukemia virus vector for analysis of virus infectivity. J. Virol. 1990, 64, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Kramme, P.M.; Schultz, R.D. Evidence for bovine leukemia virus in mammary epithelial cells of infected cows. Lab. Investig. 1994, 71, 359–365. [Google Scholar]
- Bushi, G.; Vajjala, S.M.; Irfan, F.B.; Aggarwal, D.; Jamil, S.; Sagar, D.; Raju, A.K.; George, M.M.; Pal, A.; Preetha, S. One health implications of bovine leukemia virus seroprevalence: A global systematic review and meta-analysis. Evidence 2024, 2. [Google Scholar] [CrossRef]
- Buehring, G.C.; DeLaney, A.; Shen, H.; Chu, D.L.; Razavian, N.; Schwartz, D.A.; Demkovich, Z.R.; Bates, M.N. Bovine leukemia virus discovered in human blood. BMC Infect. Dis. 2019, 19, 297. [Google Scholar] [CrossRef]
- Buehring, G.C.; Philpott, S.M.; Choi, K.Y. Humans have antibodies reactive with Bovine leukemia virus. AIDS Res. Hum. Retroviruses 2003, 19, 1105–1113. [Google Scholar] [CrossRef]
- Khalilian, M.; Hosseini, S.M.; Madadgar, O. Bovine leukemia virus detected in the breast tissue and blood of Iranian women. Microb. Pathog. 2019, 135, 103566. [Google Scholar] [CrossRef] [PubMed]
- Canova, R.; Weber, M.N.; Budaszewski, R.F.; da Silva, M.S.; Schwingel, D.; Canal, C.W.; Kreutz, L.C. Bovine leukemia viral DNA found on human breast tissue is genetically related to the cattle virus. One Health 2021, 13, 100252. [Google Scholar] [CrossRef]
- Corredor-Figueroa, A.P.; Olaya-Galán, N.N.; Velandia-Álvarez, S.; Muñoz, M.; Salas-Cárdenas, S.P.; Ibáñez-Pinilla, M.; Patarroyo, M.A.; Gutiérrez, M.F. Co-Circulation of Bovine Leukemia Virus Haplotypes among Humans, Animals, and Food Products: New Insights of Its Zoonotic Potential. Int. J. Env. Res. Public Health 2021, 18, 4883. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, D.; Andreolla, A.P.; Erpen, L.M.S.; Frandoloso, R.; Kreutz, L.C. Bovine leukemia virus DNA associated with breast cancer in women from South Brazil. Sci. Rep. 2019, 9, 2949. [Google Scholar] [CrossRef]
- Olaya-Galán, N.N.; Salas-Cárdenas, S.P.; Rodriguez-Sarmiento, J.L.; Ibáñez-Pinilla, M.; Monroy, R.; Corredor-Figueroa, A.P.; Rubiano, W.; de la Peña, J.; Shen, H.; Buehring, G.C.; et al. Risk factor for breast cancer development under exposure to bovine leukemia virus in Colombian women: A case-control study. PLoS ONE 2021, 16, e0257492. [Google Scholar] [CrossRef]
- Baltzell, K.A.; Shen, H.M.; Krishnamurthy, S.; Sison, J.D.; Nuovo, G.J.; Buehring, G.C. Bovine leukemia virus linked to breast cancer but not coinfection with human papillomavirus: Case-control study of women in Texas. Cancer 2018, 124, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect. Agents Cancer 2017, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, J.; Lian, S.; Wang, H.; Wu, R. The Global Epidemiology of Bovine Leukemia Virus: Current Trends and Future Implications. Animals 2024, 14, 297. [Google Scholar] [CrossRef]
- Maertens, G.N.; Engelman, A.N.; Cherepanov, P. Structure and function of retroviral integrase. Nat. Rev. Microbiol. 2022, 20, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sagata, N.; Yasunaga, T.; Tsuzuku-Kawamura, J.; Ohishi, K.; Ogawa, Y.; Ikawa, Y. Complete nucleotide sequence of the genome of bovine leukemia virus: Its evolutionary relationship to other retroviruses. Proc. Natl. Acad. Sci. USA 1985, 82, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, O.Y. Structural characteristics of the bovine leukemia virus genome: A mini review. Food Syst. 2023, 6, 283–287. [Google Scholar] [CrossRef]
- Plant, E.; Bellefroid, M.; Van Lint, C. A complex network of transcription factors and epigenetic regulators involved in bovine leukemia virus transcriptional regulation. Retrovirology 2023, 20, 11. [Google Scholar] [CrossRef]
- Yoshinaka, Y.; Katoh, I.; Copeland, T.D.; Smythers, G.W.; Oroszlan, S. Bovine leukemia virus protease: Purification, chemical analysis, and in vitro processing of gag precursor polyproteins. J. Virol. 1986, 57, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Moratorio, G.; Fischer, S.; Bianchi, S.; Tomé, L.; Rama, G.; Obal, G.; Carrión, F.; Pritsch, O.; Cristina, J. A detailed molecular analysis of complete bovine leukemia virus genomes isolated from B-cell lymphosarcomas. Vet. Res. 2013, 44, 19. [Google Scholar] [CrossRef] [PubMed]
- Lairmore, M.D. Animal models of bovine leukemia virus and human T-lymphotrophic virus type-1: Insights in transmission and pathogenesis. Annu. Rev. Anim. Biosci. 2014, 2, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.M.; Golemba, M.D.; Campos, R.H.; Trono, K.; Jones, L.R. Bovine leukemia virus can be classified into seven genotypes: Evidence for the existence of two novel clades. J. Gen. Virol. 2009, 90, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Balić, D.; Lojkić, I.; Periškić, M.; Bedeković, T.; Jungić, A.; Lemo, N.; Roić, B.; Cač, Z.; Barbić, L.; Madić, J. Identification of a new genotype of bovine leukemia virus. Arch. Virol. 2012, 157, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, E.J.; Ratthanophart, J.; Vitoonpong, R.; Kim, B.H.; Cho, I.S.; Song, J.Y.; Lee, K.K.; Shin, Y.K. Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infect. Genet. Evol. 2016, 41, 245–254. [Google Scholar] [CrossRef]
- Polat, M.; Takeshima, S.N.; Hosomichi, K.; Kim, J.; Miyasaka, T.; Yamada, K.; Arainga, M.; Murakami, T.; Matsumoto, Y.; de la Barra Diaz, V.; et al. A new genotype of bovine leukemia virus in South America identified by NGS-based whole genome sequencing and molecular evolutionary genetic analysis. Retrovirology 2016, 13, 4. [Google Scholar] [CrossRef]
- Yu, C.; Wang, X.; Zhou, Y.; Wang, Y.; Zhang, X.; Zheng, Y. Genotyping bovine leukemia virus in dairy cattle of Heilongjiang, northeastern China. BMC Vet. Res. 2019, 15, 179. [Google Scholar] [CrossRef]
- Robek, M.D.; Wong, F.H.; Ratner, L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 1998, 72, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Boros, I.M.; Tie, F.; Giam, C.Z. Interaction of bovine leukemia virus transactivator Tax with bZip proteins. Virology 1995, 214, 207–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arainga, M.; Takeda, E.; Aida, Y. Identification of bovine leukemia virus tax function associated with host cell transcription, signaling, stress response and immune response pathway by microarray-based gene expression analysis. BMC Genom. 2012, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Heremans, H.; Chen, G.; Portetelle, D.; Billiau, A.; Burny, A.; Kettmann, R. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. Embo J. 1990, 9, 1577–1581. [Google Scholar] [CrossRef]
- Pluta, A.; Jaworski, J.P.; Douville, R.N. Regulation of Expression and Latency in BLV and HTLV. Viruses 2020, 12, 1079. [Google Scholar] [CrossRef]
- Willems, L.; Kerkhofs, P.; Dequiedt, F.; Portetelle, D.; Mammerickx, M.; Burny, A.; Kettmann, R. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc. Natl. Acad. Sci. USA 1994, 91, 11532–11536. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, P.; Heremans, H.; Burny, A.; Kettmann, R.; Willems, L. In vitro and in vivo oncogenic potential of bovine leukemia virus G4 protein. J. Virol. 1998, 72, 2554–2559. [Google Scholar] [CrossRef]
- Rosewick, N.; Momont, M.; Durkin, K.; Takeda, H.; Caiment, F.; Cleuter, Y.; Vernin, C.; Mortreux, F.; Wattel, E.; Burny, A.; et al. Deep sequencing reveals abundant noncanonical retroviral microRNAs in B-cell leukemia/lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.A.; Hamaidia, M.; de Brogniez, A.; Gutiérrez, G.; Renotte, N.; Reichert, M.; Trono, K.; Willems, L. Bovine Leukemia Virus Small Noncoding RNAs Are Functional Elements That Regulate Replication and Contribute to Oncogenesis In Vivo. PLoS Pathog. 2016, 12, e1005588. [Google Scholar] [CrossRef]
- Kincaid, R.P.; Burke, J.M.; Sullivan, C.S. RNA virus microRNA that mimics a B-cell oncomiR. Proc. Natl. Acad. Sci. USA 2012, 109, 3077–3082. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, C.H.; Xia, W.; Yang, J.Y.; Xiong, Y.; Li, C.W.; Yu, W.H.; Rehman, S.K.; Hsu, J.L.; Lee, H.H.; et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 2011, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Danza, K.; De Summa, S.; Pinto, R.; Pilato, B.; Palumbo, O.; Carella, M.; Popescu, O.; Digennaro, M.; Lacalamita, R.; Tommasi, S. TGFbeta and miRNA regulation in familial and sporadic breast cancer. Oncotarget 2017, 8, 50715–50723. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Shi, W.; Tang, T.; Wang, Y.; Yin, X.; Chen, Y.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis. 2019, 10, 176. [Google Scholar] [CrossRef]
- Murakami, H.; Yamada, T.; Suzuki, M.; Nakahara, Y.; Suzuki, K.; Sentsui, H. Bovine leukemia virus integration site selection in cattle that develop leukemia. Virus Res. 2011, 156, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.P. Intracellular trafficking of retroviral genomes during the early phase of infection: Viral exploitation of cellular pathways. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2001, 3, 517–528. [Google Scholar] [CrossRef]
- Forlani, G.; Shallak, M.; Ramia, E.; Tedeschi, A.; Accolla, R.S. Restriction factors in human retrovirus infections and the unprecedented case of CIITA as link of intrinsic and adaptive immunity against HTLV-1. Retrovirology 2019, 16, 34. [Google Scholar] [CrossRef]
- Murakami, H.; Suzuki, T.; Tsuchiya, K.; Gatanaga, H.; Taura, M.; Kudo, E.; Okada, S.; Takei, M.; Kuroda, K.; Yamamoto, T. Protein arginine N-methyltransferases 5 and 7 promote HIV-1 production. Viruses 2020, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.-B.; Defoiche, J.; Burny, A. Mechanisms of leukemogenesis induced by bovine leukemia virus: Prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef]
- Derse, D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J. Virol. 1987, 61, 2462–2471. [Google Scholar] [CrossRef]
- Inabe, K.; Nishizawa, M.; Tajima, S.; Ikuta, K.; Aida, Y. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J. Virol. 1999, 73, 1293–1301. [Google Scholar] [CrossRef]
- Tajima, S.; Takahashi, M.; Takeshima, S.N.; Konnai, S.; Yin, S.A.; Watarai, S.; Tanaka, Y.; Onuma, M.; Okada, K.; Aida, Y. A mutant form of the tax protein of bovine leukemia virus (BLV), with enhanced transactivation activity, increases expression and propagation of BLV in vitro but not in vivo. J. Virol. 2003, 77, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Burny, A.; Cleuter, Y.; Kettmann, R.; Mammerickx, M.; Marbaix, G.; Portetelle, D.; van den Broeke, A.; Willems, L.; Thomas, R. Bovine leukaemia: Facts and hypotheses derived from the study of an infectious cancer. Vet. Microbiol. 1988, 17, 197–218. [Google Scholar] [CrossRef]
- Mammerickx, M.; Portetelle, D.; de Clercq, K.; Burny, A. Experimental transmission of enzootic bovine leukosis to cattle, sheep and goats: Infectious doses of blood and incubation period of the disease. Leuk. Res. 1987, 11, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Galán, N.N.; Corredor-Figueroa, A.P.; Velandia-Álvarez, S.; Vargas-Bermudez, D.S.; Fonseca-Ahumada, N.; Nuñez, K.; Jaime, J.; Gutiérrez, M.F. Evidence of bovine leukemia virus circulating in sheep and buffaloes in Colombia: Insights into multispecies infection. Arch. Virol. 2022, 167, 807–817. [Google Scholar] [CrossRef]
- Altanerova, V.; Ban, J.; Kettmann, R.; Altaner, C. Induction of leukemia in chicken by bovine leukemia virus due to insertional mutagenesis. Arch. Geschwulstforsch. 1990, 60, 89–96. [Google Scholar] [PubMed]
- Wyatt, C.R.; Wingett, D.; White, J.S.; Buck, C.D.; Knowles, D.; Reeves, R.; Magnuson, N.S. Persistent infection of rabbits with bovine leukemia virus associated with development of immune dysfunction. J. Virol. 1989, 63, 4498–4506. [Google Scholar] [CrossRef]
- Boris-Lawrie, K.; Altanerova, V.; Altaner, C.; Kucerova, L.; Temin, H.M. In vivo study of genetically simplified bovine leukemia virus derivatives that lack tax and rex. J. Virol. 1997, 71, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Shen, H.M.; Jensen, H.M.; Jin, D.L.; Hudes, M.; Block, G. Exposure to Bovine Leukemia Virus Is Associated with Breast Cancer: A Case-Control Study. PLoS ONE 2015, 10, e0134304. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Pierce, C.M.; Robinson, L.A. Impact of viral presence in tumor on gene expression in non-small cell lung cancer. BMC Cancer 2018, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; DiGiacomo, R.F.; Ferrer, J.F.; Parish, S.M. Transmission of bovine leukosis virus by blood inoculation. Am. J. Vet. Res. 1986, 47, 1885–1887. [Google Scholar] [CrossRef]
- Ferrer, J.F.; Piper, C.E. Role of colostrum and milk in the natural transmission of the bovine leukemia virus. Cancer Res. 1981, 41, 4906–4909. [Google Scholar] [PubMed]
- Kuczewski, A.; Orsel, K.; Barkema, H.W.; Mason, S.; Erskine, R.; van der Meer, F. Invited review: Bovine leukemia virus-Transmission, control, and eradication. J. Dairy Sci. 2021, 104, 6358–6375. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.P.; Porta, N.G.; Gutierrez, G.; Politzki, R.P.; Álvarez, I.; Galarza, R.; Abdala, A.; Calvinho, L.; Trono, K.G. Short communication: Relationship between the level of bovine leukemia virus antibody and provirus in blood and milk of cows from a naturally infected herd. J. Dairy Sci. 2016, 99, 5629–5634. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, S.; Takeshima, S.N.; Borjigin, L.; Sato, H.; Bai, L.; Murakami, H.; Sato, R.; Ishizaki, H.; Matsumoto, Y.; Aida, Y. Visualizing bovine leukemia virus (BLV)-infected cells and measuring BLV proviral loads in the milk of BLV seropositive dams. Vet. Res. 2019, 50, 102. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.P.; Saito, S.; Hara, Y.; Matsuura, R.; Takeshima, S.N.; Hosomichi, K.; Matsumoto, Y.; Furuta, R.A.; Takei, M.; Aida, Y. No evidence of bovine leukemia virus proviral DNA and antibodies in human specimens from Japan. Retrovirology 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Carrillo-Beltrán, D.; Muñoz, J.P.; Corvalán, A.H.; Calaf, G.M.; Aguayo, F. Human Papillomavirus in Breast Carcinogenesis: A Passenger, a Cofactor, or a Causal Agent? Biology 2021, 10, 804. [Google Scholar] [CrossRef]
- Mendoza, W.; Isaza, J.P.; López, L.; López-Herrera, A.; Gutiérrez, L.A. Bovine leukemia virus detection in humans: A systematic review and meta-analysis. Virus Res. 2023, 335, 199186. [Google Scholar] [CrossRef]
- Schwartz, I.; Bensaid, A.; Polack, B.; Perrin, B.; Berthelemy, M.; Levy, D. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J. Virol. 1994, 68, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sato, H.; Kubo, Y.; Wada, S.; Aida, Y. CAT1/SLC7A1 acts as a cellular receptor for bovine leukemia virus infection. Faseb. J. 2019, 33, 14516–14527. [Google Scholar] [CrossRef]
- Altaner, C.; Altanerová, V.; Bán, J.; Niwa, O.; Yokoro, K. Human cells of neural origin are permissive for bovine leukemia virus. Neoplasma 1989, 36, 691–695. [Google Scholar] [PubMed]
- Olaya-Galán, N.N.; Blume, S.; Tong, K.; Shen, H.; Gutierrez, M.F.; Buehring, G.C. In vitro Susceptibility of Human Cell Lines Infection by Bovine Leukemia Virus. Front. Microbiol. 2022, 13, 793348. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cruz, A.; Uribe, A.; Gutiérrez, M. Estudio del potencial zoonótico del virus de la leucosis bovina y su presencia en casos de cáncer de seno. Univ. Sci. 2006, 11, 31–40. [Google Scholar]
- Graves, D.C.; Ferrer, J.F. In vitro transmission and propagation of the bovine leukemia virus in monolayer cell cultures. Cancer Res. 1976, 36, 4152–4159. [Google Scholar] [PubMed]
- You, S.; Han, X.; Xu, Y.; Yao, Q. Research progress on the role of cationic amino acid transporter (CAT) family members in malignant tumors and immune microenvironment. Amino. Acids 2023, 55, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Bai, L.; Borjigin, L.; Aida, Y. Overexpression of bovine leukemia virus receptor SLC7A1/CAT1 enhances cellular susceptibility to BLV infection on luminescence syncytium induction assay (LuSIA). Virol. J. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; He, J.; Liao, X.; Liang, T.; Zhu, J.; Wei, W.; He, Y.; Zhou, X.; Peng, T. A comprehensive analysis of the diagnostic and prognostic value associated with the SLC7A family members in breast cancer. Gland. Surg. 2022, 11, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Craft, B.; Swatloski, T.; Cline, M.; Morozova, O.; Diekhans, M.; Haussler, D.; Zhu, J. The UCSC Cancer Genomics Browser: Update 2015. Nucleic. Acids Res. 2015, 43, D812–D817. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Rickard, J.A.; McDonald, W.J.; Thomas, L.N.; Too, C.K. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J. Cell Biochem. 2011, 112, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Le Borgne, R.; Alconada, A.; Bauer, U.; Hoflack, B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998, 273, 29451–29461. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Nile, A.; Oh, J.-W. Role of adaptin protein complexes in intracellular trafficking and their impact on diseases. Bioengineered 2021, 12, 8259–8278. [Google Scholar] [CrossRef] [PubMed]
- Corredor, A.P.; González, J.; Baquero, L.A.; Curtidor, H.; Olaya-Galán, N.N.; Patarroyo, M.A.; Gutiérrez, M.F. In silico and in vitro analysis of boAP3d1 protein interaction with bovine leukaemia virus gp51. PLoS ONE 2018, 13, e0199397. [Google Scholar] [CrossRef]
- Ban, J.; Portetelle, D.; Altaner, C.; Horion, B.; Milan, D.; Krchnak, V.; Burny, A.; Kettmann, R. Isolation and characterization of a 2.3-kilobase-pair cDNA fragment encoding the binding domain of the bovine leukemia virus cell receptor. J. Virol. 1993, 67, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Matsubara, Y.; Kitani, H.; Ikeda, H. Evaluation of the delta subunit of bovine adaptor protein complex 3 as a receptor for bovine leukaemia virus. J. Gen. Virol. 2003, 84, 1309–1316. [Google Scholar] [CrossRef]
- Gruel, N.; Lucchesi, C.; Raynal, V.; Rodrigues, M.J.; Pierron, G.; Goudefroye, R.; Cottu, P.; Reyal, F.; Sastre-Garau, X.; Fourquet, A.; et al. Lobular invasive carcinoma of the breast is a molecular entity distinct from luminal invasive ductal carcinoma. Eur. J. Cancer 2010, 46, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Khatami, A.; Pormohammad, A.; Farzi, R.; Saadati, H.; Mehrabi, M.; Kiani, S.J.; Ghorbani, S. Bovine Leukemia virus (BLV) and risk of breast cancer: A systematic review and meta-analysis of case-control studies. Infect. Agents Cancer 2020, 15, 48. [Google Scholar] [CrossRef]
- Saeedi-Moghaddam, F.; Mohammaditabar, M.; Mozhgani, S.H. Bovine leukemia virus (BLV) and risk of breast cancer; a systematic review and meta-analysis. Retrovirology 2024, 21, 20. [Google Scholar] [CrossRef]
- Buehring, G.C.; Shen, H.; Schwartz, D.A.; Lawson, J.S. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PLoS ONE 2017, 12, e0179367. [Google Scholar] [CrossRef]
- Lendez, P.A.; Martinez Cuesta, L.; Nieto Farías, M.V.; Shen, H.M.; Dolcini, G.L.; Buehring, G.C.; Ceriani, M.C. Bovine leukemia virus presence in breast tissue of Argentinian females and its association with cell proliferation and prognosis markers. Multidiscip. Cancer Investig. 2018, 2, 16–24. [Google Scholar]
- Khan, Z.; Abubakar, M.; Arshed, M.J.; Aslam, R.; Sattar, S.; Shah, N.A.; Javed, S.; Tariq, A.; Bostan, N.; Manzoor, S. Molecular investigation of possible relationships concerning bovine leukemia virus and breast cancer. Sci. Rep. 2022, 12, 4161. [Google Scholar] [CrossRef]
- Elmatbouly, A.; Badr, R.I.; Masallat, D.T.; Youssef, M.Y.; Omar, N.S. Prevalence of Bovine leukemia virus in Egyptian women’s breast cancer tissues. Egypt. J. Basic Appl. Sci. 2023, 10, 824–834. [Google Scholar] [CrossRef]
- Delarmelina, E.; Buzelin, M.A.; Souza, B.S.; Souto, F.M.; Bicalho, J.M.; Câmara, R.J.F.; Resende, C.F.; Bueno, B.L.; Victor, R.M.; Galinari, G.C.F.; et al. High positivity values for bovine leukemia virus in human breast cancer cases from Minas Gerais, Brazil. PLoS ONE 2020, 15, e0239745. [Google Scholar] [CrossRef] [PubMed]
- James, L.M.; Georgopoulos, A.P. Breast cancer, viruses, and human leukocyte antigen (HLA). Sci. Rep. 2024, 14, 16179. [Google Scholar] [CrossRef]
- Gillet, N.A.; Willems, L. Whole genome sequencing of 51 breast cancers reveals that tumors are devoid of bovine leukemia virus DNA. Retrovirology 2016, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, J.; Sun, W.; Zhang, J.; Huang, K.; Gu, X.; Yang, Y.; Xu, X.; Shi, Y.; Wang, C. Lack of association between bovine leukemia virus and breast cancer in Chinese patients. Breast Cancer Res. 2016, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Giovanna, M.; Carlos, U.J.; María, U.A.; Gutierrez, M.F. Bovine leukemia virus gene segment detected in human breast tissue. Open J. Med. Microbiol. 2013, 3, 84–90. [Google Scholar] [CrossRef]
- Khasawneh, A.I.; Himsawi, N.; Sammour, A.; Alorjani, M.; Al-Momani, H.; Shahin, U.; Alotaibi, M.R.; Al Shboul, S.; Saleh, T. Are Mouse Mammary Tumor Virus and Bovine Leukemia Virus Linked to Breast Cancer among Jordanian Women? Microbiol. Res. 2024, 15, 914–925. [Google Scholar] [CrossRef]
- Amato, S.; Ramsey, J.; Ahern, T.P.; Rovnak, J.; Barlow, J.; Weaver, D.; Eyasu, L.; Singh, R.; Cintolo-Gonzalez, J. Exploring the presence of bovine leukemia virus among breast cancer tumors in a rural state. Breast Cancer Res. Treat 2023, 202, 325–334. [Google Scholar] [CrossRef]
- Motton, D.D.; Buehring, G.C. Bovine leukemia virus alters growth properties and casein synthesis in mammary epithelial cells. J. Dairy Sci. 2003, 86, 2826–2838. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Buehring, G.C. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: Role of tax gene. J. Natl. Cancer Inst. 1999, 91, 933–942. [Google Scholar] [CrossRef]
- Martinez Cuesta, L.; Nieto Farias, M.V.; Lendez, P.A.; Barone, L.; Pérez, S.E.; Dolcini, G.L.; Ceriani, M.C. Stable infection of a bovine mammary epithelial cell line (MAC-T) with bovine leukemia virus (BLV). Virus Res. 2018, 256, 11–16. [Google Scholar] [CrossRef]
- Martinez Cuesta, L.; Nieto Farias, M.V.; Lendez, P.A.; Rowland, R.R.R.; Sheahan, M.A.; Cheuquepán Valenzuela, F.A.; Marin, M.S.; Dolcini, G.; Ceriani, M.C. Effect of bovine leukemia virus on bovine mammary epithelial cells. Virus Res. 2019, 271, 197678. [Google Scholar] [CrossRef]
- Shukrun, M.; Jabareen, A.; Abou-Kandil, A.; Chamias, R.; Aboud, M.; Huleihel, M. HTLV-1 Tax oncoprotein inhibits the estrogen-induced-ER α-Mediated BRCA1 expression by interaction with CBP/p300 cofactors. PLoS ONE 2014, 9, e89390. [Google Scholar] [CrossRef] [PubMed]
- Jabareen, A.; Abu-Jaafar, A.; Abou-Kandil, A.; Huleihel, M. Effect of TPA and HTLV-1 Tax on BRCA1 and ERE controlled genes expression. Cell Cycle 2017, 16, 1336–1344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huleihel, M.; Shukron, M.; Gabarin, A.; Abukandil, A.; Aboud, M. Antagonistic effects of HTLV-1 Tax oncoprotein on BRCA1 expression and function. Retrovirology 2014, 11, 88. [Google Scholar] [CrossRef]
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Katoh, I.; Yoshinaka, Y.; Ikawa, Y. Bovine leukemia virus trans-activator p38tax activates heterologous promoters with a common sequence known as a cAMP-responsive element or the binding site of a cellular transcription factor ATF. Embo J. 1989, 8, 497–503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).