Identification of a Novel Genotype of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in Northern Hebei Province, China
Abstract
1. Introduction
2. Materials and Methods
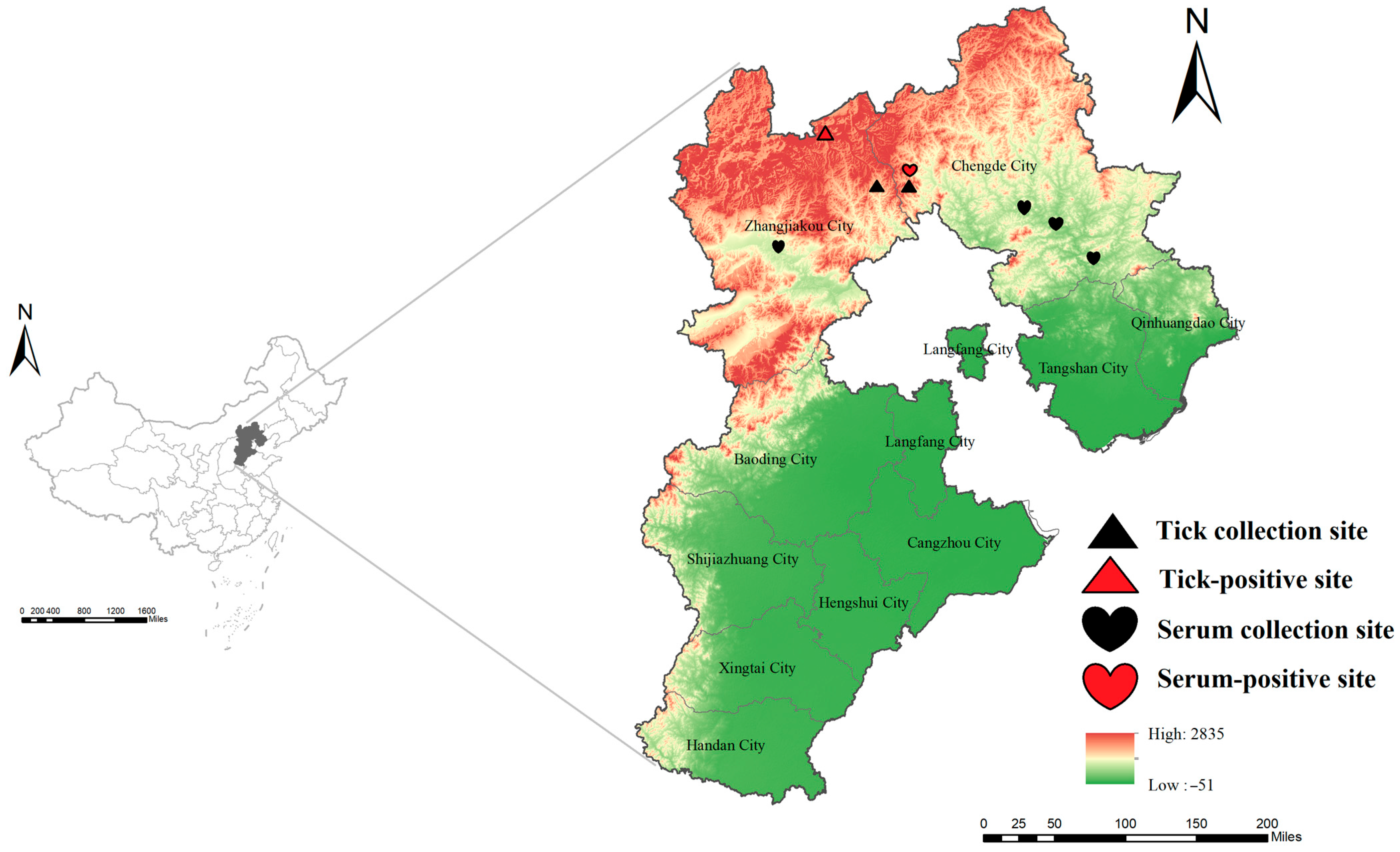
2.1. Sample Collection
2.2. RNA Extraction and qRT-PCR Detection of SFTSV
2.3. Virus Isolation and Whole-Genome Sequencing
2.4. Phylogenetic and Evolutionary Analysis
2.5. Reassortment and Recombination
2.6. Phylogenetic Analyses by BEAST
3. Results
3.1. Detection of SFTSV RNA in Patients and Ticks
3.2. Genomic Characteristics of SFTSV Isolates
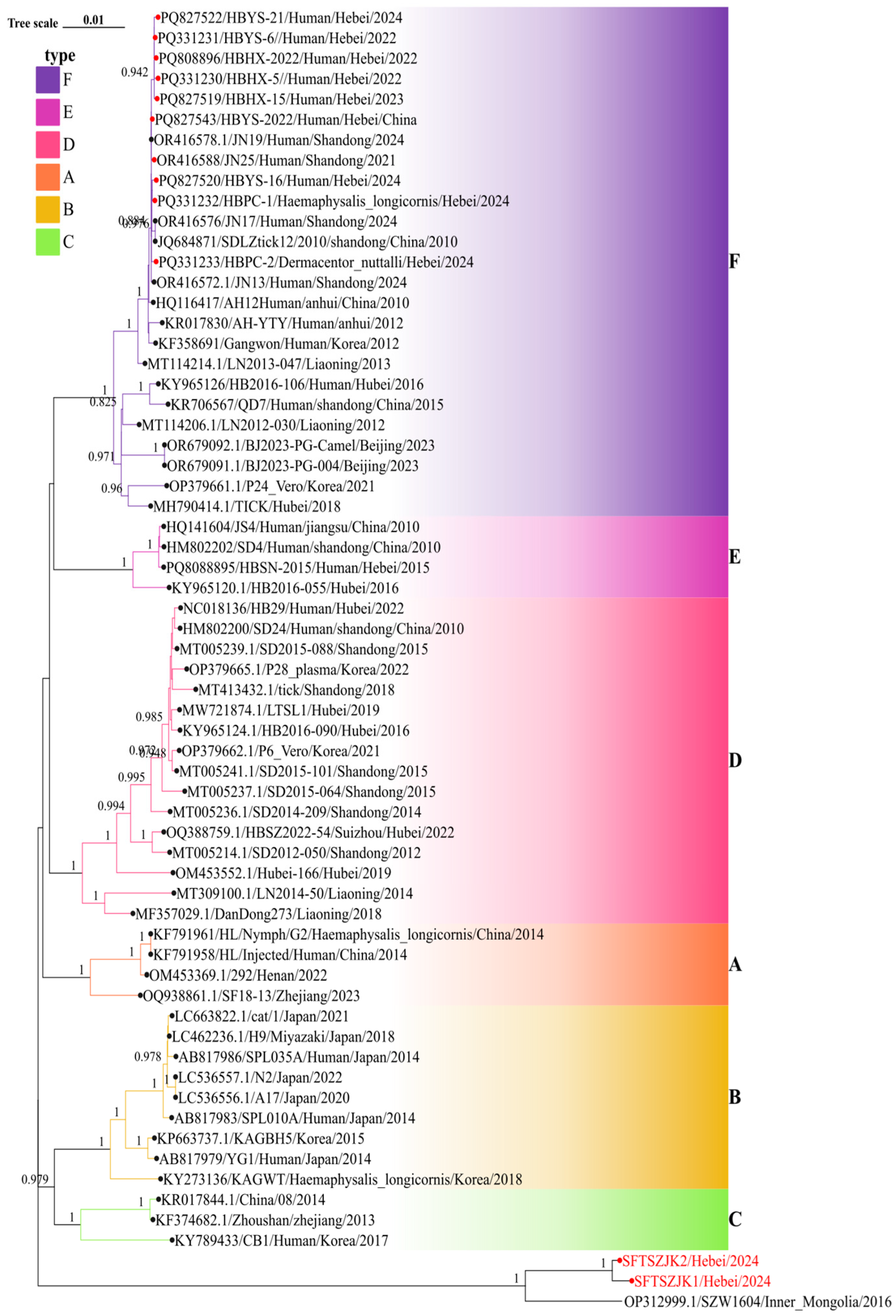
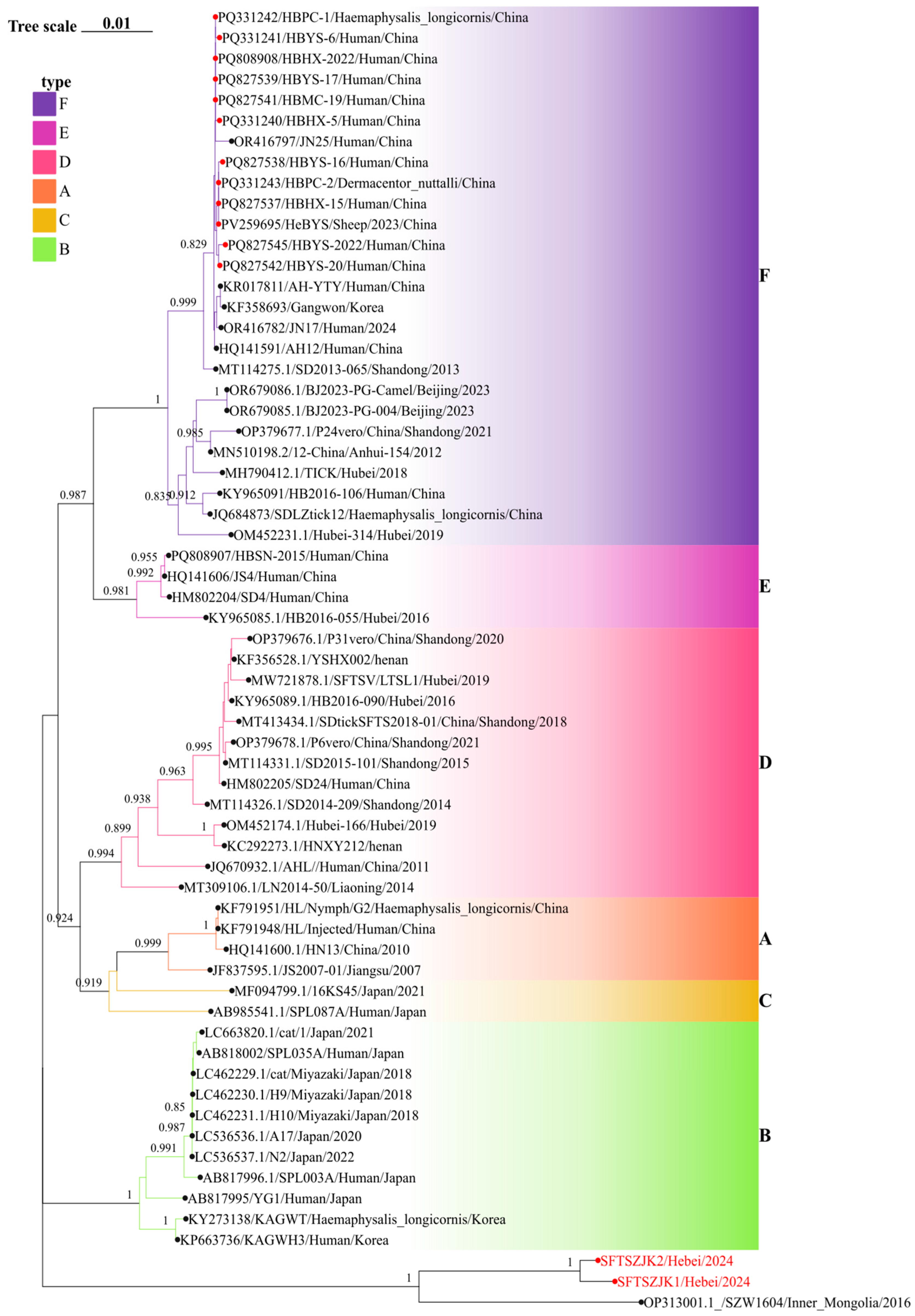
3.3. Phylogenetic Analysis of SFTSV
3.4. Recombination Analysis
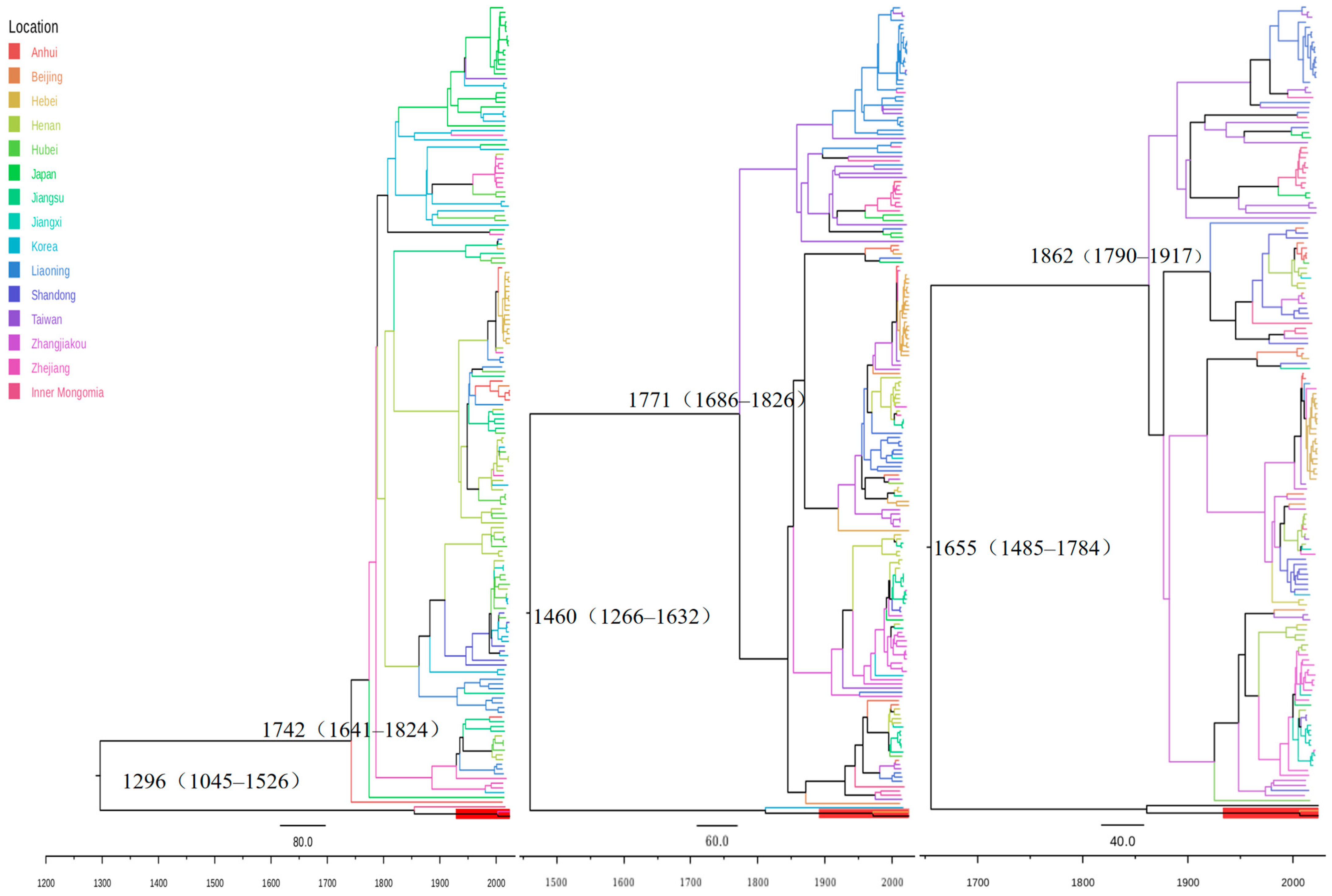
3.5. Evolutionary History and Divergence Time Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kamthania, M. A new emerging pandemic of severe fever with thrombocytopenia syndrome (SFTS). Virusdisease 2021, 32, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tian, T.; Li, A.; Du, S.; Wang, S.; Li, D.; Huang, X.; Li, J. Epidemiological characteristics of human-to-human transmission of severe fever with thrombocytopenia syndrome in China from 1996 to 2023. PLoS Negl. Trop. Dis. 2025, 19, e0013283. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Li, A.; Wang, S.; Li, D. Epidemiological Characteristics of Severe Fever with Thrombocytopenia Syndrome from 2010 to 2019 in Mainland China. Int. J. Environ. Res. Public Health 2021, 18, 3092. [Google Scholar] [CrossRef]
- Kim, K.H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.H.; Choe, P.G.; Kim, N.J.; Lee, J.K.; Oh, M.D. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 2013, 19, 1892–1894. [Google Scholar] [CrossRef]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef]
- Tran, X.C.; Yun, Y.; Van An, L.; Kim, S.H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.H.; Lee, K.H. Endemic Severe Fever with Thrombocytopenia Syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029–1031. [Google Scholar] [CrossRef]
- Tian, B.; Qu, D.; Sasaki, A.; Chen, J.; Deng, B. Acute pancreatitis in patients with severe fever with thrombocytopenia syndrome virus infection. Pancreatology 2020, 20, 1631–1636. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, B.; Yan, H.; Wu, A.L.; Yao, W.W.; Chen, K.; Pan, J.H.; Li, Z.X.; Mao, H.Y.; Zhang, Y.J. Patient with severe fever with thrombocytopenia syndrome virus infection and central nervous system disturbance in Dongyang, Zhejiang Province, China, 2017. Virol. J. 2019, 16, 129. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Hu, W.; Wu, J.; Wang, Y.; Mei, L.; Walker, D.H.; Ren, J.; Wang, Y.; Yu, X.-J. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012, 12, 156–160. [Google Scholar] [CrossRef]
- Seo, J.-W.; Kim, D.; Yun, N.; Kim, D.-M. Clinical Update of Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 1213. [Google Scholar] [CrossRef]
- Chen, H.; Hu, K.; Zou, J.; Xiao, J. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int. J. Infect. Dis. 2013, 17, e206–e208. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Jiang, Y.; Liu, X.; Wang, B.; Shi, J.; Su, Z.; Wang, H.; Wang, T.; Tang, S.; Liu, H.; et al. A Cluster of Symptomatic and Asymptomatic Infections of Severe Fever with Thrombocytopenia Syndrome Caused by Person-to-Person Transmission. Am. J. Trop. Med. Hyg. 2017, 97, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, Y.; Li, L.; Geng, M.; Zheng, Y.; Zhang, X.; Han, Z.; Zhang, Y.; Xu, Y.; Han, X.; et al. Identification of Endemic Region for Severe Fever with Thrombocytopenia Syndrome in an Alluvial Plain of Hebei Province, China. Viruses 2025, 17, 854. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, L.; Wu, W.; Liu, Y.; Huang, X.; Li, C.; Liang, M. Molecular evolution and genetic diversity analysis of SFTS virus based on next-generation sequencing. Biosaf. Health 2021, 3, 105–115. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Wang, Z.; He, B.; Wang, S.; Wei, F.; Tu, C.; Liu, Q. The first molecular evidence of severe fever with thrombocytopenia syndrome virus in ticks in Jilin, Northeastern China. Ticks Tick-Borne Dis. 2016, 7, 1280–1283. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Chen, F.; Liu, Z.; Wang, W.; Yang, S.; Qian, K.; Zhan, Z.; Guo, Y.; Li, H.; et al. Severe fever with thrombocytopenia syndrome virus was found in Northern Jiangxi Province, China. Front. Microbiol. 2024, 15, 1500146. [Google Scholar] [CrossRef]
- Thawng, C.N.; Smith, G.B. Transcriptome software results show significant variation among different commercial pipelines. BMC Genom. 2023, 24, 662. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Y.; Han, X.; Han, Z.; Zhang, Y.; Xu, Y.; Li, Q. Genetic diversity and molecular evolution of Seoul virus in Hebei province, China. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2023, 114, 105503. [Google Scholar] [CrossRef]
- Samson, S.; Lord, É.; Makarenkov, V. SimPlot: A Python application for representing sequence similarity and detecting recombination. Bioinformatics 2022, 38, 3118–3120. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Hill, V.; Baele, G. Bayesian Estimation of Past Population Dynamics in BEAST 1.10 Using the Skygrid Coalescent Model. Mol. Biol. Evol. 2019, 36, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Stephan, K.E. Markov chain Monte Carlo methods for hierarchical clustering of dynamic causal models. Hum. Brain Mapp. 2021, 42, 2973–2989. [Google Scholar] [CrossRef]
- Sheng, R.; Cheng, T.; Wang, Y.; Wen, H. Molecular evolution and geographic migration of severe fever with thrombocytopenia syndrome virus in Asia. PLoS Pathog. 2025, 21, e1012970. [Google Scholar] [CrossRef]
- Chevenet, F.; Fargette, D.; Bastide, P.; Vitré, T.; Guindon, S. EvoLaps 2: Advanced phylogeographic visualization. Virus Evol. 2024, 10, vead078. [Google Scholar] [CrossRef]
- Liu, J.-W.; Zhao, L.; Luo, L.-M.; Liu, M.-M.; Sun, Y.; Su, X.; Yu, X.-J. Molecular Evolution and Spatial Transmission of Severe Fever with Thrombocytopenia Syndrome Virus Based on Complete Genome Sequences. PLoS ONE 2016, 11, e0151677. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, G.; Jiang, L.; Wang, P.; Zhang, S.; Zheng, X.; Li, Y. Metatranscriptomics Reveals the Diversity of the Tick Virome in Northwest China. Microbiol. Spectr. 2022, 10, e0111522. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, J.; Yao, M.; Sun, Y.; Xu, H.; Liu, C.; Chen, H.; Guo, J.; Nie, X.; He, L.; et al. Isolation and Identification of Severe Fever with Thrombocytopenia Syndrome Virus from Farmed Mink in Shandong, China. Transbound. Emerg. Dis. 2024, 2024, 9604673. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, L.; Ojcius, D.M.; Lou, X.; Wang, C.; Feng, C.; Sun, Y.; Wang, Z.; Li, S.; Zhang, Y. Characterization of severe fever with thrombocytopenia syndrome in rural regions of Zhejiang, China. PLoS ONE 2014, 9, e111127. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, Q.; Fu, Y.; Li, S.; Zhang, Z.; Zhang, Z.; Yu, X. Molecular identification of severe fever with thrombocytopenia syndrome viruses from tick and bitten patient in Southeast China. Virol. J. 2020, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, S.; Shi, J.; Su, Z.; Li, M.; Zhang, W.; Li, M.; Hu, Z.; Peng, C.; Zheng, X.; et al. Isolation, characterization, and phylogenic analysis of three new severe fever with thrombocytopenia syndrome bunyavirus strains derived from Hubei Province, China. Virol. Sin. 2017, 32, 89–96. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.; Zhang, Z.; Man, S.; Li, X.; Zhang, W.; Zhang, C.; Cheng, X. Phylogeographic analysis of severe fever with thrombocytopenia syndrome virus from Zhoushan Islands, China: Implication for transmission across the ocean. Sci. Rep. 2016, 6, 19563. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Liu, W.; Bowden, T.A.; Cui, N.; Zhuang, L.; Liu, K.; Zhang, Y.-Y.; Cao, W.-C.; Pybus, O.G. Evolutionary and molecular analysis of the emergent severe fever with thrombocytopenia syndrome virus. Epidemics 2013, 5, 1–10. [Google Scholar] [CrossRef]
- Oh, S.-S.; Chae, J.-B.; Kang, J.-G.; Kim, H.-C.; Chong, S.-T.; Shin, J.-H.; Hur, M.-S.; Suh, J.-H.; Oh, M.-D.; Jeong, S.-M.; et al. Detection of Severe Fever with Thrombocytopenia Syndrome Virus from Wild Animals and Ixodidae Ticks in the Republic of Korea. Vector Borne Zoonotic Dis. 2016, 16, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, L.; Du, Y.; Wu, W.; Wang, H.; Su, J.; Tang, X.; Liu, Q.; Yang, Y.; Jiang, Y.; et al. The evolutionary history and spatiotemporal dynamics of the fever, thrombocytopenia and leukocytopenia syndrome virus (FTLSV) in China. PLoS Negl. Trop. Dis. 2014, 8, e3237. [Google Scholar] [CrossRef]
- Freire, C.C.M.; Iamarino, A.; Soumaré, P.O.L.; Faye, O.; Sall, A.A.; Zanotto, P.M.A. Reassortment and distinct evolutionary dynamics of Rift Valley Fever virus genomic segments. Sci. Rep. 2015, 5, 11353. [Google Scholar] [CrossRef]
- Park, J.Y.; Sivasankar, C.; Kirthika, P.; Prabhu, D.; Lee, J.H. Non-Structural Protein-W61 as a Novel Target in Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV): An In-Vitro and In-Silico Study on Protein-Protein Interactions with Nucleoprotein and Viral Replication. Viruses 2023, 15, 1963. [Google Scholar] [CrossRef]
- Xing, X.; Guan, X.; Liu, L.; Zhan, J.; Jiang, H.; Liu, L.; Li, G.; Xiong, J.; Tan, L.; Xu, J.; et al. Natural Transmission Model for Severe Fever With Thrombocytopenia Syndrome Bunyavirus in Villages of Hubei Province, China. Medicine 2016, 95, e2533. [Google Scholar] [CrossRef]
| SFTSV Strain | Source | GenBank Accession No. (L/M/S) | Sequence Similarity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L Segment | M Segment | S Segment | |||||||
| Nt | RDRP | Nt | GP | Nt | NSs | NP | |||
| SFTSZJK1/Hebei/2024 | Haemaphysalis verticalis | PX226566/PX226564/ PX226562 | 88.53 | 97.36 | 87.44 | 94.13 | 89.20 | 91.47 | 96.36 |
| SFTSZJK2/Hebei/2024 | Haemaphysalis verticalis | PX226567/PX226565/ PX226563 | 88.24 | 97.41 | 87.38 | 94.04 | 89.55 | 91.47 | 96.36 |
| Gene | L | M |
|---|---|---|
| Recombinant Sequence(s) | SFTSZJK1/Hebei/2024 | SFTSZJK2/Hebei/2024 |
| Beginning | 66 | 1607 |
| Ending | 6322 | 1730 |
| RDPRCS | 0.446 | 0.436 |
| RDP | 8.39 × 10−6 | 4.02 × 10−4 |
| GENECONV | NS | NS |
| Bootscan | NS | NS |
| Maxchi | NS | 1.42 × 10−4 |
| Chimaera | NS | NS |
| SiSscan | NS | NS |
| 3Seq | 5.50 × 10−4 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, M.; Wang, X.; Wei, Y.; Li, Y.; Cai, Y.; Li, J.; Jiang, C.; Zhang, X.; Wu, W.; Guo, N.; et al. Identification of a Novel Genotype of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in Northern Hebei Province, China. Viruses 2025, 17, 1534. https://doi.org/10.3390/v17121534
Geng M, Wang X, Wei Y, Li Y, Cai Y, Li J, Jiang C, Zhang X, Wu W, Guo N, et al. Identification of a Novel Genotype of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in Northern Hebei Province, China. Viruses. 2025; 17(12):1534. https://doi.org/10.3390/v17121534
Chicago/Turabian StyleGeng, Minghao, Xueqi Wang, Yamei Wei, Yan Li, Yanan Cai, Jiandong Li, Caixiao Jiang, Xinyang Zhang, Wentao Wu, Nana Guo, and et al. 2025. "Identification of a Novel Genotype of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in Northern Hebei Province, China" Viruses 17, no. 12: 1534. https://doi.org/10.3390/v17121534
APA StyleGeng, M., Wang, X., Wei, Y., Li, Y., Cai, Y., Li, J., Jiang, C., Zhang, X., Wu, W., Guo, N., Han, G., Han, X., Liu, T., Li, Q., & Wang, S. (2025). Identification of a Novel Genotype of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in Northern Hebei Province, China. Viruses, 17(12), 1534. https://doi.org/10.3390/v17121534





