1. Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for approximately 80% of cases, and remains the sixth most prevalent malignancy and the third leading cause of cancer-related deaths worldwide, according to the World Health Organization’s 2022 global cancer statistics [
1]. The relative 5-year survival rate for HCC is only ~18%, reflecting its high lethality. Current therapeutic options for HCC are stage-dependent. In very-early- or early-stage disease, surgical resection, liver transplantation, and local ablative therapies offer the greatest chance of cure [
2,
3]. However, due to the asymptomatic nature of early HCC, most patients present at intermediate or advanced stages, when curative interventions are no longer feasible. At these later stages, transarterial chemoembolization (TACE) or radioembolization (TARE) can provide disease control in intermediate disease, while advanced disease is mainly treated with systemic molecular-targeted therapies such as sorafenib [
4]. Nevertheless, despite continuous advances in conventional therapies, the high postoperative recurrence rate (often exceeding 50% within 2 years) and the emergence of drug resistance severely limit long-term survival [
5], underscoring an urgent need for novel therapeutic approaches with improved efficacy and selectivity.
Oncolytic virotherapy (OV) has emerged as a promising anticancer strategy, using wild-type or genetically engineered viruses to selectively replicate within and lyse tumor cells without harming normal cells [
6,
7]. Among OV platforms, oncolytic adenoviruses (oAds) are the most extensively studied in clinical trials, representing approximately 42% of all OV-related trials. Their clinical safety profile has been well documented, with Oncorine—approved in China in 2005—being the first oAd to reach clinical use [
8]. oAds offer several advantages, including strong induction of antitumor immunity, antiangiogenic effects, high-level transgene expression, and synergistic potential when combined with chemotherapy, radiotherapy, or immunotherapy [
9].
Apoptin, encoded by the VP3 gene of the chicken anemia virus (CAV), is a small tumor-selective apoptosis-inducing protein with a unique mechanism of action: it triggers apoptosis through a p53- and Bcl-2-independent but caspase-dependent pathway, allowing it to effectively kill tumor cells with p53 mutations or loss of function—frequent events in HCC—while sparing normal cells [
10,
11]. This selectivity is largely attributed to its subcellular localization: in normal cells, Apoptin is retained in the cytoplasm and is non-toxic, whereas in tumor cells, it translocates to the nucleus and activates apoptotic cascades [
12]. Compared to other pro-apoptotic agents, Apoptin’s independence from common apoptotic escape mechanisms makes it an attractive candidate for inclusion in oncolytic virotherapy platforms [
10,
13]. Apoptin can promote the phosphorylation of Nur77, triggering its translocation from the nucleus to the mitochondria. The C-terminal ligand-binding domain (LBD) of Nur77 interacts with the BH3 domain of Bcl-2, inducing a conformational change that disrupts the Bcl-2/Bax heterodimer [
10,
14]. This facilitates Bax oligomerization and mitochondrial outer membrane permeabilization (MOMP), leading to the release of cytochrome c (Cyt-c) and apoptosis-inducing factor (AIF) [
15,
16]. Within promyelocytic leukemia nuclear bodies (Pml-NBs), Apoptin associates with the sequestered anaphase-promoting complex/cyclosome (APC/C) to induce apoptosis; however, it can also efficiently induce apoptosis in cells with reduced or absent Pml expression [
17,
18]. Apoptin-mediated apoptosis also involves Bcl-2–dependent activation of the apoptotic protease activating factor-1 (Apaf-1) apoptosome [
14,
19]. Upon release, Cyt-c binds to Apaf-1, leading to apoptosome assembly, activation of caspase-9 and caspase-3, and ultimately the execution of apoptosis. In addition to its pro-apoptotic function, Apoptin can inhibit tumor cell proliferation by perturbing the cell cycle, causing arrest at the G2/M phase. Through interactions with multiple proteins, Apoptin directly or indirectly modulates the function of cell cycle regulators, thereby enforcing G2/M arrest [
10]. Specifically, Apoptin binds to protein kinase C β1 (PKCβ1) and activates it, which in turn phosphorylates Apoptin [
10,
20]. This phosphorylation not only promotes its nuclear accumulation but may also regulate the phosphorylation state of other cell cycle-related proteins, contributing to cell cycle control.
Adenoviruses are among the most efficient gene delivery systems, possessing large genome capacity, high packaging titers, and ease of production, which together facilitate robust transgene expression with reduced risk of insertional mutagenesis compared to integrating vectors [
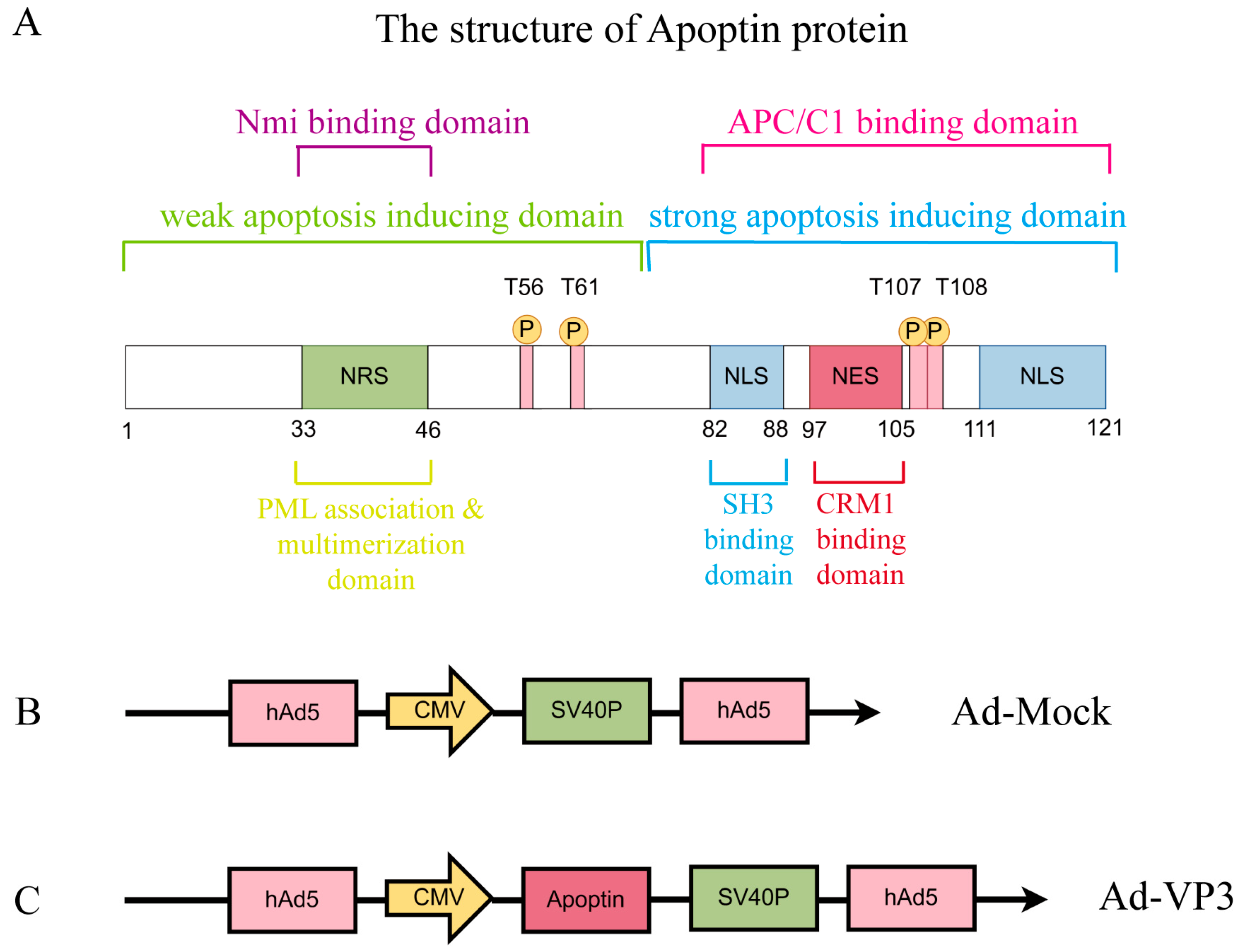
9]. In our previous work, we constructed the recombinant oncolytic adenovirus Ad-VP3 based on a human adenovirus type 5 backbone, placing the Apoptin gene under the control of the strong CMV promoter to ensure robust expression in tumor cells [
21]. The replication-defective adenovirus used in this study (E1a-deleted) Ad-VP3, as well as the empty control vector Ad-Mock, are schematically shown in
Figure 1A. Ad-VP3 has demonstrated potent inhibitory effects in several tumor types, including lung, breast, and colorectal cancers [
22,
23,
24]. Accumulating evidence indicates that cancer stem cells (CSCs) play a pivotal role in tumor initiation and progression due to their enhanced tumorigenic capacity and resistance to conventional anticancer therapies [
25]. Therefore, novel cancer therapies should be evaluated not only for their ability to induce tumor regression but also for their efficacy in targeting CSCs. In our previous study, we showed that Ad-VP3, which induced tumor regression in an immunodeficient mouse xenograft model of the human breast cancer cell line MCF-7, was also capable of effectively targeting and eliminating CSCs within these tumors [
22].
In this study, we sought to investigate the antitumor potential of Ad-VP3 in human HCC cells, using the Hep3B cell line as a model. We systematically evaluated its effects on cell viability, proliferation, and stemness through CCK-8, crystal violet, Hoechst 33342 staining, CFSE-based proliferation assays, and tumor sphere formation assays. Apoptotic induction and mitochondrial membrane potential (MMP) changes were examined by Annexin V staining, JC-1 and TMRM probes, and Western blotting. Furthermore, the impact of Ad-VP3 on migration and invasion was assessed using wound-healing assays, Transwell migration and BioCoat invasion models, alongside analysis of EMT- and stemness-related protein expression. This comprehensive approach not only evaluates Ad-VP3’s antitumor efficacy in HCC but also explores its potential mechanisms of action, providing a rationale for further preclinical development.
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
Hep3B cells were obtained from the Institute of Virology, Wenzhou University. Cells were maintained in Minimum Essential Medium (MEM; Gibco, Shanghai, China) supplemented with 15% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Hyclone, Logan, UT, USA). Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. The recombinant adenoviruses Ad-VP3 and Ad-Mock were constructed at the Molecular Virology and Immunology Laboratory, Military Veterinary Research Institute, Academy of Military Medical Sciences, Changchun, and preserved at the Institute of Virology, Wenzhou University. The titer of the recombinant adenovirus is expressed in plaque-forming units (PFU), calculated as:
PFU = (number of plaques × dilution factor)/volume of virus inoculum. In infection experiments, viral dose is expressed as the multiplicity of infection (MOI), which refers to the ratio of the number of infectious viral particles to the number of target cells at the time of infection, with units of PFU per cell (PFU/cell).
2.2. CCK-8 Assay
Hep3B cells were seeded into 96-well plates at a density of 5 × 103 cells per well in 200 μL medium. An additional cell-free group served as a blank control. After 12–24 h incubation at 37 °C with 5% CO2, cells were treated with Ad-Mock or Ad-VP3 at multiplicities of infection (MOIs) of 100, or 200 (5 × l05 PFU, 1 × l06 PFU), and cultured for 24–96 h. At 24, 48, 72, and 96 h post-infection, 10 μL of CCK-8 solution (Cell Counting Kit-8 purchased from Tongren, Dojindo Laboratories, Kumamoto, Japan) was added to each well under light-protected conditions and incubated for 4 h. Absorbance was measured at 450 nm using a microplate reader.
The cell viability calculation formula is:
2.3. Crystal Violet Staining Assay
Hep3B cells were seeded into 6-well plates at a density of 5 × 105 cells per well in 2 mL medium and incubated for 12–24 h at 37 °C with 5% CO2. Cells were then treated with Ad-Mock or Ad-VP3 at MOIs of 100, or 200 (5 × l07 PFU, 1 × l08 PFU), and cultured for 24–96 h. At each time point (24, 48, 72, and 96 h), the medium was removed, and cells were rinsed with PBS, fixed with 4% paraformaldehyde, and stained with crystal violet (4% paraformaldehyde and crystal violet staining solution purchased from Beyotime, Shanghai, China). Images were captured under a microscope for analysis.
2.4. Hoechst 33342 Staining Assay
Hep3B cells were seeded into 6-well plates at 3.5 × 105 cells per well in 2 mL medium and incubated for 12–24 h. Three groups were established: Ad-VP3, Ad-Mock, and untreated control. Cells were treated with 100 MOI (3.5 × 107 PFU) of Ad-VP3 or Ad-Mock, and at 24, 48, and 72 h post-infection, Hoechst 33342 dye (10 μL) (purchased from Beyotime, Shanghai, China)was added to each well. After 10 min incubation at 37 °C in the dark, cells were washed twice with PBS and examined using a fluorescence microscope.
2.5. CFSE Staining Assay
Hep3B single-cell suspensions were labeled with CFSE (CFDA SE dye purchased from Beyotime, Shanghai, China) and seeded into 6-well plates at a density of 3.5 × 105 cells per well in 2 mL medium. Staining efficiency was confirmed by flow cytometry prior to infection. Cells were then divided into Ad-VP3, Ad-Mock, and control groups, and infected with 100 MOI (3.5 × 107 PFU) of Ad-VP3 or Ad-Mock. At 24, 48, and 72 h post-infection, cells were harvested, resuspended in 500 μL PBS, and analyzed by flow cytometry to assess cell proliferation.
2.6. Cell Cycle Analysis
Hep3B cells were seeded into 6-well plates at 3.5 × 105 cells per well in 2 mL medium and incubated for 12–24 h. Cells were assigned to Ad-VP3, Ad-Mock, and control groups, and treated with 100 MOI (3.5 × 107 PFU) of Ad-VP3 or Ad-Mock. At 24, 48, and 72 h post-infection, cells were harvested, resuspended in 500 μL of Vybrant® DyeCycle™ Orange stain (purchased from Invitrogen, Waltham, MA, USA) (prepared by diluting 1 μL Vybrant® DyeCycle™ Orange stain dye in 1 mL PBS), and incubated at 37 °C for 30 min. Cell cycle distribution was analyzed by flow cytometry.
2.7. Tumor Sphere Formation Assay
Hep3B cells were seeded in 12-well plates at a density of 2 × 105 cells (1ml/well) and incubated at 37 °C with 5% CO2 for 12–24 h. The cells were then treated with 100 MOI (2 × 107 PFU) of recombinant adenovirus Ad-VP3. Control groups included the Ad-Mock virus group and a blank control group. At 24, 48, and 72 h post-treatment, single-cell suspensions of Hep3B cells were collected, and live cell counts were performed. Subsequently, 2000 cells per well were plated into untreated 6-well plates. The cells were cultured in serum-free DMEM/F12 medium supplemented with 20 ng/mL bFGF, 20 ng/mL EGF, 2% B27, 1% N-2, and 1% penicillin-streptomycin (EGF, B27, and N-2 purchased from Invitrogen, Waltham, MA, USA; bFGF purchased from PeproTech, Rocky Hill, NJ, USA). Cells were observed every two days, and images were taken approximately one week after plating.
2.8. Annexin V Analysis
Hep3B cells were collected and seeded in 6-well plates at a density of 3.5 × 105 cells per well in 2 mL medium. The plates were incubated at 37 °C with 5% CO2 for 12–24 h. The experimental groups, including the Ad-VP3, Ad-Mock, and control groups, were established. In each group, Hep3B cells were treated with 100 MOI (3.5 × 107 PFU) of Ad-VP3 or Ad-Mock. At 24, 48, and 72 h post-treatment, one 6-well plate from each group was collected, and cells were dissociated into a single-cell suspension. The cells were resuspended in 500 μL of 1× Binding Buffer (from the Annexin V-FITC/PI Kit) and stained using the Annexin V-FITC/PI staining kit (purchased from BD Biosciences, Franklin Lakes, NJ, USA). Each sample was incubated with 5 μL of Annexin V-FITC and 5 μL of PI, and the samples were gently mixed. The cells were then incubated in the dark at room temperature for 15 min. After staining, cell apoptosis levels were assessed using both flow cytometry and fluorescence microscopy.
2.9. TMRM Staining Assay
Hep3B cells were collected and seeded in 6-well plates at a density of 3.5 × 105 cells per well in 2 mL medium and incubated at 37 °C with 5% CO2 for 24 h. Subsequently, Ad-VP3 and Ad-Mock were added at a multiplicity of infection (MOI) of 100 (3.5 × 107 PFU). At 24, 48, and 72 h post-infection, one 6-well plate from each group was collected, and cells were dissociated into a single-cell suspension. The cells were then incubated with 500 μL of TMRM dye, diluted at a 1:500 ratio (1 μL of TMRM and 500 μL of TMRM detection buffer), and incubated in the dark at 37 °C for 45 min. After incubation, the cell slides were inverted and observed under a fluorescence microscope. Following this treatment, the cell samples were transferred to flow cytometry tubes and analyzed using a flow cytometer.
2.10. JC-1 Staining Assay
JC-1 dye (purchased from Life Technologies, Carlsbad, CA, USA) was used to assess both qualitative and quantitative changes in mitochondrial membrane potential (MMP). Hep3B cells were collected and seeded in 6-well plates at a density of 3.5 × 105 cells per well in 2 mL medium and incubated at 37 °C with 5% CO2 for 24 h. Subsequently, Ad-VP3 and Ad-Mock were added at a multiplicity of infection (MOI) of 100 (3.5 × 107 PFU). At 24, 48, and 72 h post-infection, one 6-well plate from each group was collected, and cells were dissociated into a single-cell suspension. The cells were then incubated with 1 mL of JC-1 dye diluted at a 1:5000 ratio (0.2 μL JC-1 dye and 1 mL culture medium) and incubated in the dark at 37 °C for 15 min. After incubation, the cell slides were inverted and observed under a fluorescence microscope. Following this treatment, the cell samples were transferred to flow cytometry tubes and analyzed using a flow cytometer.
2.11. Western Blot
Hep3B cells were collected and seeded in 6-well plates at a density of 3.5 × 105 cells per well in 2 mL medium. The plates were then incubated at 37 °C with 5% CO2 for 12–24 h. The experimental groups included the Ad-VP3 group, Ad-Mock group, and control group. In each group, Hep3B cells were treated with 100 MOI (3.5 × 107 PFU) of Ad-VP3 or Ad-Mock. At 24, 48, and 72 h post-treatment, one 6-well plate was collected from each group, and the cells were harvested. Total protein was extracted using the Minute™ Total Protein Extraction Kit (purchased from Invent Biotechnologies, Eden Prairie, MN, USA). To each well, 200 μL of pre-chilled SC-001 cell lysis buffer (pre-added with protease and phosphatase inhibitors) was added. The cells were thoroughly lysed by grinding with a 1 mL pipette tip on ice. After lysis for 3–5 min on ice, the lysate was transferred to a pre-chilled column from the Invent kit. The sample was then centrifuged at 14,000 rpm for 1 min at 4 °C, and the column was discarded. The liquid in the collection tube was collected as the total protein solution. The protein concentration was determined using the BCA Protein Assay Kit (purchased from Beyotime Biotechnology, Shanghai, China). Protein quantification, concentration determination, and sample preparation were then performed. Western blotting was used to analyze the expression levels of Apoptin and apoptosis-related proteins, including Cleaved-caspase3, Caspase3, PARP, Survivin, and Bcl-2. In addition, migration and invasion-related proteins such as Slug, Vimentin, Snail, Twist1, and MMP-9, as well as stemness-related proteins ALDH1A1, KLF4, and SOX2, were also analyzed. All Western blot results were normalized to GAPDH and quantified relative to the control group. The anti-Apoptin antibody was purchased from Abcam (Cambridge, UK). The antibodies for Cleaved-caspase3, PARP, Caspase3, Survivin, GAPDH, Bcl-2, Snail, Slug, Twist1, Vimentin, MMP9, ALDH1A1, KLF4, and Sox2 were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.12. Wound Healing Assay
Hep3B cells were seeded at a density of 5 × 105 cells per well in a 6-well plate with 2 mL per well and cultured in a 37 °C, 5% CO2 incubator for 12–24 h. When the cells reached 80–90% confluence, three parallel lines were scratched in the center and on both sides of the well bottom using the tip of a 200 μL pipette, creating a rectangular area devoid of cells. The area of the rectangular scratch was recorded by capturing images under a microscope (denoted as 0 h). After discarding the original culture medium, serum-free medium was added, followed by the addition of 20 MOI (1 × 107 PFU) of recombinant adenovirus Ad-VP3 and Ad-Mock. Four hours later, MEM medium containing 2% serum was added. At 12 and 24 h post-infection, one 6-well plate from each group was selected. The same area photographed at 0 h was located under the microscope, and images were captured to calculate the change in scratch area.
The cell migration rate was calculated as:
2.13. Transwell Migration and Invasion Assay
Hep3B cells were collected and seeded at a density of 5 × 105 cells per well in a 6-well plate with 2 mL per well, and cultured in a 37 °C, 5% CO2 incubator for 12–24 h. Three groups were set up: Ad-VP3, Ad-Mock, and Control. Afterward, 100 MOI (5 × 107 PFU) of Ad-VP3 and Ad-Mock were added to the respective wells. At 24, 48, and 72 h post-treatment, cells were collected from one 6-well plate at each time point. Next, at 24, 48, and 72 h post-infection, a new 24-well plate was prepared. To the lower chamber of each well, 500 μL of 10% DMEM medium was added, while the upper chamber was seeded with 200 μL of cell suspension at the same cell density. After 24 h of incubation, non-invasive cells in the upper chamber were removed with a sterile cotton swab. In the dark, 60 μL of CCK-8 reagent was added to the lower chamber, and the Transwell and Biocoat chambers (Transwell Permeable Supports, Matrigel Matrix purchased from Corning, Corning, NY, USA) were reinserted into the wells. The plate was incubated at 37 °C in the dark for 4 h. After incubation, the 24-well plate was removed, and the chambers were taken out. The OD value at 450 nm was measured using a microplate reader. The chambers were then stained with crystal violet, and the number of cells that had migrated through the membrane was quantified by capturing images under a microscope.
2.14. Statistical Processing
Each experiment was repeated three times or more. The obtained data were analyzed using GraphPad Prism 10 software, and all data are expressed as the mean ± standard deviation. Statistical comparisons between two groups were performed using a t-test, while comparisons among three or more groups were conducted using one-way analysis of variance (ANOVA). A p-value > 0.05 indicates no statistical significance, * p < 0.05 indicates statistical significance, ** p < 0.01, and *** p < 0.001 indicate highly significant differences.
4. Discussion
Oncolytic virotherapy is being extensively developed in clinical settings, and adenoviruses were among the first gene therapy vectors evaluated in clinical trials, with their safety profiles widely assessed and documented [
26,
27]. Oncorine, approved in China in 2005, was the first oncolytic virus (OV) licensed for clinical use and serves as a landmark demonstration of the feasibility of oncolytic adenoviruses (oAds) in clinical application [
8]. The major advantages of oAds include their ability to elicit potent antitumor immune responses, exert anti-angiogenic effects, achieve high transgene expression, and synergize with other therapeutic modalities. Notably, oAds are the most frequently used OV type in clinical trials, accounting for approximately 42% of all studies [
26].
Building on this foundation, our group previously engineered the recombinant adenovirus Ad-VP3, based on a human adenovirus type 5 backbone, with the Apoptin gene under the control of a CMV promoter to enable robust expression of the tumor-selective apoptosis-inducing protein [
21]. Using the human hepatocellular carcinoma cell line Hep3B as a model, we evaluated the cytotoxic, antiproliferative, and pro-apoptotic effects of Ad-VP3. Initial CCK-8 and crystal violet assays revealed that Ad-Mock exerted minimal inhibitory effects on cell viability, whereas Ad-VP3 displayed clear tumor-suppressive activity in a time- and dose-dependent manner. Hoechst 33342 staining further showed that Ad-VP3-infected cells exhibited nuclear condensation and fragmentation, indicative of apoptosis. CFSE-based flow cytometry demonstrated that Ad-VP3 markedly inhibited Hep3B cell proliferation, while Ad-Mock had no such effect. Further cell cycle analysis revealed that Ad-VP3 increased the proportion of cells in G2/M phase, inducing cell cycle arrest—findings consistent with similar observations in other cancer models [
28]. Cancer stem cells (CSCs) play a central role in tumor initiation and progression. This subpopulation of cancer cells with stem cell-like properties is capable of self-renewal and differentiation into multiple cell lineages, and is critically involved in tumor onset, progression, therapeutic resistance, and metastasis [
25,
29]. With increasing evidence supporting the pivotal role of CSCs in cancer evolution and resistance to current therapies, the evaluation of novel treatment strategies must consider not only their ability to induce tumor regression but also their efficacy in targeting CSCs. With respect to stemness, Ad-VP3 exerted a marked inhibitory effect on both tumorsphere-forming capacity and the expression of stemness-associated proteins in Hep3B cells. We observed a pronounced reduction in the number of tumorspheres in the Ad-VP3 group, and a time-dependent decrease in the expression of the stemness markers ALDH1A1, KLF4, and Sox2 at 48 and 72 h post-infection. ALDH1A1 (aldehyde dehydrogenase 1 family member A1) is one of the most commonly used markers of CSCs; its high activity is closely associated with intracellular aldehyde metabolism, resistance to oxidative stress, and drug tolerance, and is often correlated with poor prognosis and increased risk of recurrence in various cancers [
30]. KLF4 (Krüppel-like factor 4) is a transcription factor involved in maintaining stem cell pluripotency and self-renewal [
31]. It is frequently overexpressed in CSCs and regulates cell cycle and differentiation through its downstream targets. Sox2 (SRY-box transcription factor 2) is another core stemness-related transcription factor that plays a key role in both embryonic stem cells and CSCs by sustaining an undifferentiated state and promoting proliferative potential [
32]. These findings suggest that Ad-VP3 may inhibit the self-renewal and differentiation capacity of CSCs by modulating the expression of stemness-related proteins, thereby attenuating tumor malignancy and reducing the risk of recurrence. This is consistent with other studies targeting the regulation of CSC stemness and further supports the role of Ad-VP3 in suppressing CSC-like properties.
Regarding cancer stemness, Ad-VP3 significantly impaired sphere-forming capacity and reduced the expression of stemness-associated proteins ALDH1A1, KLF4, and Sox2 in a time-dependent manner at 48 h and 72 h post-infection. This suggests that Ad-VP3 may suppress tumor stem cell self-renewal and differentiation by downregulating key stemness regulators, thereby potentially reducing tumor aggressiveness and recurrence risk. These findings align with prior research on targeting cancer stem cell properties as a therapeutic strategy.
A hallmark of cancer is the combination of uncontrolled proliferation and evasion of apoptosis [
33]. Apoptosis is essential for removing abnormal cells and maintaining tissue homeostasis, but tumor cells often escape this fate through diverse mechanisms, including downregulation of tumor suppressors (e.g., p53, PTEN) and overexpression of anti-apoptotic factors (e.g., Bcl-2, Bcl-XL, IAPs) [
34]. The loss of apoptotic control confers survival advantages, allowing accumulation of genetic mutations that drive tumor progression, aggressiveness, and poor differentiation. For example, p53 plays a central role in preventing oncogenic transformation, and its tumor-suppressive function is partly mediated through apoptosis induction [
35]. Loss of p53 function is a common selection during tumorigenesis. Similarly, overexpression of anti-apoptotic proteins such as Bcl-2 and Bcl-XL, often due to chromosomal translocations, contributes to cancer development and treatment resistance [
36]. Many chemotherapeutic agents fail in tumors harboring p53 loss or overexpressed anti-apoptotic proteins. Apoptin is a tumor-selective apoptosis inducer that acts independently of p53 and Bcl-2, enabling it to kill tumor cells with p53 mutations or loss of function.
Previous studies have reported that Ad-VP3 exerts a significant inhibitory effect on HepG2 cells, with the apoptosis rate reaching 43.02% at 72 h [
37]. It is known that HepG2 cells harbor wild-type p53, whereas Hep3B cells are p53-deficient [
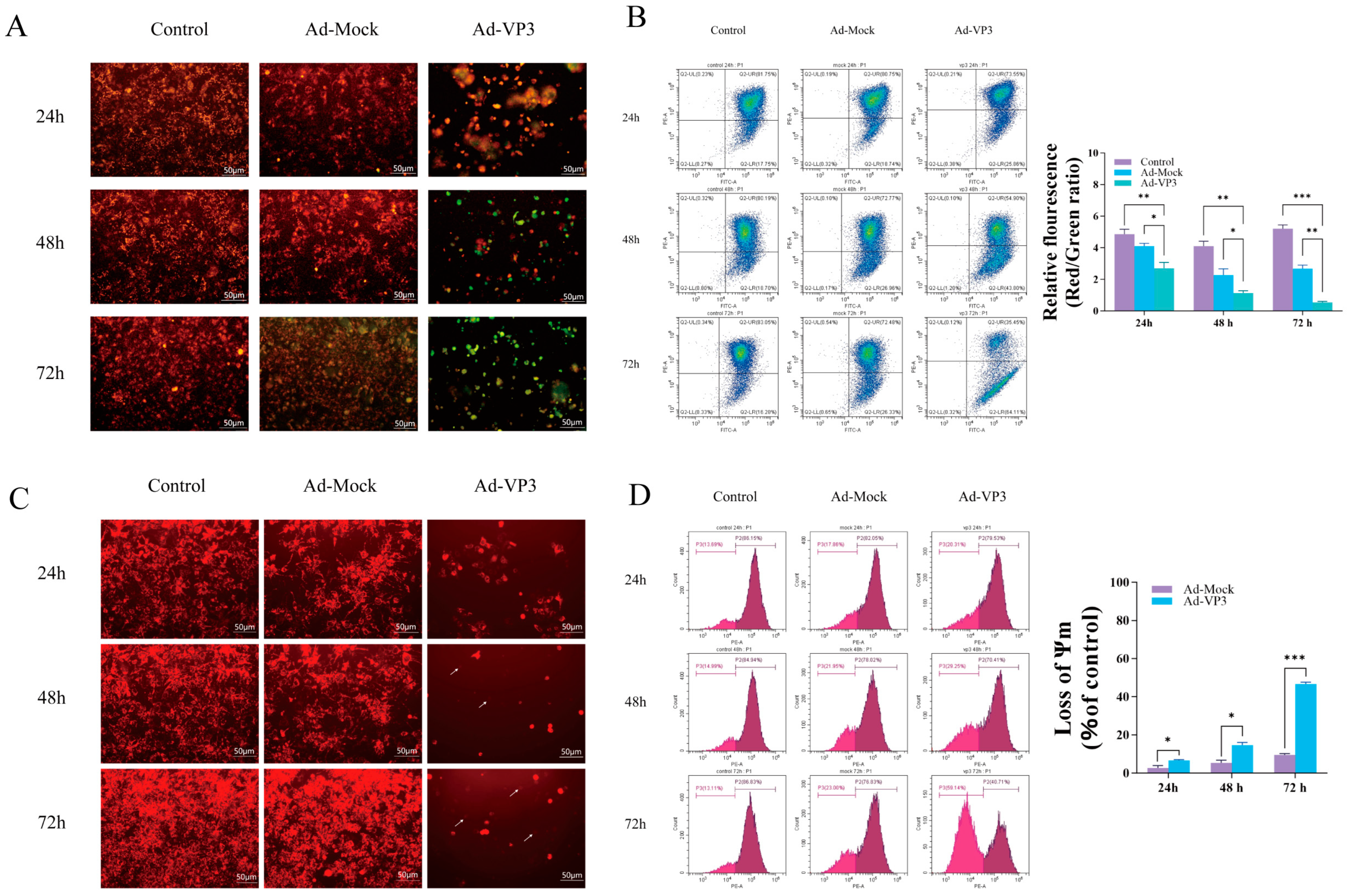
38]. Therefore, in the present study, we investigated whether the recombinant adenovirus Ad-VP3 could also induce apoptosis in p53-deficient Hep3B cells. In our study, AnnexinV-FITC/PI staining and flow cytometry confirmed that Ad-VP3 effectively induced apoptosis in Hep3B cells, with apoptotic cells evident early after infection and increasing markedly over time, reaching 44.25% at 72 h. This result stands in sharp contrast to the Ad-Mock group and strongly demonstrates that Ad-VP3 possesses p53-independent apoptosis-inducing activity. Loss of mitochondrial membrane potential (MMP) is a pivotal event in apoptosis. Using JC-1 and TMRM flow cytometry, we observed that Ad-VP3-induced MMP reduction correlated positively with treatment duration. This phenomenon suggests that Ad-VP3–induced apoptosis in Hep3B cells is most likely mediated through a mitochondria-dependent apoptotic pathway. MMP loss can lead to mitochondrial permeability transition pore opening, cytochrome c release, and activation of the downstream caspase cascade [
34]. While Ad-Mock caused only transient and minimal MMP changes—likely representing a mild stress response—Ad-VP3 induced sustained mitochondrial disruption.
Western blot analysis provided molecular evidence for the apoptotic mechanism: Ad-VP3 upregulated the executioner caspase cleaved-caspase-3 and its substrate cleaved-PARP, while downregulating procaspase-3, the survival factor Survivin, and the anti-apoptotic protein Bcl-2. Cleaved caspase-3 is a defining step in apoptosis execution, and increased cleaved-PARP further corroborates apoptosis progression. The downregulation of Survivin and Bcl-2 suggests suppression of survival signaling and dismantling of anti-apoptotic defenses. Together, these findings indicate that Ad-VP3 induces apoptosis in Hep3B cells primarily via the intrinsic pathway through dual modulation of pro- and anti-apoptotic proteins.
Tumor metastasis is a complex, multistep process that severely impacts patient prognosis [
39]. In this study, wound-healing, Transwell migration, and BioCoat invasion assays showed that Ad-VP3 significantly reduced Hep3B cell migration and invasion. Correspondingly, Western blotting revealed that Ad-VP3 downregulated EMT- and invasion-associated proteins, including Snail, Twist1, Slug, Vimentin, and MMP-9, in a time-dependent manner. Snail and Twist1 are key EMT transcription factors whose downregulation may restore epithelial marker expression (e.g., E-cadherin) and strengthen cell–cell adhesion, thereby reducing migratory and invasive potential. MMP-9, a matrix metalloproteinase that degrades extracellular matrix components, was also reduced, potentially limiting tumor cell dissemination [
40,
41,
42]. In summary, Ad-VP3 inhibits migration, invasion, sphere formation, and stemness in Hep3B cells by downregulating proteins such as Snail, Twist1, MMP-9, ALDH1A1, KLF4, and Sox2.
This study has limitations. While we identified changes in key protein expression, the upstream regulatory pathways through which Ad-VP3 exerts these effects remain unclear. Future work should elucidate these signaling cascades and investigate potential intermediary regulators. Moreover, the current study was conducted exclusively in vitro; in vivo validation in animal models is essential to assess therapeutic efficacy and safety, thereby providing a more robust foundation for clinical translation. Despite these limitations, our findings provide strong theoretical support for further development of Ad-VP3 as a potential therapeutic agent for hepatocellular carcinoma and highlight its promise as a novel cancer treatment strategy.