Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats
Abstract
1. Introduction
2. Materials and Methods
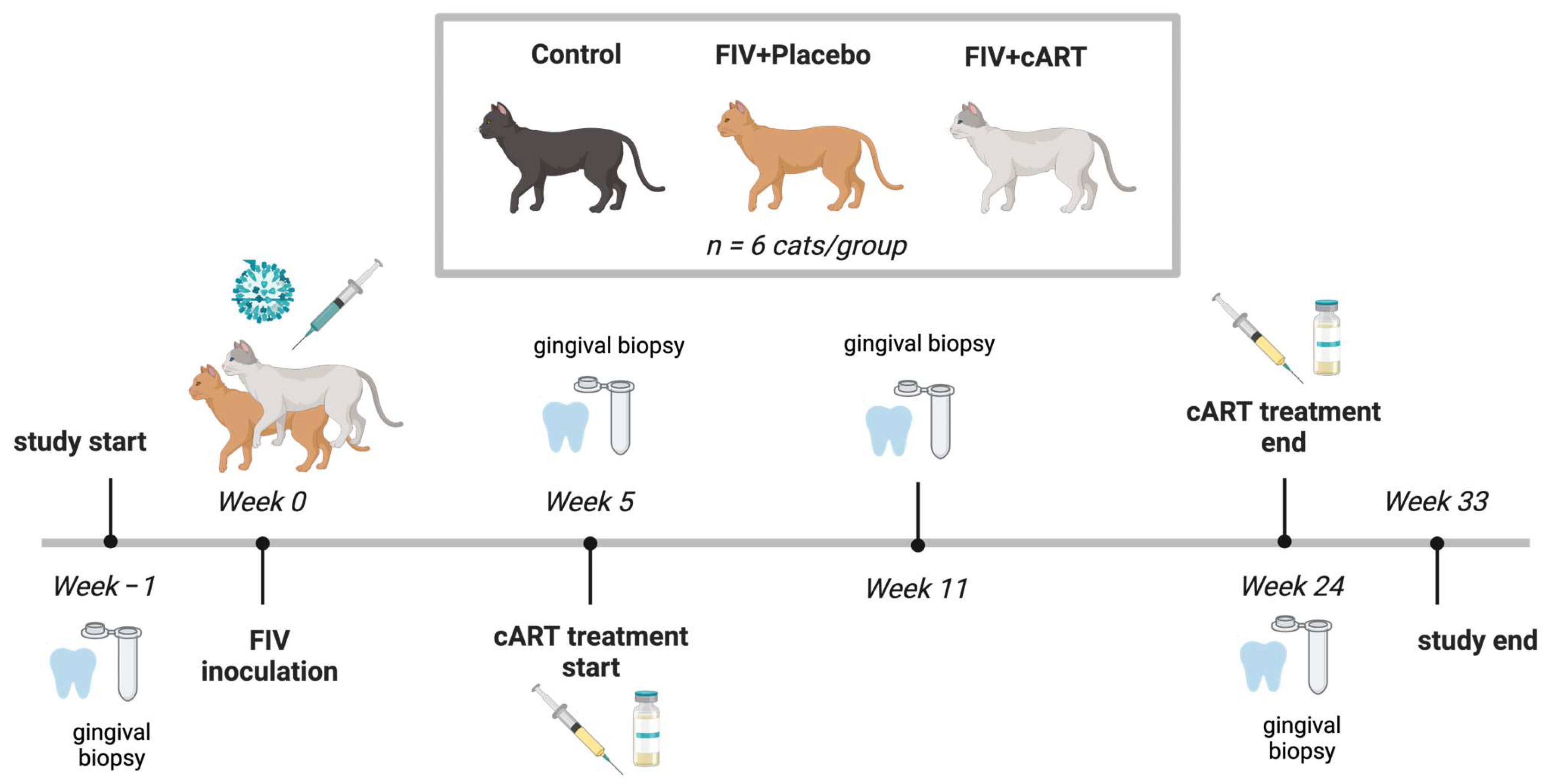
2.1. Study Population
2.2. FIV Inoculation and Treatment Groups
2.3. Oral Health Data and Sample Acquisition
2.4. Oral Health Analyses
2.5. DNA Isolation and 16S Amplicon Sequencing
2.6. Bioinformatics Analyses
3. Results
3.1. Assessment of Feline Oral Health
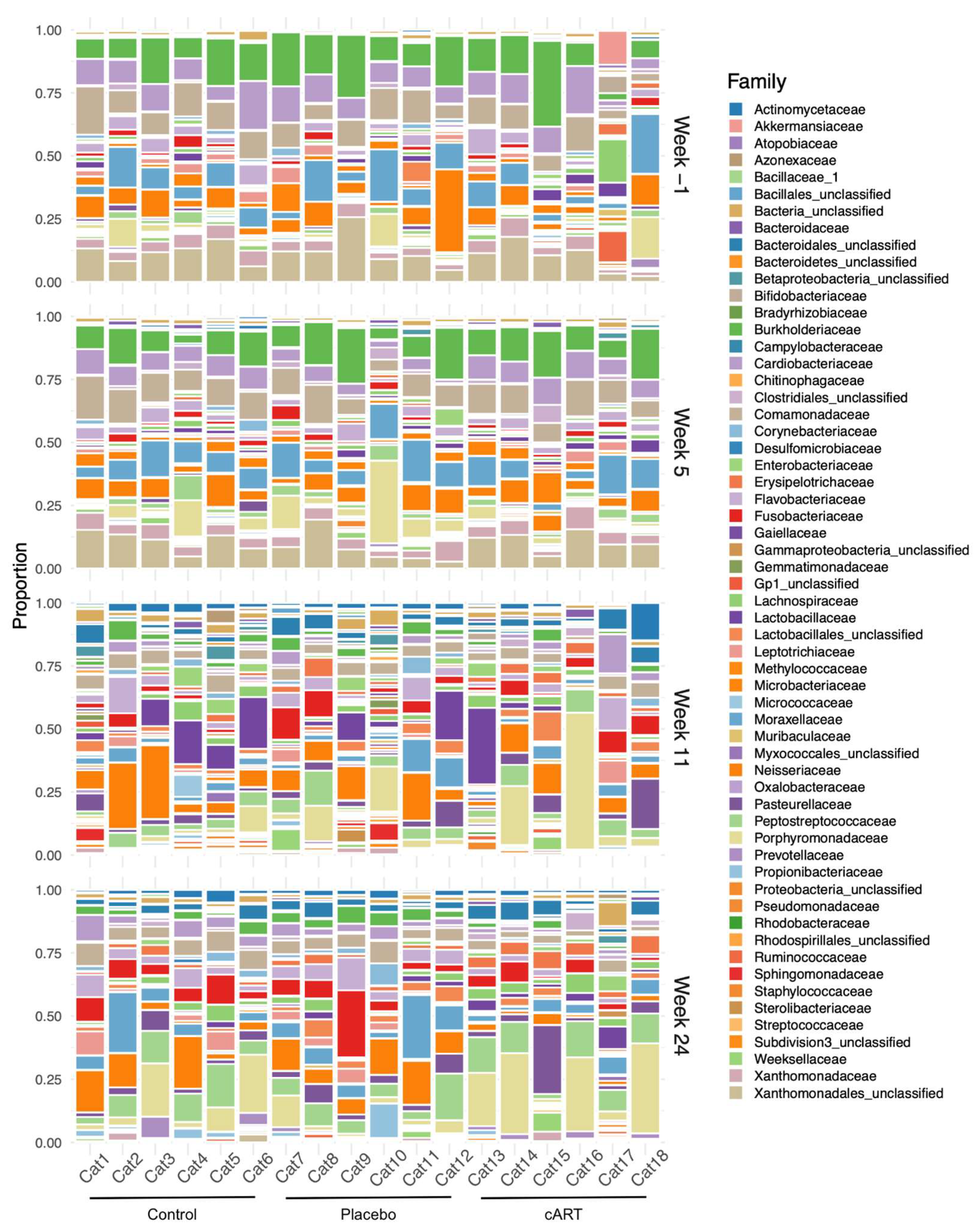
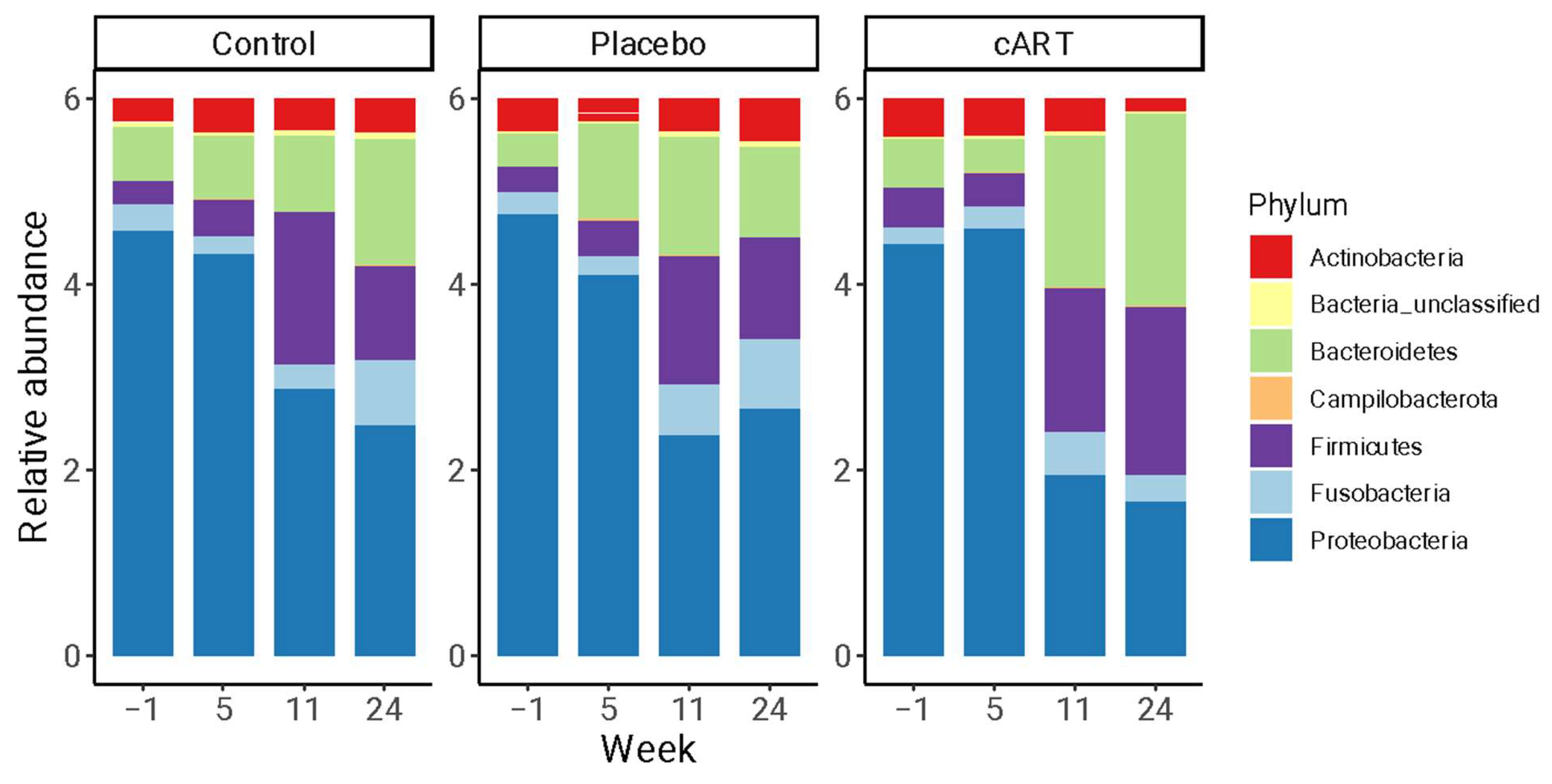
3.2. 16 rRNA Sequencing and Alpha Diversity of the Oral Microbiome
3.3. Beta Diversity of the Oral Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, C.; Abdo, Z.; Ericsson, A.; Elder, J.; VandeWoude, S. Applications of the FIV Model to Study HIV Pathogenesis. Viruses 2018, 10, 206. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a T-Lymphotropic Virus from Domestic Cats with an Immunodeficiency-like Syndrome. Science 1987, 235, 790–793. [Google Scholar] [CrossRef]
- Kolenda-Roberts, H.M.; Kuhnt, L.A.; Jennings, R.N.; Mergia, A.; Gengozian, N.; Johnson, C.M. Immunopathogenesis of Feline Immunodeficiency Virus Infection in the Fetal and Neonatal Cat. Front. Biosci. 2007, 12, 3668–3682. [Google Scholar] [CrossRef] [PubMed]
- Kornya, M.R.; Little, S.E.; Scherk, M.A.; Sears, W.C.; Bienzle, D. Association between Oral Health Status and Retrovirus Test Results in Cats. J. Am. Vet. Med. Assoc. 2014, 245, 916–922. [Google Scholar] [CrossRef]
- Mataftsi, M.; Skoura, L.; Sakellari, D. HIV Infection and Periodontal Diseases: An Overview of the Post-HAART Era. Oral. Dis. 2011, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Croft, J.; O’Flynn, C.; Deusch, O.; Colyer, A.; Allsopp, J.; Milella, L.; Davis, I.J. A Pyrosequencing Investigation of Differences in the Feline Subgingival Microbiota in Health, Gingivitis and Mild Periodontitis. PLoS ONE 2015, 10, e0136986. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The Subgingival Microbial Community of Feline Periodontitis and Gingivostomatitis: Characterization and Comparison between Diseased and Healthy Cats. Sci. Rep. 2019, 9, 12340. [Google Scholar] [CrossRef]
- Older, C.E.; Gomes, M.d.O.S.; Hoffmann, A.R.; Policano, M.D.; Reis, C.A.C.d.; Carregaro, A.B.; Ambrósio, C.E.; Carregaro, V.M.L. Influence of the FIV Status and Chronic Gingivitis on Feline Oral Microbiota. Pathogens 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Weese, S.J.; Nichols, J.; Jalali, M.; Litster, A. The Oral and Conjunctival Microbiotas in Cats with and without Feline Immunodeficiency Virus Infection. Vet. Res. 2015, 46, 21. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Oberti, L.; Lucchese, A.; Di Stasio, D.; Conese, M.; Carinci, F. Oral Manifestations in HIV-Positive Children: A Systematic Review. Pathogens 2020, 9, 88. [Google Scholar] [CrossRef]
- Freeman, A.D.; Liberali, S.A.; Coates, E.A.; Logan, R.M. Oral Health in Australian HIV Patients since the Advent of Combination Antiretroviral Therapy. Aust. Dent. J. 2012, 57, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Miziara, I.D.; Weber, R. Oral Lesions as Predictors of Highly Active Antiretroviral Therapy Failure in Brazilian HIV-Infected Children. J. Oral. Pathol. Med. 2008, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Okunseri, C.; Badner, V.; Wiznia, A.; Rosenberg, M. Prevalence of Oral Lesions and Percent CD4+ T-Lymphocytes in HIV-Infected Children on Antiretroviral Therapy. AIDS Patient Care STDS 2003, 17, 5–11. [Google Scholar] [CrossRef]
- Soares, L.F.; de Araújo Castro, G.F.B.; de Souza, I.P.R.; Pinheiro, M. Pediatric HIV-Related Oral Manifestations: A Five-Year Retrospective Study. Braz. Oral. Res. 2004, 18, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Vaseliu, N.; Carter, A.B.; Kline, N.E.; Kozinetz, C.; Cron, S.G.; Matusa, R.; Kline, M.W. Longitudinal Study of the Prevalence and Prognostic Implications of Oral Manifestations in Romanian Children Infected with Human Immunodeficiency Virus Type 1. Pediatr. Infect. Dis. J. 2005, 24, 1067–1071. [Google Scholar] [CrossRef]
- Friend, S.C.; Birch, C.J.; Lording, P.M.; Marshall, J.A.; Studdert, M.J. Feline Immunodeficiency Virus: Prevalence, Disease Associations and Isolation. Aust. Vet. J. 1990, 67, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Lehmann, R.; Berger, M.; Sigrist, B.; Schawalder, P.; Lutz, H. Feline Immunodeficiency Virus (FIV) Infection Leads to Increased Incidence of Feline Odontoclastic Resorptive Lesions (FORL). Vet. Immunol. Immunopathol. 1998, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.E.; Coggins, S.J.; van Dorsselaer, M.; Norris, J.M.; Squires, R.A.; Thompson, M.; Malik, R. Feline Immunodeficiency Virus (FIV) Infection in Domestic Pet Cats in Australia and New Zealand: Guidelines for Diagnosis, Prevention and Management. Aust. Vet. J. 2022, 100, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Earl, D.D.; Yamamoto, J.K. Is AZT/3TC Therapy Effective against FIV Infection or Immunopathogenesis? Vet. Immunol. Immunopathol. 2002, 85, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Bisset, L.R.; Lutz, H.; Böni, J.; Hofmann-Lehmann, R.; Lüthy, R.; Schüpbach, J. Combined Effect of Zidovudine (ZDV), Lamivudine (3TC) and Abacavir (ABC) Antiretroviral Therapy in Suppressing in Vitro FIV Replication. Antivir. Res. 2002, 53, 35–45. [Google Scholar] [CrossRef]
- Egberink, H.; Borst, M.; Niphuis, H.; Balzarini, J.; Neu, H.; Schellekens, H.; De Clercq, E.; Horzinek, M.; Koolen, M. Suppression of Feline Immunodeficiency Virus Infection in Vivo by 9-(2-Phosphonomethoxyethyl)Adenine. Proc. Natl. Acad. Sci. USA 1990, 87, 3087–3091. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Behzadi, E.S.; Nehring, M.; Carver, S.; Cowan, S.R.; Conry, M.K.; Rawlinson, J.E.; VandeWoude, S.; Miller, C.A. Combination Antiretroviral Therapy and Immunophenotype of Feline Immunodeficiency Virus. Viruses 2023, 15, 822. [Google Scholar] [CrossRef] [PubMed]
- de Rozìeres, S.; Thompson, J.; Sundstrom, M.; Gruber, J.; Stump, D.S.; de Parseval, A.P.; VandeWoude, S.; Elder, J.H. Replication Properties of Clade A/C Chimeric Feline Immunodeficiency Viruses and Evaluation of Infection Kinetics in the Domestic Cat. J. Virol. 2008, 82, 7953–7963. [Google Scholar] [CrossRef]
- de Rozières, S.; Mathiason, C.K.; Rolston, M.R.; Chatterji, U.; Hoover, E.A.; Elder, J.H. Characterization of a Highly Pathogenic Molecular Clone of Feline Immunodeficiency Virus Clade C. J. Virol. 2004, 78, 8971–8982. [Google Scholar] [CrossRef]
- Magden, E.; Miller, C.; MacMillan, M.; Bielefeldt-Ohmann, H.; Avery, A.; Quackenbush, S.L.; Vandewoude, S. Acute Virulent Infection with Feline Immunodeficiency Virus (FIV) Results in Lymphomagenesis via an Indirect Mechanism. Virology 2013, 436, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Bielefeldt-Ohmann, H.; MacMillan, M.; Huitron-Resendiz, S.; Henriksen, S.; Elder, J.; VandeWoude, S. Strain-Specific Viral Distribution and Neuropathology of Feline Immunodeficiency Virus. Vet. Immunol. Immunopathol. 2011, 143, 282–291. [Google Scholar] [CrossRef]
- Lommer, M.J. Efficacy of Cyclosporine for Chronic, Refractory Stomatitis in Cats: A Randomized, Placebo-Controlled, Double-Blinded Clinical Study. J. Vet. Dent. 2013, 30, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E. Application of the Total Mouth Periodontal Score System for Use in Cats. In Proceedings of the Veterinary Dental Forum 2009, Phoenix, AZ, USA, 29 October 2009. [Google Scholar]
- Harvey, C.E.; Laster, L.; Shofer, F.S. Validation of Use of Subsets of Teeth When Applying the Total Mouth Periodontal Score (TMPS) System in Dogs. J. Vet. Dent. 2012, 29, 222–226. [Google Scholar] [CrossRef]
- Harvey, C.E.; Laster, L.; Shofer, F.; Miller, B. Scoring the Full Extent of Periodontal Disease in the Dog: Development of a Total Mouth Periodontal Score (TMPS) System. J. Vet. Dent. 2008, 25, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E. Development and Application of a Weighted Gingivitis and Periodontitis (W-G/P) Score System in Cats. J. Vet. Dent. 2024, 41, 376–381. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O'Hara, R.B.; Simpson, G.L.; Sólymos, P.; Stevens, M.H.H.; Wagner, H. vegan: Community Ecology Package. Software. 2012. Available online: http://CRAN.R-project.org/package=vegan (accessed on 22 January 2025).
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential Abundance Analysis for Microbial Marker-Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Narayanan, A.; Giske, C.G.; Neogi, U.; Sönnerborg, A.; Nowak, P. Altered Gut Microbiome under Antiretroviral Therapy: Impact of Efavirenz and Zidovudine. ACS Infect. Dis. 2021, 7, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Tao, J.; Abu, Y.; Sussman, D.A.; Girotra, M.; Franceschi, D.; Roy, S. HIV-Positive Patients on Antiretroviral Therapy Have an Altered Mucosal Intestinal but Not Oral Microbiome. Microbiol. Spectr. 2023, 11, e0247222. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, J.; Su, B.; Wei, H.; Chen, F.; Liu, H.; Wei, J.; Yang, X.; Zhang, Q.; Xia, W.; et al. Alteration in Oral Microbiome Among Men Who Have Sex With Men With Acute and Chronic HIV Infection on Antiretroviral Therapy. Front. Cell Infect. Microbiol. 2021, 11, 695515. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.J.; Lilly, E.A.; Granada, C.; Treas, K.; Dubois, K.R.; Hashmi, S.B.; Vazquez, J.A.; Hagensee, M.E.; Griffen, A.L.; Leys, E.J.; et al. Independent Effects of HIV and Antiretroviral Therapy on the Oral Microbiome Identified by Multivariate Analyses. mBio 2023, 14, e0040923. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Thompson, Z.A.; Beall, C.J.; Lilly, E.A.; Granada, C.; Treas, K.D.; DuBois, K.R.; Hashmi, S.B.; Mukherjee, C.; Gilliland, A.E.; et al. Significant Effect of HIV/HAART on Oral Microbiota Using Multivariate Analysis. Sci. Rep. 2019, 9, 19946. [Google Scholar] [CrossRef] [PubMed]
- Shilaih, M.; Angst, D.C.; Marzel, A.; Bonhoeffer, S.; Günthard, H.F.; Kouyos, R.D. Antibacterial Effects of Antiretrovirals, Potential Implications for Microbiome Studies in HIV. Antivir. Ther. 2018, 23, 91–94. [Google Scholar] [CrossRef]
- Miller, C.; Boegler, K.; Carver, S.; MacMillan, M.; Bielefeldt-Ohmann, H.; VandeWoude, S. Pathogenesis of Oral FIV Infection. PLoS ONE 2017, 12, e0185138. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.K.; Vester Boler, B.; Gardner, C.; Li, Q. Development of the Oral Microbiome in Kittens. In Proceedings of the Companion Animal Nutrition (CAN) Summit: The Nexus of Pet and Human Nutrition: Focus on Cognition and Microbiome, Vancouver, BC, Canada, 4 May 2017. [Google Scholar]
- Adler, C.J.; Malik, R.; Browne, G.V.; Norris, J.M. Diet May Influence the Oral Microbiome Composition in Cats. Microbiome 2016, 4, 23. [Google Scholar] [CrossRef]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.R.; Henker, L.C.; Rodrigues Hoffmann, A. The Feline Cutaneous and Oral Microbiota Are Influenced by Breed and Environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the Oral Microbiota of Healthy Cats Using Next-Generation Sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Reiter, A.M.; Pohl, J.C.; Tang, S.; Kim, Y.J.; Linde, A.; Prem, A.; Melgarejo, T. Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens 2021, 10, 904. [Google Scholar] [CrossRef] [PubMed]



| Treatment | sWeek * Control | sWeek * Placebo | sWeek * cART | Deviance (%) | ||
|---|---|---|---|---|---|---|
| Oral Health | ||||||
| Gingival | F | 0.99 | 3.82 | 7.13 | 4.41 | 47.5 |
| Inflammation | P | 0.376 | 0.054 a | <0.001 a | 0.039 b | |
| Attachment Loss | F | 0.75 | 3.78 | 19.1 | 3.22 | 64.7 |
| P | 0.476 | 0.026 ab | <0.001 a | 0.015 b | ||
| Lymph Node Size | F | 6.62 | 5.30 | 15.38 | 9.95 | 64.5 |
| Right | P | 0.002 | 0.024 a | 0.002 a | <0.001 b | |
| Lymph Node Size | F | 5.79 | 2.03 | 5.98 | 7.31 | 57.7 |
| Left | P | 0.004 | 0.121 a | <0.001 a | <0.001 b | |
| Microbiome | ||||||
| Shannon Diversity | F | 1.17 | 2.26 | 7.25 | 5.08 | 36.1 |
| P | 0.318 | 0.138 a | 0.001 b | 0.028 a | ||
| Inverse Simpson | F | 1.75 | 0.63 | 3.64 | 0.70 | 17.8 |
| Diversity | P | 0.181 | 0.431 a | 0.029 a | 0.408 a | |
| Vegan Richness | F | 0.31 | 0.67 | 6.23 | 1.69 | 28.6 |
| P | 0.733 | 0.448 a | 0.002 b | 0.198 a | ||
| PERMANOVA | F | 1.08 | Week * Treatment: F = 7.70, p < 0.001 | 27.7 | ||
| P | <0.001 | a | b | c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashor, L.; Rawlinson, J.E.; Kozakiewicz, C.P.; Behzadi, E.; Miller, C.; Kim, J.; Cierzan, M.; Nehring, M.; Carver, S.; Abdo, Z.; et al. Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats. Viruses 2025, 17, 257. https://doi.org/10.3390/v17020257
Bashor L, Rawlinson JE, Kozakiewicz CP, Behzadi E, Miller C, Kim J, Cierzan M, Nehring M, Carver S, Abdo Z, et al. Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats. Viruses. 2025; 17(2):257. https://doi.org/10.3390/v17020257
Chicago/Turabian StyleBashor, Laura, Jennifer E. Rawlinson, Christopher P. Kozakiewicz, Elisa Behzadi, Craig Miller, Jeffrey Kim, Megan Cierzan, Mary Nehring, Scott Carver, Zaid Abdo, and et al. 2025. "Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats" Viruses 17, no. 2: 257. https://doi.org/10.3390/v17020257
APA StyleBashor, L., Rawlinson, J. E., Kozakiewicz, C. P., Behzadi, E., Miller, C., Kim, J., Cierzan, M., Nehring, M., Carver, S., Abdo, Z., & VandeWoude, S. (2025). Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats. Viruses, 17(2), 257. https://doi.org/10.3390/v17020257







