Transcriptional Upregulation of HERV-env Genes Under Simulated Microgravity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Simulated Microgravity
2.3. RNA Extraction, Reverse Transcription, and Real-Time PCR
2.4. Statistical Analysis
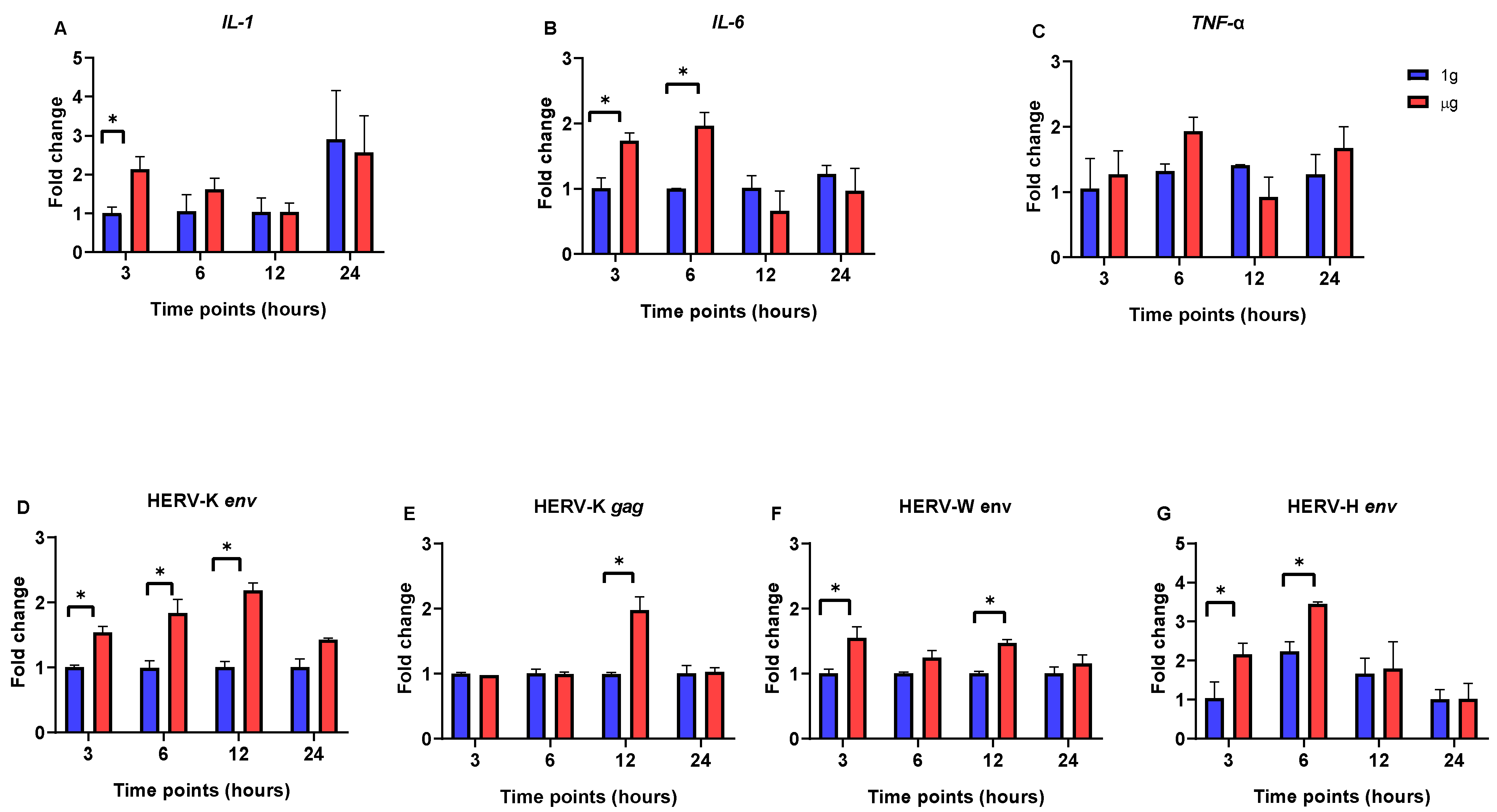
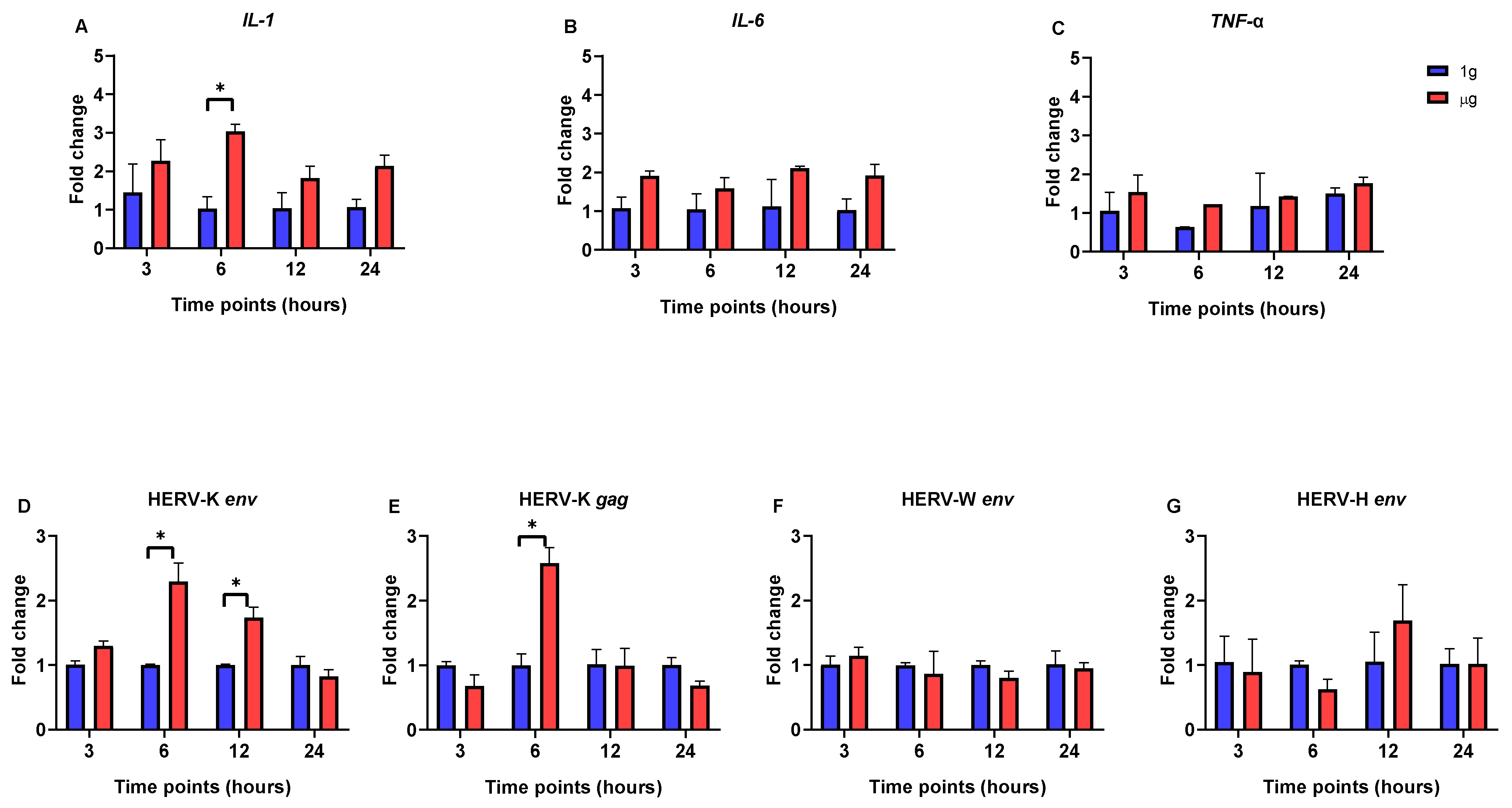
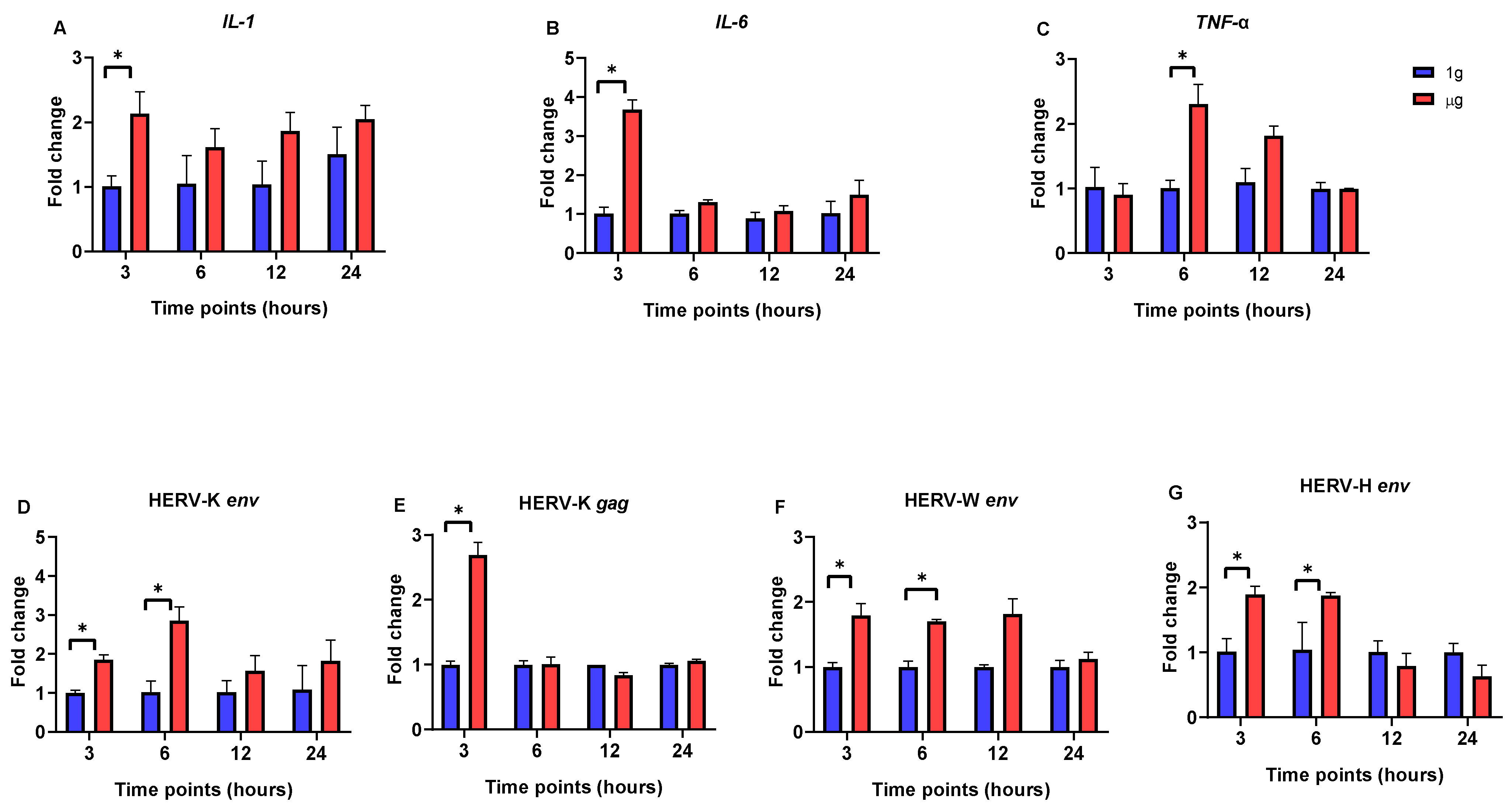
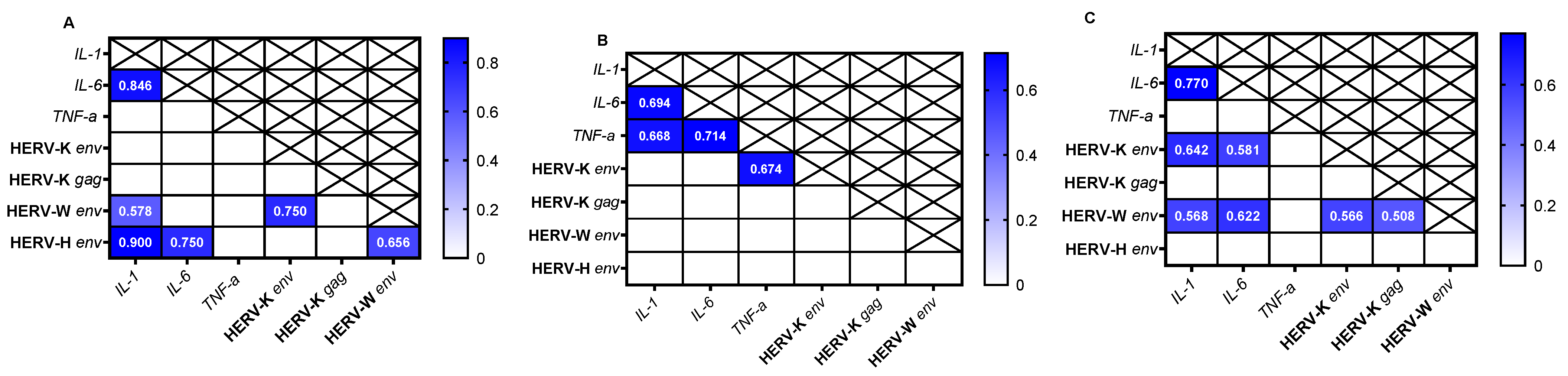
3. Results
3.1. The Impact of Simulated Microgravity on Pro-Inflammatory Cytokine and HERV Gene Expression in SH-SY5Y, Caco-2, and HEp-2 Cells
3.2. Correlation Analysis of Gene Expression in Different Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Antony, J.M.; DesLauriers, A.M.; Bhat, R.K.; Ellestad, K.K.; Power, C. Human endogenous retroviruses and multiple sclerosis: Innocent bystanders or disease determinants? Biochim. Et Biophys. Acta-Mol. Basis Dis. 2011, 1812, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Balada, E.; Ordi-Ros, J.; Vilardell-Tarrés, M. Molecular mechanisms mediated by human endogenous retroviruses (HERVs) in autoimmunity. Rev. Med. Virol. 2009, 19, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, Q.; Cong, Y.S. Human endogenous retroviruses in development and disease. Comput. Struct. Biotechnol. J. 2021, 19, 5978–5986. [Google Scholar] [CrossRef] [PubMed]
- Rangel, S.C.; da Silva, M.D.; da Silva, A.L.; Dos Santos, J.D.M.B.; Neves, L.M.; Pedrosa, A.; Rodrigues, F.M.; Trettel, C.D.S.; Furtado, G.E.; de Barros, M.P.; et al. Human endogenous retroviruses and the inflammatory response: A vicious circle associated with health and illness. Front. Immunol. 2022, 13, 1057791. [Google Scholar] [CrossRef]
- Bannert, N.; Kurth, R. The Evolutionary Dynamics of Human Endogenous Retroviral Families. Annu. Rev. Genom. Hum. Genet. 2006, 7, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Geis, F.K.; Goff, S.P. Silencing and Transcriptional Regulation of Endogenous Retroviruses: An Overview. Viruses 2020, 12, 884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posso-Osorio, I.; Tobón, G.J.; Cañas, C.A. Human endogenous retroviruses (HERV) and non-HERV viruses incorporated into the human genome and their role in the development of autoimmune diseases. J. Transl. Autoimmun. 2021, 4, 100137. [Google Scholar] [CrossRef] [PubMed]
- Küry, P.; Nath, A.; Créange, A.; Dolei, A.; Marche, P.; Gold, J.; Giovannoni, G.; Hartung, H.-P.; Perron, H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 2018, 24, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Dembny, P.; Newman, A.G.; Singh, M.; Hinz, M.; Szczepek, M.; Krüger, C.; Adalbert, R.; Dzaye, O.; Trimbuch, T.; Wallach, T. Human endogenous retrovirus HERV-K (HML-2) RNA causes neurodegeneration through Toll-like receptors. JCI Insight 2020, 5, e131093. [Google Scholar] [CrossRef]
- Rolland, A.; Jouvin-Marche, E.; Viret, C.; Faure, M.; Perron, H.; Marche, P.N. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 2006, 176, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef] [PubMed]
- Rangel, S.C.; da Silva, M.D.; Natrielli Filho, D.G.; Santos, S.N.; do Amaral, J.B.; Victor, J.R.; Silva, K.C.N.; Tuleta, I.D.; França, C.N.; Shio, M.T.; et al. HERV-W upregulation expression in bipolar disorder and schizophrenia: Unraveling potential links to systemic immune/inflammation status. Retrovirology 2024, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.P.; Magiorkinis, G. Activation of the innate immune response by endogenous retroviruses. J. Gen. Virol. 2015, 96, 1207–1218. [Google Scholar] [CrossRef]
- Hicks, J.; Olson, M.; Mitchell, C.; Juran, C.M.; Paul, A.M. The impact of microgravity on immunological states. Immunohorizons 2023, 7, 670–682. [Google Scholar] [PubMed]
- Corydon, T.J.; Schulz, H.; Richter, P.; Strauch, S.M.; Böhmer, M.; Ricciardi, D.A.; Wehland, M.; Krüger, M.; Erzinger, G.S.; Lebert, M.; et al. Current Knowledge about the Impact of Microgravity on Gene Regulation. Cells 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-M.; Kim, H.-M.; Kim, H.-S. Expression of the human endogenous retrovirus HERV-W family in various human tissues and cancer cells. J. Gen. Virol. 2004, 85, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, P.W.; Quansah, D.N.K.; Kumar, N.; Steiner, J.P.; Nath, A.; Geiger, J.D. HERV-K (HML-2) Envelope Protein Induces Mitochondrial Depolarization and Neurotoxicity via Endolysosome Iron Dyshomeostasis. J. Neurosci. 2024, 44, e0826232024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, L.; Cafora, M.; Rota, F.; Hoxha, M.; Iodice, S.; Tarantini, L.; Dolci, M.; Delbue, S.; Pistocchi, A.; Bollati, V. Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses. Int. J. Mol. Sci. 2019, 20, 3669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogetseder, W.; Feng, J.; Haibach, C.; Mayerl, W.; Dierich, M.P. Detection of a 67-kD glycoprotein in human tumor cell lines by a monoclonal antibody established against a recombinant human endogenous retrovirus-K envelope-gene-encoded protein. Exp. Clin. Immunogenet. 1995, 12, 96–102. [Google Scholar] [PubMed]
- Manis, C.; Manca, A.; Murgia, A.; Uras, G.; Caboni, P.; Congiu, T.; Faa, G.; Pantaleo, A.; Cao, G. Understanding the Behaviour of Human Cell Types under Simulated Microgravity Conditions: The Case of Erythrocytes. Int. J. Mol. Sci. 2022, 23, 6876. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem. Cell Res. Ther. 2022, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, S.; Santovito, A.; Simula, E.R.; Noli, M.; Manca, M.A.; Sechi, L.A. Bisphenols induce human genomic damage and modulate HERVs/env expression. Environ. Mol. Mutagen. 2022, 63, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhu, H.; Li, N.; Yu, C.; Song, Z.; Wang, S.; Sun, Y.; Zheng, L.; Wang, G.; Huang, Y.; et al. Stevioside Activates AMPK to Suppress Inflammation in Macrophages and Protects Mice from LPS-Induced Lethal Shock. Molecules 2021, 26, 858. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.S.; Hauschild, S.; Tauber, S.; Paulsen, K.; Raig, C.; Raem, A.; Biskup, J.; Gutewort, A.; Hürlimann, E.; Unverdorben, F.; et al. Identification of reference genes in human myelomonocytic cells for gene expression studies in altered gravity. Biomed. Res. Int. 2015, 2015, 363575, Epub 13 January 2015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiel, C.S.; Huge, A.; Hauschild, S.; Tauber, S.; Lauber, B.A.; Polzer, J.; Paulsen, K.; Lier, H.; Engelmann, F.; Schmitz, B.; et al. Stability of gene expression in human T cells in different gravity environments is clustered in chromosomal region 11p15.4. NPJ Microgravity 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asslih, S.; Damri, O.; Agam, G. Neuroinflammation as a Common Denominator of Complex Diseases (Cancer, Diabetes Type 2, and Neuropsychiatric Disorders). Int. J. Mol. Sci. 2021, 22, 6138. [Google Scholar] [CrossRef]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M. Impact of Simulated Microgravity on Cell Cycle Control and Cytokine Release by U937 Cells. Int. J. Immunopathol. Pharmacol. 2006, 19, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. 2015, 64, 303–311. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Zhu, L.; Yang, F.; Chu, Z.; Tian, H.; Feng, M.; Zhao, Y.; Shang, P. Microgravity inhibition of lipopolysaccharide-induced tumor necrosis factor-α expression in macrophage cells. Inflamm. Res. 2014, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-L.; Chao, P.-D.L.; Fang, S.-H. Morin sulphates/glucuronides enhance macrophage function in microgravity culture system. Eur. J. Clin. Investig. 2005, 35, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Dietrichs, D.; Grimm, D.; Sahana, J.; Melnik, D.; Corydon, T.J.; Wehland, M.; Krüger, M.; Vermeesen, R.; Baselet, B.; Baatout, S.; et al. Three-Dimensional Growth of Prostate Cancer Cells Exposed to Simulated Microgravity. Front. Cell Dev. Biol. 2022, 10, 841017. [Google Scholar] [CrossRef]
- La Barbera, G.; Capriotti, A.L.; Michelini, E.; Piovesana, S.; Calabretta, M.M.; Zenezini Chiozzi, R.; Roda, A.; Laganà, A. Proteomic analysis and bioluminescent reporter gene assays to investigate effects of simulated microgravity on Caco-2 cells. Proteomics 2017, 17, 1700081. [Google Scholar] [CrossRef]
- Li, P.; Shi, J.; Zhang, P.; Wang, K.; Li, J.; Liu, H.; Zhou, Y.; Xu, X.; Hao, J.; Sun, X.; et al. Simulated microgravity disrupts intestinal homeostasis and increases colitis susceptibility. FASEB J. 2015, 29, 3263–3273. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.S.; Good, T.A. Effect of culture in a rotating wall bioreactor on the physiology of differentiated neuron-like PC12 and SH-SY5Y cells. J. Cell. Biochem. 2001, 83, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Qu, L.N.; Huang, Z.M.; Wang, C.Y.; Li, Q.; Tan, Y.J.; Li, Y.H. Effects of parabolic flight on redox status in SH-SY5Y cells. Sheng Li Xue Bao 2009, 61, 445–450. (In Chinese) [Google Scholar] [PubMed]
- Faucard, R.; Madeira, A.; Gehin, N.; Authier, F.-J.; Panaite, P.-A.; Lesage, C.; Burgelin, I.; Bertel, M.; Bernard, C.; Curtin, F.; et al. Human Endogenous Retrovirus and Neuroinflammation in Chronic Inflammatory Demyelinating Polyradiculoneuropathy. eBioMedicine 2016, 6, 190–198. [Google Scholar] [CrossRef]
| Target Gene | Sequence 5′ to 3′ (Forward) | Sequence 5′ to 3′ (Reverse) |
|---|---|---|
| IL-1B | F: GCACGATGCACCTGTACGAT | R: AGACATCACCAAGCTTTTTTGCT |
| IL-6 | F: CCAGGAGCCCAGCTATGAAC | R: GAGCAGCCCCAGGGAGAA |
| TNF-α | F: CAGAGGGAAGAGTTCCCCAG | R: CCTTGGTCTGGTAGGAGACG |
| GAPDH | F: CAAGGAGTAAGACCCCTGGAC | R: TCTACATGGCAACTGTGAGGAG |
| HERV-H env | F: CCCATATTTGGACCTCTCAC | R: TGTGTAGTTGGGCTTTGGAG |
| HERV-W env | F: CCTATTTAATACCACCCTCACTG | R: AGTTGTTCCATTGTTCAGGT |
| HERV-K env | F: GCTGTCTCTTCGGAGCTGTT | R: CTGAGGCAATTGCAGGAGTT |
| HERV-K gag | F: CCCATGGTTTCCAGAACAAGG | R: AAGCTGCTTTAATAATGGCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasemi, S.; Simula, E.R.; Pantaleo, A.; Sechi, L.A. Transcriptional Upregulation of HERV-env Genes Under Simulated Microgravity. Viruses 2025, 17, 306. https://doi.org/10.3390/v17030306
Jasemi S, Simula ER, Pantaleo A, Sechi LA. Transcriptional Upregulation of HERV-env Genes Under Simulated Microgravity. Viruses. 2025; 17(3):306. https://doi.org/10.3390/v17030306
Chicago/Turabian StyleJasemi, Seyedesomaye, Elena Rita Simula, Antonella Pantaleo, and Leonardo A. Sechi. 2025. "Transcriptional Upregulation of HERV-env Genes Under Simulated Microgravity" Viruses 17, no. 3: 306. https://doi.org/10.3390/v17030306
APA StyleJasemi, S., Simula, E. R., Pantaleo, A., & Sechi, L. A. (2025). Transcriptional Upregulation of HERV-env Genes Under Simulated Microgravity. Viruses, 17(3), 306. https://doi.org/10.3390/v17030306







