Tobacco Mosaic Virus Movement: From Capsid Disassembly to Transport Through Plasmodesmata
Abstract
1. Introduction
2. TMV Capsid Disassembly and Aspects of Virus Replication Pertinent to TMV Movement
2.1. Capsid Disassembly
2.2. Formation of a Replication Complex and Genomic RNA Accumulation
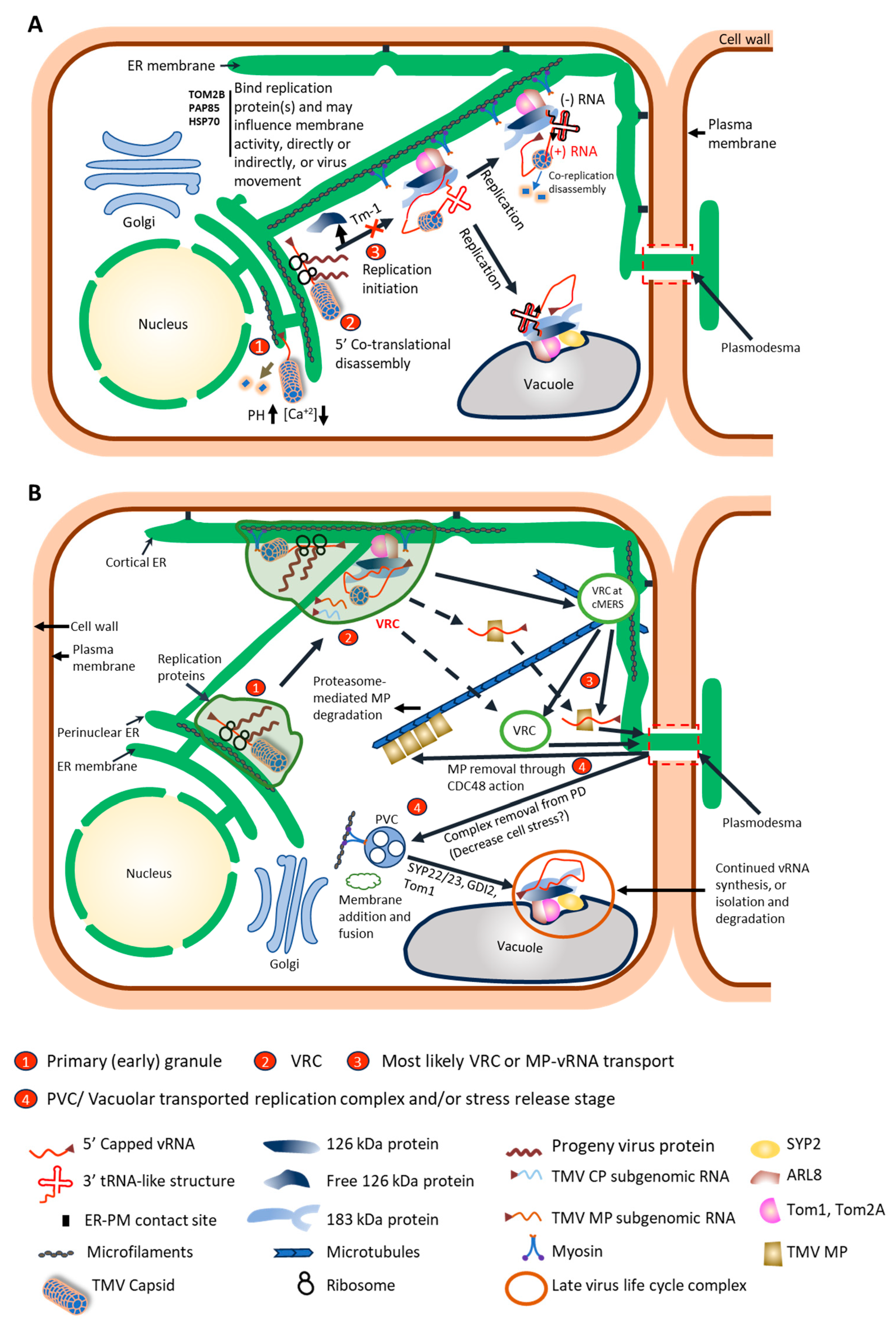
3. Intracellular Movement to the Plasmodesmata
3.1. Virus Components for Intracellular Movement
3.2. Host Components for Intracellular Movement
3.2.1. TMV and Cytoskeleton
3.2.2. TMV and Prevacuolar Compartment/Vacuole Association
4. Intercellular Movement of TMV
4.1. MP-Facilitated TMV Intercellular Movement
4.2. MP Targeting to Plasmodesmata
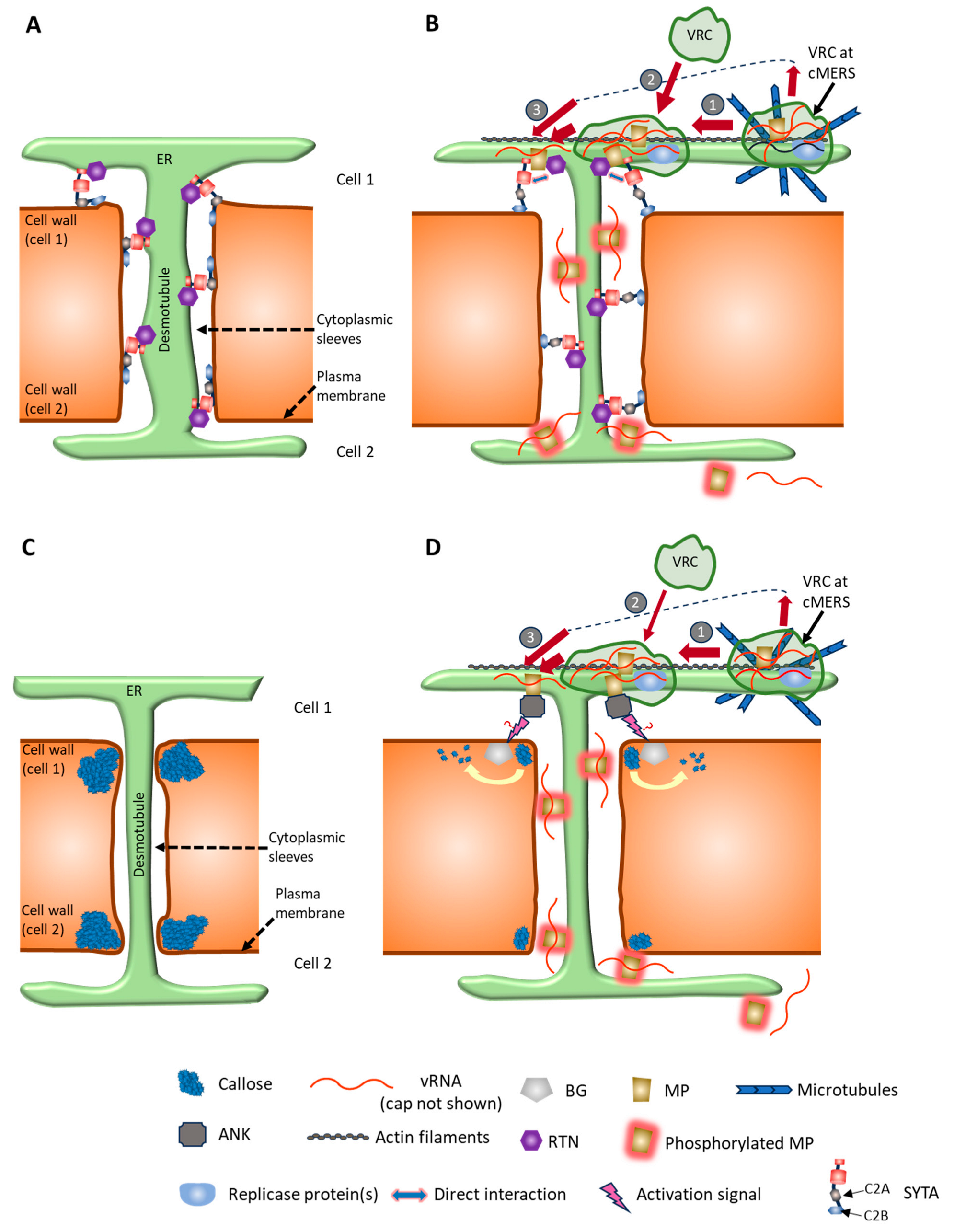
4.3. MP and Modulation of PD Permeability
4.4. MP Phosphorylation
4.5. Cytoskeletal Proteins
4.6. MP Homeostasis
4.7. Replication Proteins
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Mayer, A. Über die mosaikkrankheit des tabaks. Landwirtsch. Vers.-Station. 1886, 32, 451–467. [Google Scholar]
- Ivanowski, D. Ueber die mosaikkrankheit der tabakspflanze. St. Petersb. Acad. Imp. Sci. Bul. 1892, 35, 67–70. [Google Scholar]
- Beijerinck, M.W. Ueber ein contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblätter. Verh. Koninklyke Akad. Wettenshappen Amst. 1898, 6, 3–21. [Google Scholar]
- Okada, Y. Historical overview of research on the tobacco mosaic virus genome: Genome organization, infectivity and gene manipulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 569–582. [Google Scholar] [CrossRef]
- Hull, R. Introduction. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 3–14. ISBN 978-0-12-384871-0. [Google Scholar]
- Goelet, P.; Lomonossoff, G.P.; Butler, P.J.; Akam, M.E.; Gait, M.J.; Karn, J. Nucleotide sequence of tobacco mosaic virus RNA. Proc. Natl. Acad. Sci. USA 1982, 79, 5818–5822. [Google Scholar] [CrossRef]
- Dawson, W.O.; Beck, D.L.; Knorr, D.A.; Grantham, G.L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc. Natl. Acad. Sci. USA 1986, 83, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Meshi, T.; Ishikawa, M.; Motoyoshi, F.; Semba, K.; Okada, Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc. Natl. Acad. Sci. USA 1986, 83, 5043–5047. [Google Scholar] [CrossRef] [PubMed]
- Hull, R. Architecture and assembly of virus particles. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 69–143. ISBN 978-0-12-384871-0. [Google Scholar]
- Hull, R. Movement of viruses within plants. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 531–603. ISBN 978-0-12-384871-0. [Google Scholar]
- Hull, R. Plant virus viromics: Involvement of genomes of three organisms—Virus, host, and vector. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 929–971. ISBN 978-0-12-384871-0. [Google Scholar]
- Saito, T.; Yamanaka, K.; Okada, Y. Long-distance movement and viral assembly of tobacco mosaic virus mutants. Virology 1990, 176, 329–336. [Google Scholar] [CrossRef]
- Palukaitis, P.; Akbarimotlagh, M.; Astaraki, S.; Shams-Bakhsh, M.; Yoon, J.-Y. The forgotten tobamovirus genes encoding the 54 kDa protein and the 4–6 kDa proteins. Viruses 2024, 16, 1680. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Yadav, D.K.; Yadav, S.; Khurana, S.M.P. Viral movement-cellular protein interaction. In Plant Virus-Host Interaction, 2nd ed.; Gaur, R.K., Khurana, S.M.P., Sharma, P., Hohn, T., Eds.; Academic Press: Boston, MA, USA, 2021; pp. 59–109. ISBN 978-0-12-821629-3. [Google Scholar]
- He, R.; Li, Y.; Bernards, M.A.; Wang, A. Manipulation of the cellular membrane-cytoskeleton network for RNA virus replication and movement in plants. Viruses 2023, 15, 744. [Google Scholar] [CrossRef] [PubMed]
- Hull, R. Symptoms and host range. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 145–198. ISBN 978-0-12-384871-0. [Google Scholar]
- Liu, J.-Z.; Richerson, K.; Nelson, R.S. Growth conditions for plant virus–host studies. Curr. Protoc. Microbiol. 2009, 14, 16A.1.1–16A.1.16. [Google Scholar] [CrossRef]
- Navarro, J.A.; Sanchez-Navarro, J.A.; Pallas, V. Key checkpoints in the movement of plant viruses through the host. Adv. Virus Res. 2019, 104, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P.; Zaitlin, M. Tobacco mosaic virus infectivity and replication. In The Plant Viruses: The Rod-Shaped Plant Viruses; Van Regenmortel, M.H.V., Fraenkel-Conrat, H., Eds.; Springer US: Boston, MA, USA, 1986; pp. 105–131. ISBN 978-1-4684-7026-0. [Google Scholar]
- Shaw, J.G. Tobacco mosaic virus and the study of early events in virus infections. Philos. Trans. R. Soc. B 1999, 354, 603–611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, T.M.A.; Plaskitt, K.A.; Watts, J.W.; Osbourn, J.K.; Watkins, P.A.C. Signals and structures involved in early interactions between plants and viruses or pseudoviruses. In Proceedings of the Recognition and Response in Plant-Virus Interactions; Fraser, R.S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 123–145. [Google Scholar]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Sun, Y.-D.; Spellman-Kruse, A.; Folimonova, S.Y. Blaze a new trail: Plant virus xylem exploitation. Int. J. Mol. Sci. 2022, 23, 8375. [Google Scholar] [CrossRef]
- Culver, J.N. Tobacco mosaic virus assembly and disassembly: Determinants in pathogenicity and resistance. Annu. Rev. Phytopathol. 2002, 40, 287–308. [Google Scholar] [CrossRef]
- Buck, K.W. Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 613–627. [Google Scholar] [CrossRef]
- Harrison, B.D.; Wilson, T.M. Milestones in the research on tobacco mosaic virus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 521–529. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ishikawa, M. Replication of tobamovirus RNA. Annu. Rev. Phytopathol. 2016, 54, 55–78. [Google Scholar] [CrossRef]
- Genus: Tobamovirus|ICTV. Available online: https://ictv.global/report/chapter/virgaviridae/virgaviridae/tobamovirus (accessed on 18 January 2025).
- Ohno, T.; Aoyagi, M.; Yamanashi, Y.; Saito, H.; Ikawa, S.; Meshi, T.; Okada, Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J. Biochem. 1984, 96, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Watanabe, E.; Watanabe, Y.; Okada, Y. Nucleotide sequence of Tobamovirus Ob which can spread systemically in N gene tobacco. J. Gen. Virol. 1993, 74, 1939–1944. [Google Scholar] [CrossRef]
- Padgett, H.S.; Epel, B.L.; Kahn, T.W.; Heinlein, M.; Watanabe, Y.; Beachy, R.N. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J. 1996, 10, 1079–1088. [Google Scholar] [CrossRef]
- Niehl, A.; Pasquier, A.; Ferriol, I.; Mély, Y.; Heinlein, M. Comparison of the oilseed rape mosaic virus and tobacco mosaic virus movement proteins (MP) reveals common and dissimilar MP functions for tobamovirus spread. Virology 2014, 456–457, 43–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levy, A.; Zheng, J.Y.; Lazarowitz, S.G. The tobamovirus turnip vein clearing virus 30-kilodalton movement protein localizes to novel nuclear filaments to enhance virus infection. J. Virol. 2013, 87, 6428–6440. [Google Scholar] [CrossRef]
- Aguilar, I.; Sánchez, F.; Martin Martin, A.; Martinez-Herrera, D.; Ponz, F. Nucleotide sequence of Chinese rape mosaic virus (oilseed rape mosaic virus), a crucifer Tobamovirus infectious on Arabidopsis thaliana. Plant Mol. Biol. 1996, 30, 191–197. [Google Scholar] [CrossRef]
- Melcher, U. Turnip vein-clearing virus, from pathogen to host expression profile. Mol. Plant Pathol. 2003, 4, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Pattanayek, R.; Stubbs, G. Visualization of protein-nucleic acid interactions in a virus: Refined structure of intact tobacco mosaic virus at 2.9 Å resolution by X-Ray fiber diffraction. J. Mol. Biol. 1989, 208, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.A. Cotranslational disassembly of tobacco mosaic virus in vitro. Virology 1984, 137, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shaw, J.G. Evidence that a viral replicase protein is involved in the disassembly of tobacco mosaic virus particles in vivo. Virology 1997, 239, 426–434. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Xu, Z.; Shaw, J.G. Uncoating of tobacco mosaic virus rna in protoplasts. Virology 1994, 200, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shaw, J. Bidirectional Uncoating of the Genomic RNA of a Helical Virus. Proc. Natl. Acad. Sci. USA 1996, 93, 2981–2984. [Google Scholar] [CrossRef]
- Christensen, N.M.; Tilsner, J.; Bell, K.F.S.; Hammann, P.; Parton, R.M.; Lacomme, C.; Oparka, K. The 5′ cap of tobacco mosaic virus (TMV) is required for virion attachment to the actin/endoplasmic reticulum network during early infection. Traffic 2009, 10, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Meshi, T.; Motoyoshi, F.; Takamatsu, N.; Okada, Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986, 14, 8291–8305. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.J.; Dawson, W.O. Functions of the 126- and 183-kDa proteins of tobacco mosaic virus. Virology 2000, 271, 90–98. [Google Scholar] [CrossRef]
- Merits, A.; Kettunen, R.; Mäkinen, K.; Lampio, A.; Auvinen, P.; Kääriäinen, L.; Ahola, T. Virus-specific capping of tobacco mosaic virus RNA: Methylation of GTP prior to formation of covalent complex p126-m7GMP. FEBS Lett. 1999, 455, 45–48. [Google Scholar] [CrossRef]
- Goregaoker, S.P.; Culver, J.N. Oligomerization and activity of the helicase domain of the tobacco mosaic virus 126- and 183-kilodalton replicase proteins. J. Virol. 2003, 77, 3549–3556. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 375–430. [Google Scholar] [CrossRef]
- Miyashita, S.; Ishibashi, K.; Kishino, H.; Ishikawa, M. Viruses roll the dice: The stochastic behavior of viral genome molecules accelerates viral adaptation at the cell and tissue levels. PLoS Biol. 2015, 13, e1002094. [Google Scholar] [CrossRef]
- Ishikawa, M.; Meshi, T.; Ohno, T.; Okada, Y. Specific cessation of minus-strand RNA accumulation at an early stage of tobacco mosaic virus infection. J. Virol. 1991, 65, 861–868. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ishikawa, M. The resistance protein Tm-1 inhibits formation of a tomato mosaic virus replication protein-host membrane protein complex. J. Virol. 2013, 87, 7933–7939. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, M.; Mori, M.; Dohi, K.; Okamura, H.; Katoh, E.; Naito, S.; Meshi, T.; Ishikawa, M. A host small GTP-binding protein ARL8 plays crucial roles in tobamovirus RNA replication. PLoS Pathog. 2011, 7, e1002409. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.S.; Liu, J.; Cheng, N.-H.; Folimonov, A.; Hou, Y.-M.; Bao, Y.; Katagi, C.; Carter, S.A.; Nelson, R.S. The tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol. Plant-Microbe Interact. 2004, 17, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Tsuda, S.; Tamai, A.; Meshi, T. Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J. Virol. 2003, 77, 11016–11026. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara-Komoda, Y.; Hirai, K.; Mochizuki, A.; Nishiguchi, M.; Meshi, T.; Ishikawa, M. Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing. Virology 2008, 376, 132–139. [Google Scholar] [CrossRef]
- Kawamura-Nagaya, K.; Ishibashi, K.; Huang, Y.-P.; Miyashita, S.; Ishikawa, M. Replication protein of tobacco mosaic virus cotranslationally binds the 5′ untranslated region of genomic RNA to enable viral replication. Proc. Natl. Acad. Sci. USA 2014, 111, E1620–E1628. [Google Scholar] [CrossRef] [PubMed]
- Osman, T.A.M.; Buck, K.W. Identification of a region of the tobacco mosaic virus 126- and 183-kilodalton replication proteins which binds specifically to the viral 3′-terminal tRNA-like structure. J. Virol. 2003, 77, 8669–8675. [Google Scholar] [CrossRef]
- Zeenko, V.V.; Ryabova, L.A.; Spirin, A.S.; Rothnie, H.M.; Hess, D.; Browning, K.S.; Hohn, T. Eukaryotic elongation factor 1A interacts with the upstream pseudoknot domain in the 3′ untranslated region of tobacco mosaic virus RNA. J. Virol. 2002, 76, 5678–5691. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, A.; Iwata, A.; Ueda, S.; Hibi, T.; Ishihama, A. Isolation from tobacco mosaic virus-infected tobacco of a solubilized template-specific RNA-dependent RNA polymerase containing a 126K/183K protein heterodimer. J. Virol. 1999, 73, 2633–2640. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Numaga, T.; Ohshima, K.; Yano, M.-A.; Ohsawa, R.; Goto, D.B.; Naito, S.; Ishikawa, M. Arabidopsis TOBAMOVIRUS MULTIPLICATION (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 2003, 22, 335–343. [Google Scholar] [CrossRef]
- Chen, C.-E.; Yeh, K.-C.; Wu, S.-H.; Wang, H.-I.; Yeh, H.-H. A vicilin-like seed storage protein, PAP85, is involved in tobacco mosaic virus replication. J. Virol. 2013, 87, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nelson, R.S. The cell biology of tobacco mosaic virus replication and movement. Front. Plant Sci. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, M. Plant virus replication and movement. Virology 2015, 479–480, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2018, 69, 117–132. [Google Scholar] [CrossRef]
- Niehl, A.; Amari, K.; Gereige, D.; Brandner, K.; Mély, Y.; Heinlein, M. Control of tobacco mosaic virus movement protein fate by CELL-DIVISION-CYCLE protein48. Plant Physiol. 2012, 160, 2093–2108. [Google Scholar] [CrossRef]
- Reichel, C.; Beachy, R.N. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J. Virol. 2000, 74, 3330–3337. [Google Scholar] [CrossRef]
- Gillespie, T.; Boevink, P.C.; Haupt, S.; Roberts, A.G.; Toth, R.L.; Valentine, T.A.; Chapman, S.; Oparka, K.J. Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell 2002, 14, 1207–1222. [Google Scholar] [CrossRef]
- Ibrahim, A.; Yang, X.; Liu, C.; Cooper, K.D.; Bishop, B.A.; Zhu, M.; Kwon, S.; Schoelz, J.E.; Nelson, R.S. Plant SNAREs SYP22 and SYP23 interact with tobacco mosaic virus 126 kDa protein and SYP2s are required for normal local virus accumulation and spread. Virology 2020, 547, 57–71. [Google Scholar] [CrossRef]
- Szécsi, J.; Ding, X.S.; Lim, C.O.; Bendahmane, M.; Cho, M.J.; Nelson, R.S.; Beachy, R.N. Development of tobacco mosaic virus infection sites in Nicotiana benthamiana. Mol. Plant-Microbe Interact. 1999, 12, 143–152. [Google Scholar] [CrossRef]
- Boevink, P.; Oparka, K.J. Virus-Host Interactions during Movement Processes. Plant Physiol. 2005, 138, 1815. [Google Scholar] [CrossRef]
- Harries, P.A.; Schoelz, J.E.; Nelson, R.S. Intracellular transport of viruses and their components: Utilizing the cytoskeleton and membrane highways. Mol. Plant-Microbe Interact. 2010, 23, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kelman, Z.; Culver, J.N. Helicase ATPase activity of the tobacco mosaic virus 126-kDa protein modulates replicase complex assembly. Virology 2010, 402, 292–302. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Komoda, K.; Yamanaka, T.; Tamai, A.; Meshi, T.; Funada, R.; Tsuchiya, T.; Naito, S.; Ishikawa, M. Subcellular localization of host and viral proteins associated with tobamovirus RNA replication. EMBO J. 2003, 22, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.R.; Goregaoker, S.P.; Culver, J.N. Association of the tobacco mosaic virus 126kDa replication protein with a GDI protein affects host susceptibility. Virology 2011, 414, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, M.; Meshi, T.; Ishikawa, M. Guanylylation-competent replication proteins of tomato mosaic virus are disulfide-linked. Virology 2012, 434, 118–128. [Google Scholar] [CrossRef]
- Nishikiori, M.; Sugiyama, S.; Xiang, H.; Niiyama, M.; Ishibashi, K.; Inoue, T.; Ishikawa, M.; Matsumura, H.; Katoh, E. Crystal structure of the superfamily 1 helicase from tomato mosaic virus. J. Virol. 2012, 86, 7565–7576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishikiori, M.; Dohi, K.; Mori, M.; Meshi, T.; Naito, S.; Ishikawa, M. Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J. Virol. 2006, 80, 8459–8468. [Google Scholar] [CrossRef]
- Yamaji, Y.; Sakurai, K.; Hamada, K.; Komatsu, K.; Ozeki, J.; Yoshida, A.; Yoshii, A.; Shimizu, T.; Namba, S.; Hibi, T. Significance of eukaryotic translation elongation factor 1A in tobacco mosaic virus infection. Arch. Virol. 2009, 155, 263–268. [Google Scholar] [CrossRef]
- Figueira, A.d.R.; Golem, S.; Goregaoker, S.P.; Culver, J.N. A Nuclear localization signal and a membrane association domain contribute to the cellular localization of the tobacco mosaic virus 126-kDa replicase protein. Virology 2002, 301, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, M.; Padgett, H.S.; Gens, J.S.; Pickard, B.G.; Casper, S.J.; Epel, B.L.; Beachy, R.N. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 1998, 10, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Komoda, K.; Naito, S.; Ishikawa, M. Replication of plant RNA virus genomes in a cell-free extract of evacuolated plant protoplasts. Proc. Natl. Acad. Sci. USA 2004, 101, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Reichel, C.; Reichel, C.A.; Beachy, R.N. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1998, 95, 11169–11174. [Google Scholar] [CrossRef]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyán, J. The P122 subunit of tobacco mosaic virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Inaba, N.; Kutsuna, N.; Takeda, A.; Tagami, Y.; Watanabe, Y. Binding of tobamovirus replication protein with small RNA duplexes. J. Gen. Virol. 2007, 88, 2347–2352. [Google Scholar] [CrossRef]
- Mérai, Z.; Kerényi, Z.; Kertész, S.; Magna, M.; Lakatos, L.; Silhavy, D. Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J. Virol. 2006, 80, 5747–5756. [Google Scholar] [CrossRef]
- Spiegelman, Z.; Dinesh-Kumar, S.P. Breaking boundaries: The perpetual interplay between tobamoviruses and plant immunity. Annu. Rev. Virol. 2023, 10, 455–476. [Google Scholar] [CrossRef]
- Conti, G.; Rodriguez, M.C.; Venturuzzi, A.L.; Asurmendi, S. Modulation of host plant immunity by tobamovirus proteins. Ann. Bot. 2017, 119, 737–747. [Google Scholar] [CrossRef]
- Ishibashi, K.; Masuda, K.; Naito, S.; Meshi, T.; Ishikawa, M. An inhibitor of viral RNA replication is encoded by a plant resistance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 13833–13838. [Google Scholar] [CrossRef]
- Ishibashi, K.; Mawatari, N.; Miyashita, S.; Kishino, H.; Meshi, T.; Ishikawa, M. Coevolution and hierarchical interactions of tomato mosaic virus and the resistance gene Tm-1. PLoS Pathog. 2012, 8, e1002975. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kezuka, Y.; Kobayashi, C.; Kato, M.; Inoue, T.; Nonaka, T.; Ishikawa, M.; Matsumura, H.; Katoh, E. Structural basis for the recognition-evasion arms race between tomato mosaic virus and the resistance gene Tm-1. Proc. Natl. Acad. Sci. USA 2014, 111, E3486–E3495. [Google Scholar] [CrossRef]
- Hýsková, V.; Bělonožníková, K.; Čeřovská, N.; Ryšlavá, H. HSP70 plays an ambiguous role during viral infections in plants. Biol. Plant. 2021, 65, 68–79. [Google Scholar] [CrossRef]
- Levy, A.; Tilsner, J. Creating contacts between replication and movement at plasmodesmata—A role for membrane contact sites in plant virus infections? Front. Plant Sci. 2020, 11, 862. [Google Scholar] [CrossRef]
- Reagan, B.C.; Burch-Smith, T.M. Viruses reveal the secrets of plasmodesmal cell biology. Mol. Plant-Microbe Interact. 2020, 33, 26–39. [Google Scholar] [CrossRef]
- Fannin, F.F.; Shaw, J.G. Evidence for concurrent spread of tobacco mosaic virus from infected epidermal cells to neighboring epidermal and mesophyll cells. Plant Sci. 1987, 51, 305–310. [Google Scholar] [CrossRef]
- Epel, B.L. Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host β-1,3-glucanases. Semin. Cell Dev. Biol. 2009, 20, 1074–1081. [Google Scholar] [CrossRef]
- Kawakami, S.; Watanabe, Y.; Beachy, R.N. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl. Acad. Sci. USA 2004, 101, 6291–6296. [Google Scholar] [CrossRef]
- Nishiguchi, M.; Motoyoshi, F.; Oshima, N. Behaviour of a temperature sensitive strain of tobacco mosaic virus in tomato leaves and protoplasts. J. Gen. Virol. 1978, 39, 53–61. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H. Susceptibility genes to plant viruses. Viruses 2018, 10, 484. [Google Scholar] [CrossRef]
- Agaoua, A.; Bendahmane, A.; Moquet, F.; Dogimont, C. Membrane trafficking proteins: A new target to identify resistance to viruses in plants. Plants 2021, 10, 2139. [Google Scholar] [CrossRef]
- Jovanović, I.; Frantová, N.; Zouhar, J. A sword or a buffet: Plant endomembrane system in viral infections. Front. Plant Sci. 2023, 14, 1226498. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, J.-F.; Zheng, H. Viral manipulation of plant host membranes. Annu. Rev. Virol. 2014, 1, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Schoelz, J.E.; Harries, P.A.; Nelson, R.S. Intracellular transport of plant viruses: Finding the door out of the cell. Mol. Plant 2011, 4, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Byalik, T.E.; Komarova, T.V. Diversity of plant virus movement proteins: What do they have in common? Processes 2020, 8, 1547. [Google Scholar] [CrossRef]
- Saito, T.; Hosokawa, D.; Meshi, T.; Meshi, T.; Okada, Y. Immunocytochemical localization of the 130K and 180K proteins (putative replicase components) of tobacco mosaic virus. Virology 1987, 160, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Linnik, O.; Christensen, N.M.; Bell, K.F.S.; Roberts, I.M.; Lacomme, C.; Oparka, K. Live-cell imaging of viral RNA genomes using a Pumilio-based reporter. Plant J. 2009, 57, 758–770. [Google Scholar] [CrossRef]
- Citovsky, V.; Knorr, D.; Schuster, G.; Zambryski, P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell 1990, 60, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Citovsky, V.; Wong, M.L.; Shaw, A.L.; Prasad, B.V.; Zambryski, P. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell 1992, 4, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Z.; Blancaflor, E.B.; Nelson, R.S. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 2005, 138, 1853–1865. [Google Scholar] [CrossRef]
- Amari, K.; Di Donato, M.; Dolja, V.V.; Heinlein, M. Myosins VIII and XI play distinct roles in reproduction and transport of tobacco mosaic virus. PLoS Pathog. 2014, 10, e1004448. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Shishikura, R.; Nyunoya, H. Formation and intracellular movement of cytoplasmic bodies of the tomato mosaic virus 126-kDa replication protein in association with the viral movement protein. J. Gen. Plant Pathol. 2014, 80, 272–281. [Google Scholar] [CrossRef]
- Guenoune-Gelbart, D.; Elbaum, M.; Sagi, G.; Levy, A.; Epel, B.L. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating tmv spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2008, 21, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, K.; Watanabe, Y. Tobamovirus replicase coding region is involved in cell-to-cell movement. J. Virol. 2001, 75, 8831–8836. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, K.; Watanabe, Y. RNA helicase domain of tobamovirus replicase executes cell-to-cell movement possibly through collaboration with its nonconserved region. J. Virol. 2003, 77, 12357–12362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, K.M.; Wood, N.T.; Roberts, A.G.; Chapman, S.; Boevink, P.C.; MacKenzie, K.; Oparka, K. Targeting of TMV movement protein to plasmodesmata requires the actin/ER network; evidence from FRAP. Traffic 2007, 8, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sambade, A.; Brandner, K.; Hofmann, C.; Seemanpillai, M.; Mutterer, J.; Heinlein, M. Transport of TMV movement protein particles associated with the targeting of RNA to plasmodesmata. Traffic 2008, 9, 2073–2088. [Google Scholar] [CrossRef]
- Luo, K.-R.; Huang, N.-C.; Chang, Y.-H.; Jan, Y.-W.; Yu, T.-S. Arabidopsis cyclophilins direct intracellular transport of mobile mRNA via organelle hitchhiking. Nat. Plants 2024, 10, 161–171. [Google Scholar] [CrossRef]
- Dawson, W.O.; Lehto, K.M. Regulation of tobamovirus gene expression. Adv. Virus Res. 1990, 38, 307–342. [Google Scholar] [CrossRef] [PubMed]
- Sztuba-Solińska, J.; Stollar, V.; Bujarski, J.J. Subgenomic messenger RNAs: Mastering regulation of (+)-strand RNA virus life cycle. Virology 2011, 412, 245–255. [Google Scholar] [CrossRef]
- Más, P.; Beachy, R.N. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 1999, 147, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.M.; Brill, L.M.; Nunn, R.S.; Kahn, T.W.; Yeager, M.; Beachy, R.N. Recombinant tobacco mosaic virus movement protein is an RNA-binding, α-helical membrane protein. Proc. Natl. Acad. Sci. USA 2000, 97, 7112–7117. [Google Scholar] [CrossRef] [PubMed]
- Peiró, A.; Martínez-Gil, L.; Tamborero, S.; Pallás, V.; Sánchez-Navarro, J.A.; Mingarro, I. The tobacco mosaic virus movement protein associates with but does not integrate into biological membranes. J. Virol. 2014, 88, 3016–3026. [Google Scholar] [CrossRef]
- Sparkes, I.; Runions, J.; Hawes, C.; Griffing, L. Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 2009, 21, 3937–3949. [Google Scholar] [CrossRef]
- Ueda, H.; Yokota, E.; Kutsuna, N.; Shimada, T.; Tamura, K.; Shimmen, T.; Hasezawa, S.; Dolja, V.V.; Hara-Nishimura, I. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6894–6899. [Google Scholar] [CrossRef] [PubMed]
- Runions, J.; Brach, T.; Kühner, S.; Hawes, C. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J. Exp. Bot. 2005, 57, 43–50. [Google Scholar] [CrossRef]
- Tagami, Y.; Watanabe, Y. Effects of Brefeldin A on the localization of Tobamovirus movement protein and cell-to-cell movement of the virus. Virology 2007, 361, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Boutant, E.; Fitterer, C.; Ritzenthaler, C.; Heinlein, M. Interaction of the tobacco mosaic virus movement protein with microtubules during the cell cycle in tobacco BY-2 cells. Protoplasma 2009, 237, 3–12. [Google Scholar] [CrossRef]
- Genovés, A.; Navarro, J.A.; Pallás, V. The intra- and intercellular movement of melon necrotic spot virus (MNSV) depends on an active secretory pathway. Mol. Plant-Microbe Interact. 2010, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kotzer, A.M.; Brandizzi, F.; Neumann, U.; Paris, N.; Moore, I.; Hawes, C. AtRabF2b (Ara7) acts on the vacuolar trafficking pathway in tobacco leaf epidermal cells. J. Cell Sci. 2004, 117, 6377–6389. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Gao, C.; Cui, Y.; Wang, J.; Ding, Y.; Cai, Y.; Ueda, T.; Nakano, A.; Jiang, L. ARA7(Q69L) expression in transgenic arabidopsis cells induces the formation of enlarged multivesicular bodies. J. Exp. Bot. 2013, 64, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Boyko, V.; Hu, Q.; Seemanpillai, M.; Ashby, J.; Heinlein, M. Validation of microtubule-associated tobacco mosaic virus RNA movement and involvement of microtubule-aligned particle trafficking. Plant J. 2007, 51, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Harries, P.A.; Park, J.-W.; Sasaki, N.; Ballard, K.D.; Maule, A.J.; Nelson, R.S. Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc. Natl. Acad. Sci. USA 2009, 106, 17594–17599. [Google Scholar] [CrossRef]
- Avisar, D.; Prokhnevsky, A.I.; Dolja, V.V. Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J. Virol. 2008, 82, 2836–2843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peña, E.J.; Heinlein, M. Cortical microtubule-associated ER sites: Organization centers of cell polarity and communication. Curr. Opin. Plant Biol. 2013, 16, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Niehl, A.; Sambade, A.; Steinmetz, A.; Heinlein, M. Inhibition of tobacco mosaic virus movement by expression of an actin-binding protein. Plant Physiol. 2009, 149, 1810–1823. [Google Scholar] [CrossRef]
- McLean, B.G.; Zupan, J.; Zambryski, P.C. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell 1995, 7, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, M.; Epel, B.L.; Padgett, H.S.; Beachy, R.N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science 1995, 270, 1983–1985. [Google Scholar] [CrossRef]
- Brandner, K.; Sambade, A.; Boutant, E.; Didier, P.; Mély, Y.; Ritzenthaler, C.; Heinlein, M. Tobacco mosaic virus movement protein interacts with green fluorescent protein-tagged microtubule end-binding protein 1. Plant Physiol. 2008, 147, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Ouko, M.O.; Sambade, A.; Brandner, K.; Niehl, A.; Peña, E.J.; Ahad, A.; Heinlein, M.; Nick, P. Tobacco mutants with reduced microtubule dynamics are less susceptible to TMV. Plant J. 2010, 62, 829–839. [Google Scholar] [CrossRef]
- Seemanpillai, M.; Elamawi, R.; Ritzenthaler, C.; Heinlein, M. Challenging the role of microtubules in tobacco mosaic virus movement by drug treatments is disputable. J. Virol. 2006, 80, 6712–6715. [Google Scholar] [CrossRef]
- Huang, C.; Heinlein, M. Function of plasmodesmata in the interaction of plants with microbes and viruses. In Plasmodesmata: Methods and Protocols; Benitez-Alfonso, Y., Heinlein, M., Eds.; Springer US: New York, NY, USA, 2022; pp. 23–54. ISBN 978-1-07-162132-5. [Google Scholar]
- Niehl, A.; Peña, E.J.; Amari, K.; Heinlein, M. Microtubules in viral replication and transport. Plant J. 2013, 75, 290–308. [Google Scholar] [CrossRef]
- Yamanaka, T.; Ohta, T.; Takahashi, M.; Meshi, T.; Schmidt, R.; Dean, C.; Naito, S.; Ishikawa, M. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. USA 2000, 97, 10107–10112. [Google Scholar] [CrossRef]
- Kim, S.-J.; Brandizzi, F. News and views into the SNARE complexity in Arabidopsis. Front. Plant Sci. 2012, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hicks, G.R.; Raikhel, N.V. Plant vacuole morphology and vacuolar trafficking. Front. Plant Sci. 2014, 5, 476. [Google Scholar] [CrossRef]
- Ebine, K.; Inoue, T.; Ito, J.; Ito, E.; Uemura, T.; Goh, T.; Abe, H.; Sato, K.; Nakano, A.; Ueda, T. Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr. Biol. 2014, 24, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Ebine, K.; Askani, J.C.; Krüger, F.; Gonzalez, Z.A.; Ito, E.; Goh, T.; Schumacher, K.; Nakano, A.; Ueda, T. Distinct sets of tethering complexes, SNARE complexes, and Rab GTPases mediate membrane fusion at the vacuole in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E2457–E2466. [Google Scholar] [CrossRef]
- Prasanth, K.R.; Kovalev, N.; de Castro Martín, I.F.; Baker, J.; Nagy, P.D. Screening a yeast library of temperature-sensitive mutants reveals a role for actin in tombusvirus RNA recombination. Virology 2016, 489, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Nagy, P.D. Enrichment of phosphatidylethanolamine in viral replication compartments via co-opting the endosomal Rab5 small GTPase by a positive-strand RNA virus. PLoS Biol. 2016, 14, e2000128. [Google Scholar] [CrossRef]
- Tee, E.E.; Faulkner, C. Plasmodesmata and intercellular molecular traffic control. New Phytol. 2024, 243, 32–47. [Google Scholar] [CrossRef]
- Zanini, A.A.; Burch-Smith, T.M. New insights into plasmodesmata: Complex ‘protoplasmic connecting threads’. J. Exp. Bot. 2024, 75, 5557–5567. [Google Scholar] [CrossRef]
- Ueki, S.; Citovsky, V. To gate, or not to gate: Regulatory mechanisms for intercellular protein transport and virus movement in plants. Mol. Plant 2011, 4, 782–793. [Google Scholar] [CrossRef]
- Barton, D.A.; Cole, L.; Collings, D.A.; Liu, D.Y.T.; Smith, P.M.C.; Day, D.A.; Overall, R.L. Cell-to-cell transport via the lumen of the endoplasmic reticulum. Plant J. 2011, 66, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Nicolas, W.; Rosado, A.; Bayer, E.M. Staying Tight: Plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annu. Rev. Plant Biol. 2016, 67, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, W.J.; Grison, M.S.; Trépout, S.; Gaston, A.; Fouché, M.; Cordelières, F.P.; Oparka, K.; Tilsner, J.; Brocard, L.; Bayer, E.M. Architecture and permeability of post-cytokinesis plasmodesmata lacking cytoplasmic sleeves. Nat. Plants 2017, 3, 17082. [Google Scholar] [CrossRef] [PubMed]
- Brault, M.L.; Petit, J.D.; Immel, F.; Nicolas, W.J.; Glavier, M.; Brocard, L.; Gaston, A.; Fouché, M.; Hawkins, T.J.; Crowet, J.; et al. Multiple C2 domains and transmembrane region proteins (MCTPs) tether membranes at plasmodesmata. EMBO Rep. 2019, 20, e47182. [Google Scholar] [CrossRef] [PubMed]
- Deom, C.M.; Oliver, M.J.; Beachy, R.N. The 30-Kilodalton gene product of tobacco mosaic virus potentiates virus movement. Sci. New Ser. 1987, 237, 389–394. [Google Scholar] [CrossRef]
- Meshi, T.; Watanabe, Y.; Saito, T.; Sugimoto, A.; Maeda, T.; Okada, Y. Function of the 30 kd protein of tobacco mosaic virus: Involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987, 6, 2557–2563. [Google Scholar] [CrossRef]
- Citovsky, V. Tobacco mosaic virus: A pioneer of cell-to-cell movement. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 637–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holt, C.A.; Beachy, R.N. In vivo complementation of infectious transcripts from mutant tobacco mosaic virus cDNAs in transgenic plants. Virology 1991, 181, 109–117. [Google Scholar] [CrossRef]
- Niehl, A.; Heinlein, M. Cellular pathways for viral transport through plasmodesmata. Protoplasma 2011, 248, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.J.; Robles Luna, G.; Heinlein, M. In vivo imaging of tagged mRNA in plant tissues using the bacterial transcriptional antiterminator BglG. Plant J. 2021, 105, 271–282. [Google Scholar] [CrossRef]
- Wolf, S.; Lucas, W.J.; Deom, C.M.; Beachy, R.N. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 1989, 246, 377–379. [Google Scholar] [CrossRef]
- Tomenius, K.; Clapham, D.; Meshi, T. Localization by immunogold cytochemistry of the virus-coded 30K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology 1987, 160, 363–371. [Google Scholar] [CrossRef]
- Oparka, K.J.; Prior, D.A.; Santa Cruz, S.; Padgett, H.S.; Beachy, R.N. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV). Plant J. 1997, 12, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Haudenshield, J.S.; Hull, R.J.; Wolf, S.; Beachy, R.N.; Lucas, W.J. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell 1992, 4, 915–928. [Google Scholar] [PubMed]
- Burch-Smith, T.M.; Stonebloom, S.; Xu, M.; Zambryski, P.C. Plasmodesmata during development: Re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 2011, 248, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. Identification of a functional plasmodesmal localization signal in a plant viral cell-to-cell-movement protein. mBio 2016, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Zeng, J.; Yu, H.; Li, Y.; Yuan, C. Identification of two additional plasmodesmata localization domains in the tobacco mosaic virus cell-to-cell-movement protein. Biochem. Biophys. Res. Commun. 2020, 521, 145–151. [Google Scholar] [CrossRef]
- Gafny, R.; Lapidot, M.; Berna, A.; Holt, C.A.; Deom, C.M.; Beachy, R.N. Effects of terminal deletion mutations on function of the movement protein of tobacco mosaic virus. Virology 1992, 187, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.-T.; Vo Phan, M.-S.; Citovsky, V. Gain-of-function mutant of movement protein allows systemic transport of a defective tobacco mosaic virus. iScience 2022, 25, 105486. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lazarowitz, S.G. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl. Acad. Sci. USA 2010, 107, 2491–2496. [Google Scholar] [CrossRef]
- Levy, A.; Zheng, J.Y.; Lazarowitz, S.G. Synaptotagmin SYTA forms ER-plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr. Biol. 2015, 25, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Fuente, F.; Botella, M.A. Biological roles of plant synaptotagmins. Eur. J. Cell Biol. 2023, 102, 151335. [Google Scholar] [CrossRef] [PubMed]
- Schapire, A.L.; Voigt, B.; Jasik, J.; Rosado, A.; Lopez-Cobollo, R.; Menzel, D.; Salinas, J.; Mancuso, S.; Valpuesta, V.; Baluska, F.; et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell 2008, 20, 3374–3388. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tamura, K.; Fukao, Y.; Shimada, T. Structural and functional relationships between plasmodesmata and plant endoplasmic reticulum–plasma membrane contact sites consisting of three synaptotagmins. New Phytol. 2020, 226, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. The plasmodesmal localization signal of TMV MP is recognized by plant synaptotagmin SYTA. mBio 2018, 9. [Google Scholar] [CrossRef]
- Hak, H.; Raanan, H.; Schwarz, S.; Sherman, Y.; Dinesh-Kumar, S.P.; Spiegelman, Z. Activation of Tm-22 resistance is mediated by a conserved cysteine essential for tobacco mosaic virus movement. Mol. Plant Pathol. 2023, 24, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.-T.; Citovsky, V. Receptor-like kinase BAM1 facilitates early movement of the tobacco mosaic virus. Commun. Biol. 2021, 4, 511. [Google Scholar] [CrossRef]
- Rosas-Diaz, T.; Zhang, D.; Fan, P.; Wang, L.; Ding, X.; Jiang, Y.; Jimenez-Gongora, T.; Medina-Puche, L.; Zhao, X.; Feng, Z.; et al. A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. USA 2018, 115, 1388–1393. [Google Scholar] [CrossRef]
- Matsushita, Y.; Deguchi, M.; Youda, M.; Nishiguchi, M.; Nyunoya, H. The tomato mosaic tobamovirus movement protein interacts with a putative transcriptional coactivator KELP. Mol. Cells 2001, 12, 57–66. [Google Scholar] [CrossRef]
- Sasaki, N.; Ogata, T.; Deguchi, M.; Nagai, S.; Tamai, A.; Meshi, T.; Kawakami, S.; Watanabe, Y.; Matsushita, Y.; Nyunoya, H. Over-expression of putative transcriptional coactivator KELP interferes with tomato mosaic virus cell-to-cell movement. Mol. Plant Pathol. 2008, 10, 161. [Google Scholar] [CrossRef]
- Pérez-Sancho, J.; Smokvarska, M.; Dubois, G.; Glavier, M.; Sritharan, S.; Moraes, T.S.; Moreau, H.; Dietrich, V.; Platre, M.P.; Paterlini, A.; et al. Plasmodesmata act as unconventional membrane contact sites regulating inter-cellular molecular exchange in plants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kriechbaumer, V.; Botchway, S.W.; Slade, S.E.; Knox, K.; Frigerio, L.; Oparka, K.; Hawes, C. Reticulomics: Protein-protein interaction studies with two plasmodesmata-localized reticulon family proteins identify binding partners enriched at plasmodesmata, endoplasmic reticulum, and the plasma membrane. Plant Physiol. 2015, 169, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Spektor, R.; Natale, D.M.; Citovsky, V. ANK, a host cytoplasmic receptor for the tobacco mosaic virus cell-to-cell movement protein, facilitates intercellular transport through plasmodesmata. PLoS Pathog. 2010, 6, e1001201. [Google Scholar] [CrossRef] [PubMed]
- German, L.; Yeshvekar, R.; Benitez-Alfonso, Y. Callose metabolism and the regulation of cell walls and plasmodesmata during plant mutualistic and pathogenic interactions. Plant Cell Environ. 2023, 46, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Amsbury, S.; Kirk, P.; Benitez-Alfonso, Y. Emerging models on the regulation of intercellular transport by plasmodesmata-associated callose. J. Exp. Bot. 2018, 69, 105–115. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins Jr, F. Movement of plant viruses is delayed in a β-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Zavaliev, R.; Levy, A.; Gera, A.; Epel, B.L. Subcellular dynamics and role of arabidopsis β-1,3-glucanases in cell-to-cell movement of tobamoviruses. Mol. Plant-Microbe Interact. 2013, 26, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Wang, X.; Cui, W.; Sager, R.; Modla, S.; Czymmek, K.; Zybaliov, B.; Van Wijk, K.; Zhang, C.; Lu, H.; et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 2011, 23, 3353–3373. [Google Scholar] [CrossRef]
- Cui, W.; Lee, J.-Y. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nat. Plants 2016, 2, 16034. [Google Scholar] [CrossRef]
- Liu, N.-J.; Zhang, T.; Liu, Z.-H.; Chen, X.; Guo, H.-S.; Ju, B.-H.; Zhang, Y.-Y.; Li, G.-Z.; Zhou, Q.-H.; Qin, Y.-M.; et al. Phytosphinganine affects plasmodesmata permeability via facilitating PDLP5-stimulated callose accumulation in Arabidopsis. Mol. Plant 2020, 13, 128–143. [Google Scholar] [CrossRef]
- Kamarova, K.A.; Ershova, N.M.; Sheshukova, E.V.; Arifulin, E.A.; Ovsiannikova, N.L.; Antimonova, A.A.; Kudriashov, A.A.; Komarova, T.V. Nicotiana benthamiana class 1 reversibly glycosylated polypeptides suppress tobacco mosaic virus infection. Int. J. Mol. Sci. 2023, 24, 12843. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Sagi, G.; Gera, A.; Epel, B.L. The constitutive expression of Arabidopsis plasmodesmal-associated class 1 reversibly glycosylated polypeptide impairs plant development and virus spread. J. Exp. Bot. 2010, 61, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Burch-Smith, T.M.; Cui, Y.; Zambryski, P.C. Reduced levels of class 1 reversibly glycosylated polypeptide increase intercellular transport via plasmodesmata. Plant Signal. Behav. 2012, 7, 62–67. [Google Scholar] [CrossRef]
- Huang, C.; Sede, A.R.; Elvira-González, L.; Yan, Y.; Rodriguez, M.E.; Mutterer, J.; Boutant, E.; Shan, L.; Heinlein, M. dsRNA-Induced Immunity Targets Plasmodesmata and Is Suppressed by Viral Movement Proteins. Plant Cell 2023, 35, 3845–3869. [Google Scholar] [CrossRef]
- Tilsner, J.; Kriechbaumer, V. Reticulons 3 and 6 interact with viral movement proteins. Mol. Plant Pathol. 2022, 23, 1807–1814. [Google Scholar] [CrossRef]
- Brandizzi, F. Maintaining the structural and functional homeostasis of the plant endoplasmic reticulum. Dev. Cell 2021, 56, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calvino, L.; Faulkner, C.; Walshaw, J.; Saalbach, G.; Bayer, E.; Benitez-Alfonso, Y.; Maule, A. Arabidopsis plasmodesmal proteome. PLoS ONE 2011, 6, e0018880. [Google Scholar] [CrossRef]
- Knox, K.; Wang, P.; Kriechbaumer, V.; Tilsner, J.; Frigerio, L.; Sparkes, I.; Hawes, C.; Oparka, K. Putting the squeeze on plasmodesmata: A role for reticulons in primary plasmodesmata formation. Plant Physiol. 2015, 168, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Bayer, E.; Lafarge, D.; Cluzet, S.; German Retana, S.; Boubekeur, T.; Leborgne-Castel, N.; Carde, J.-P.; Lherminier, J.; Noirot, E.; et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell 2009, 21, 1541–1555. [Google Scholar] [CrossRef]
- Gouguet, P.; Gronnier, J.; Legrand, A.; Perraki, A.; Jolivet, M.-D.; Deroubaix, A.-F.; German-Retana, S.; Boudsocq, M.; Habenstein, B.; Mongrand, S.; et al. Connecting the dots: From nanodomains to physiological functions of REMORINs. Plant Physiol. 2021, 185, 632–649. [Google Scholar] [CrossRef]
- Perraki, A.; Binaghi, M.; Mecchia, M.A.; Gronnier, J.; German-Retana, S.; Mongrand, S.; Bayer, E.; Zelada, A.M.; Germain, V. StRemorin1.3 hampers potato virus X TGBp1 ability to increase plasmodesmata permeability, but does not interfere with its silencing suppressor activity. FEBS Lett. 2014, 588, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Perraki, A.; Gronnier, J.; Gouguet, P.; Boudsocq, M.; Deroubaix, A.-F.; Simon, V.; German-Retana, S.; Legrand, A.; Habenstein, B.; Zipfel, C.; et al. REM1.3′s phospho-status defines its plasma membrane nanodomain organization and activity in restricting PVX cell-to-cell movement. PLoS Pathog. 2018, 14, e1007378. [Google Scholar] [CrossRef]
- Ma, T.; Fu, S.; Wang, K.; Wang, Y.; Wu, J.; Zhou, X. Palmitoylation is indispensable for remorin to restrict tobacco mosaic virus cell-to-cell movement in Nicotiana benthamiana. Viruses 2022, 14, 1324. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Takashima, E.; Nyunoya, H. Altered subcellular localization of a tobacco membrane raft-associated remorin protein by tobamovirus infection and transient expression of viral replication and movement proteins. Front. Plant Sci. 2018, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Raiola, A.; Cervone, F.; Bellincampi, D. How do pectin methylesterases and their inhibitors affect the spreading of tobamovirus? Plant Signal. Behav. 2014, 9, e972863. [Google Scholar] [CrossRef]
- Bayer, E.M.; Benitez-Alfonso, Y. Plasmodesmata: Channels under pressure. Annu. Rev. Plant Biol. 2024, 75, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Mäkinen, K.; Frolova, O.Y.; Merits, A.; Saarinen, J.; Kalkkinen, N.; Atabekov, J.G.; Saarma, M. A novel function for a ubiquitous plant enzyme pectin methylesterase: The host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett. 1999, 461, 223–228. [Google Scholar] [CrossRef]
- Chen, M.-H.; Sheng, J.; Hind, G.; Handa, A.K.; Citovsky, V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 2000, 19, 913–920. [Google Scholar] [CrossRef]
- Lionetti, V.; Raiola, A.; Cervone, F.; Bellincampi, D. Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol. Plant Pathol. 2014, 15, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef] [PubMed]
- Sheshukova, E.V.; Kamarova, K.A.; Ershova, N.M.; Komarova, T.V. Nicotiana benthamiana methanol-inducible gene (MIG) 21 encodes a nucleolus-localized protein that stimulates viral intercellular transport and downregulates nuclear import. Plants 2024, 13, 279. [Google Scholar] [CrossRef]
- Jia, X.-Y.; He, L.-H.; Jing, R.-L.; Li, R.-Z. Calreticulin: Conserved protein and diverse functions in plants. Physiol. Plant. 2009, 136, 127–138. [Google Scholar] [CrossRef]
- Chen, M.-H.; Tian, G.-W.; Gafni, Y.; Citovsky, V. Effects of calreticulin on viral cell-to-cell movement. Plant Physiol. 2005, 138, 1866–1876. [Google Scholar] [CrossRef]
- Holdaway-Clarke, T.L.; Walker, N.A.; Hepler, P.K.; Overall, R.L. Physiological elevations in cytoplasmic free calcium by cold or ion injection result in transient closure of higher plant plasmodesmata. Planta 2000, 210, 329–335. [Google Scholar] [CrossRef]
- Crowley, N.C.; Hanson, J.B. The infection of apical meristems of tomato roots with tobacco mosaic virus after treatment with ethylenediaminetetraacetic acid. Virology 1960, 12, 603–606. [Google Scholar] [CrossRef]
- Yoshii, A.; Shimizu, T.; Yoshida, A.; Hamada, K.; Sakurai, K.; Yamaji, Y.; Suzuki, M.; Namba, S.; Hibi, T.; Hibi, T. NTH201, a novel class II KNOTTED1-like protein, facilitates the cell-to-cell movement of tobacco mosaic virus in tobacco. Mol. Plant-Microbe Interact. 2008, 21, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Nookaraju, A.; Pandey, S.K.; Ahlawat, Y.K.; Joshi, C.P. Understanding the modus operandi of class II KNOX transcription factors in secondary cell wall biosynthesis. Plants 2022, 11, 493. [Google Scholar] [CrossRef]
- Shimizu, T.; Yoshii, A.; Sakurai, K.; Hamada, K.; Yamaji, Y.; Suzuki, M.; Namba, S.; Hibi, T. Identification of a novel tobacco DnaJ-like protein that interacts with the movement protein of tobacco mosaic virus. Arch. Virol. 2009, 154, 959–967. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, J.; Chen, T.; Liu, Q.; Zhang, H.; Wang, Y.; Hong, Y.; Xiao, F.; Zhang, L.; Shen, Q.; et al. Type I J-domain NbMIP1 proteins are required for both tobacco mosaic virus infection and plant innate immunity. PLoS Pathog. 2013, 9, e1003659. [Google Scholar] [CrossRef]
- Rajan, V.B.V.; D’Silva, P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct. Integr. Genom. 2009, 9, 433–446. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, T.; Wu, X.; Hong, Y.; Fan, Z.; Li, H. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Mol. Plant Pathol. 2008, 9, 809–817. [Google Scholar] [CrossRef]
- Aoki, K.; Kragler, F.; Xoconostle-Cázares, B.; Lucas, W.J. A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl. Acad. Sci. USA 2002, 99, 16342. [Google Scholar] [CrossRef]
- Citovsky, V.; McLean, B.G.; Zupan, J.R.; Zambryski, P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993, 7, 904–910. [Google Scholar] [CrossRef]
- Waigmann, E.; Chen, M.-H.; Bachmaier, R.; Ghoshroy, S.; Citovsky, V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 2000, 19, 4875–4884. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Taoka, K.; Yoo, B.-C.; Ben-Nissan, G.; Kim, D.-J.; Lucas, W.J. Plasmodesmal-associated protein kinase in tobacco and Arabidopsis recognizes a subset of non-cell-autonomous proteins. Plant Cell 2005, 17, 2817–2831. [Google Scholar] [CrossRef]
- Trutnyeva, K.; Bachmaier, R.; Waigmann, E. Mimicking carboxyterminal phosphorylation differentially effects subcellular distribution and cell-to-cell movement of tobacco mosaic virus movement protein. Virology 2005, 332, 563–577. [Google Scholar] [CrossRef][Green Version]
- Tyulkina, L.G.; Karger, E.M.; Sheveleva, A.A.; Atabekov, J.G. Binding of monoclonal antibodies to the movement protein (mp) of tobacco mosaic virus: Influence of subcellular mp localization and phosphorylation. J. Gen. Virol. 2010, 91, 1621–1628. [Google Scholar] [CrossRef]
- Karger, E.M.; Frolova, O.Y.; Fedorova, N.V.; Baratova, L.A.; Ovchinnikova, T.V.; Susi, P.; Makinen, K.; Ronnstrand, L.; Dorokhov, Y.L.; Atabekov, J.G. Dysfunctionality of a tobacco mosaic virus movement protein mutant mimicking threonine 104 phosphorylation. J. Gen. Virol. 2003, 84, 727–732. [Google Scholar] [CrossRef]
- Haley, A.; Hunter, T.; Kiberstis, P.; Zimmern, D. Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J. 1995, 8, 715–724. [Google Scholar] [CrossRef]
- Karpova, O.V.; Ivanov, K.I.; Rodionova, N.P.; Dorokhov, Y.L.; Atabekov, J.G. Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology 1997, 230, 11–21. [Google Scholar] [CrossRef]
- Karpova, O.V.; Rodionova, N.P.; Ivanov, K.I.; Kozlovsky, S.V.; Dorokhov, Y.L.; Atabekov, J.G. Phosphorylation of tobacco mosaic virus movement protein abolishes its translation repressing ability. Virology 1999, 261, 20–24. [Google Scholar] [CrossRef]
- Blackman, L.M.; Overall, R.L. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 1998, 14, 733–741. [Google Scholar] [CrossRef]
- Krasavina, M.S.; Ktitorova, I.N.; Burmistrova, N.A. Electrical conductance of cell-to-cell junctions and the cytoskeleton of plant cells. Russ. J. Plant Physiol. 2001, 48, 741–748. [Google Scholar] [CrossRef]
- White, R.G.; Barton, D.A. The cytoskeleton in plasmodesmata: A role in intercellular transport? J. Exp. Bot. 2011, 62, 5249–5266. [Google Scholar] [CrossRef][Green Version]
- White, R.G.; Badelt, K.; Overall, R.L.; Vesk, M. Actin associated with plasmodesmata. Protoplasma 1994, 180, 169–184. [Google Scholar] [CrossRef]
- Ding, B.; Kwon, M.-O.; Warnberg, L. Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J. 1996, 10, 157–164. [Google Scholar] [CrossRef]
- Radford, J.E.; White, R.G. Localization of a myosin-like protein to plasmodesmata. Plant J. Cell Mol. Biol. 1998, 14, 743–750. [Google Scholar] [CrossRef]
- Su, S.; Liu, Z.; Chen, C.; Zhang, Y.; Wang, X.; Zhu, L.; Miao, L.; Wang, X.-C.; Yuan, M. Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell 2010, 22, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ren, S.; Wang, Q.; Qian, L.; Shen, J.; Liu, Y.; Huang, S. Arabidopsis formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. eLife 2018, 7, e36316. [Google Scholar] [CrossRef]
- Kragler, F.; Curin, M.; Trutnyeva, K.; Gansch, A.; Waigmann, E. MPB2C, a Microtubule-associated plant protein binds to and interferes with cell-to-cell transport of tobacco mosaic virus movement protein. Plant Physiol. 2003, 132, 1870–1883. [Google Scholar] [CrossRef]
- Curin, M.; Ojangu, E.-L.; Trutnyeva, K.; Ilau, B.; Truve, E.; Waigmann, E. MPB2C, a microtubule-associated plant factor, is required for microtubular accumulation of tobacco mosaic virus movement protein in plants. Plant Physiol. 2007, 143, 801–811. [Google Scholar] [CrossRef]
- Inès, D.; Courty, P.-E.; Wendehenne, D.; Rosnoblet, C. CDC48 in plants and its emerging function in plant immunity. Trends Plant Sci. 2024, 29, 786–798. [Google Scholar] [CrossRef]
- Nicolas-Francès, V.; Besson-Bard, A.; Meschini, S.; Klinguer, A.; Bonnotte, A.; Héloir, M.-C.; Citerne, S.; Inès, D.; Hichami, S.; Wendehenne, D.; et al. CDC48 regulates immunity pathway in tobacco plants. Plant Physiol. Biochem. 2024, 211, 108714. [Google Scholar] [CrossRef]
- Peña, E.J.; Ferriol, I.; Sambade, A.; Buschmann, H.; Niehl, A.; Elena, S.F.; Rubio, L.; Heinlein, M. Experimental virus evolution reveals a role of plant microtubule dynamics and TORTIFOLIA1/SPIRAL2 in RNA trafficking. PLoS ONE 2014, 9, e105364. [Google Scholar] [CrossRef]
- Das, P.P.; Macharia, M.W.; Lin, Q.; Wong, S.-M. In planta proximity-dependent biotin identification (BioID) identifies a TMV replication co-chaperone NbSGT1 in the vicinity of 126 kDa replicase. J. Proteomics 2019, 204, 103402. [Google Scholar] [CrossRef]
- Andronov, L.; Han, M.; Zhu, Y.; Balaji, A.; Roy, A.R.; Barentine, A.E.S.; Patel, P.; Garhyan, J.; Qi, L.S.; Moerner, W.E. Nanoscale cellular organization of viral RNA and proteins in SARS-CoV-2 replication organelles. Nat. Commun. 2024, 15, 4644. [Google Scholar] [CrossRef]
- Sathanantham, P.; Zhao, W.; He, G.; Murray, A.; Fenech, E.; Diaz, A.; Schuldiner, M.; Wang, X. A conserved viral amphipathic helix governs the replication site-specific membrane association. PLoS Pathog. 2022, 18, e1010752. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.; Sasaki, N.; Schoelz, J.E.; Nelson, R.S. Tobacco Mosaic Virus Movement: From Capsid Disassembly to Transport Through Plasmodesmata. Viruses 2025, 17, 214. https://doi.org/10.3390/v17020214
Ibrahim A, Sasaki N, Schoelz JE, Nelson RS. Tobacco Mosaic Virus Movement: From Capsid Disassembly to Transport Through Plasmodesmata. Viruses. 2025; 17(2):214. https://doi.org/10.3390/v17020214
Chicago/Turabian StyleIbrahim, Amr, Nobumitsu Sasaki, James E. Schoelz, and Richard S. Nelson. 2025. "Tobacco Mosaic Virus Movement: From Capsid Disassembly to Transport Through Plasmodesmata" Viruses 17, no. 2: 214. https://doi.org/10.3390/v17020214
APA StyleIbrahim, A., Sasaki, N., Schoelz, J. E., & Nelson, R. S. (2025). Tobacco Mosaic Virus Movement: From Capsid Disassembly to Transport Through Plasmodesmata. Viruses, 17(2), 214. https://doi.org/10.3390/v17020214





