Viral Respiratory Infections in Djibouti: Insights from Two Years of Pilot Surveillance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Setting
2.2. Collection and Transportation of Clinical Samples
2.3. Nucleic Acid Extraction and Screening of Respiratory Viruses
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics of Enrolled Patients
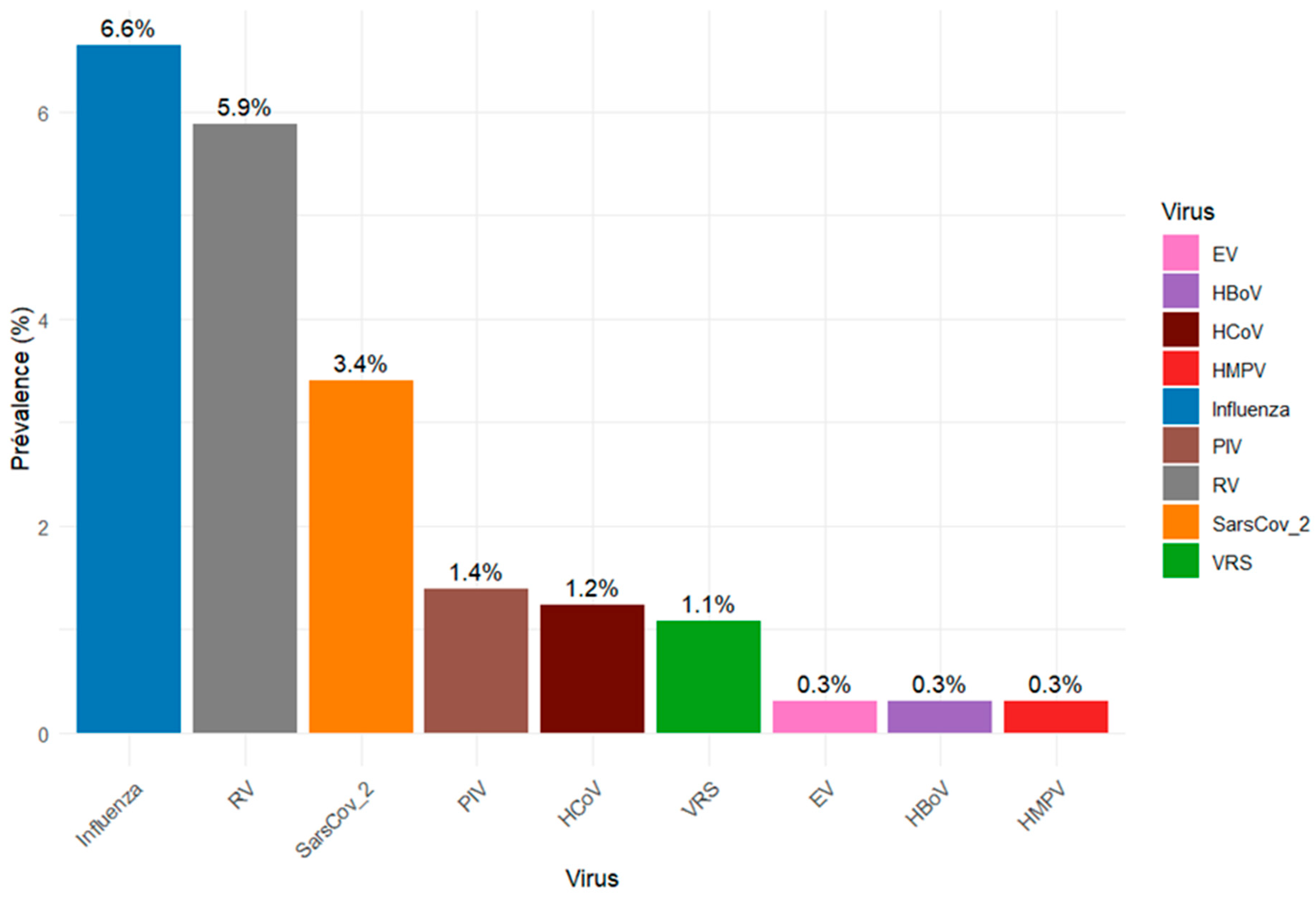
3.2. Detection of Respiratory Viruses
3.3. Clinical and Demographic Characteristics of Patients with Confirmed VRI
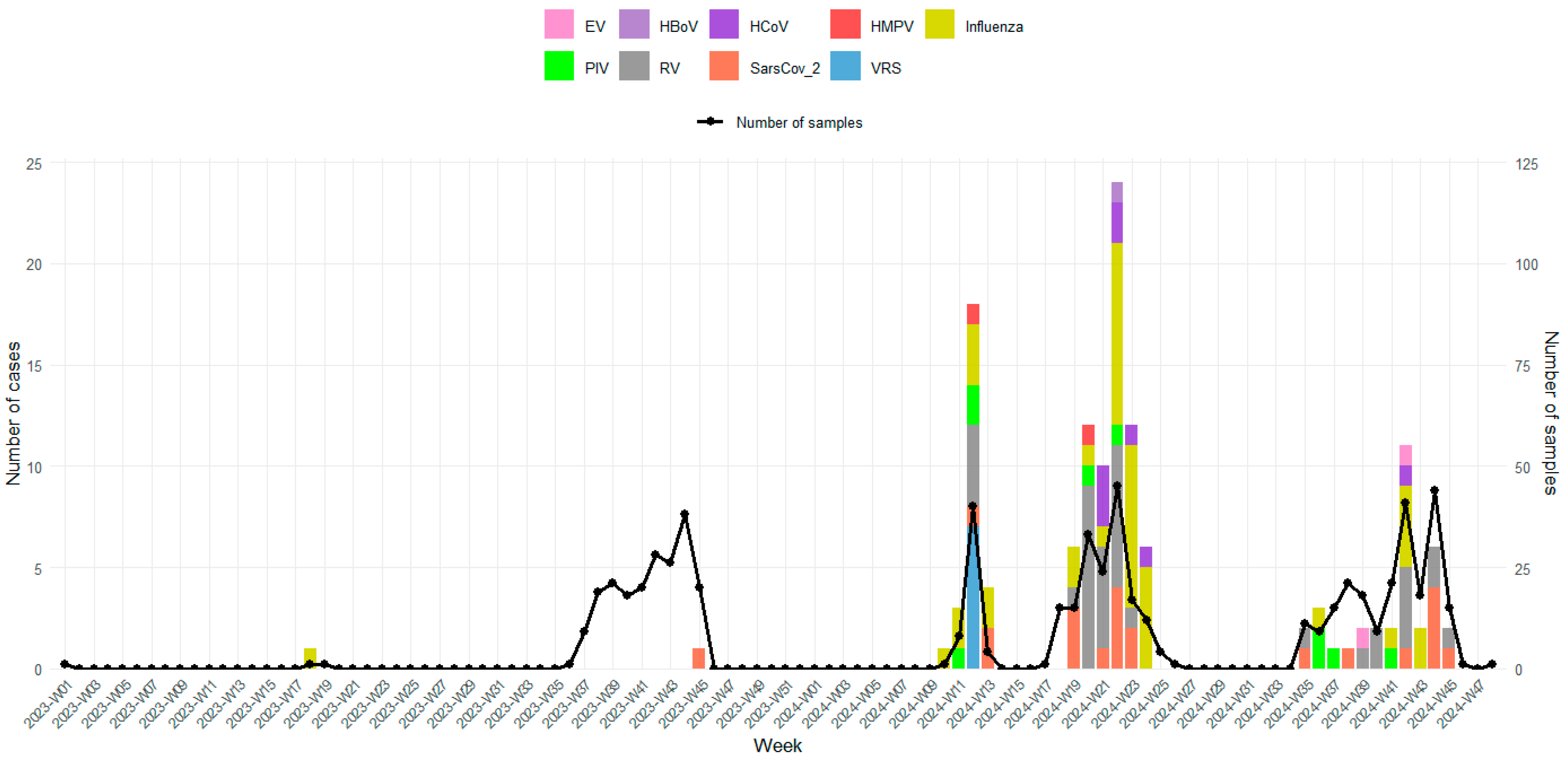
3.4. Temporal Distribution of Viral Confirmed Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Alberton, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- García-Arroyo, L.; Prim, N.; Del Cuerpo, M.; Marín, P.; Roig, M.C.; Esteban, M.; Labeaga, R.; Marti, N.; Berengua, C.; Gich, I.; et al. Prevalence and seasonality of viral respiratory infections in a temperate climate region: A 24-year study (1997–2020). Influenza Other Respir. Viruses 2022, 16, 756–766. [Google Scholar] [CrossRef]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L.; et al. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]
- Williams, B.G.; Gouws, E.; Boschi-Pinto, C.; Bryce, J.; Dye, C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2002, 2, 25–32. [Google Scholar] [CrossRef]
- Elhakim, T.S.; Abdul, H.S.; Romero, C.P.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. CP 2020, 13, e239489. [Google Scholar] [CrossRef]
- Elhakim, M.; Tourab, S.B.; Salem, F.; Van De Weerdt, R. Epidemiology of the first and second waves of COVID-19 pandemic in Djibouti and the vaccination strategy developed for the response. BMJ Glob. Health 2022, 7 (Suppl. 3), e008157. [Google Scholar] [CrossRef]
- WHO. Global Epidemiological Surveillance Standards for Influenza; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Jallow, M.M.; Diagne, M.M.; Sagne, S.N.; Tall, F.; Diouf, J.B.N.; Boiro, D.; Mendy, M.P.; Ndiaye, N.K.; Kiori, D.; Sy, S.; et al. Respiratory syncytial virus in pediatric patients with severe acute respiratory infections in Senegal: Findings from the 2022 sentinel surveillance season. Sci. Rep. 2023, 13, 20404. [Google Scholar] [CrossRef]
- Bechini, A.; Salvati, C.; Bonito, B.; Del Riccio, M.; Stancanelli, E.; Bruschi, M.; Ionita, G.; Iamarino, J.A.; Bentivegna, D.; Buscemi, P.; et al. Costs and healthcare utilisation due to respiratory syncytial virus disease in paediatric patients in Italy: A systematic review. Public Health 2024, 227, 103–111. [Google Scholar]
- Houde, L.; Law, A.W.; Averin, A.; Weycker, D.; Cane, A.; Pugh, S.; Shea, K.M. Annual clinical and economic burden of medically attended lower respiratory tract illnesses due to respiratory syncytial virus among US infants aged < 12 months. J. Infect. Dis. 2005, 231, 1318–1326. [Google Scholar]
- Hanage, W.P.; Schaffner, W. Burden of acute respiratory infections caused by influenza virus, respiratory syncytial virus, and SARS-CoV-2 with consideration of older adults: A narrative review. Infect. Dis. Ther. 2025, 14 (Suppl. 1), 5–37. [Google Scholar] [CrossRef]
- Fall, A.; Dieng, A.; Wade, S.F.; Diop, A.; Diouf, J.B.N.; Boiro, D.; Keita, Y.; Sylla, A.; Ndiaye, O.; Boye, C.S.B.; et al. Children under five years of age in Senegal: A group highly exposed to respiratory viruses infections. Virol. Res. Rev. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Singleton, R.J.; Bulkow, L.R.; Miernyk, K.; DeByle, C.; Pruitt, L.; Hummel, K.B.; Bruden, D.; Englund, J.A.; Anderson, L.J.; Lucher, L.; et al. Viral respiratory infections in hospitalized and community control children in Alaska. J. Med. Virol. 2010, 82, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Juliana, A.E.; Tang, M.J.; Kemps, L.; Noort, A.C.; Hermelijn, S.; Plötz, F.B.; Wilschut, J. Viral causes of severe acute respiratory infection in hospitalized children and association with outcomes: A two-year prospective surveillance study in Suriname. PLoS ONE 2021, 16, e0247000. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Nadjm, B.; Thomas, S.; Agustiningsih; Malik, S.; Nguyen, D.N.T.; Vu, D.V.T.; Nguyen, K.V.; Nguyen, C.V.V.; Nguyen, L.T.; et al. Viral and atypical bacterial aetiologies of infection in hospitalised patients admitted with clinical suspicion of influenza in Thailand, Vietnam and Indonesia. Influenza Other Respir. Viruses 2015, 9, 315–322. [Google Scholar] [CrossRef]
- Ahmed, J.A.; Katz, M.A.; Auko, E.; Njenga, M.K.; Weinberg, M.; Kapella, B.K.; Burke, H.; Nyoka, R.; Gichangi, A.; Waiboci, L.W.; et al. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007–2010. BMC Infect. Dis. 2012, 12, 7. [Google Scholar] [CrossRef]
- Lekana-Douki, S.E.; Nkoghe, D.; Drosten, C.; Ngoungou, E.B.; Drexler, J.F.; Leroy, E.M. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infect. Dis. 2014, 14, 373. [Google Scholar] [CrossRef]
- Jarju, S.; Greenhalgh, K.; Wathuo, M.; Banda, M.; Camara, B.; Mendy, S.; Sowe, G.; Dahaba, P.O.; Jammeh, L.; Bajinka, Y.; et al. Viral etiology, clinical features and antibiotic use in children < 5 years of age in The Gambia presenting with influenza-like illness. Pediatr. Infect. Dis. J. 2020, 39, 925–930. [Google Scholar]
- Tchatchouang, S.; Kenmoe, S.; Nzouankeu, A.; Njankouo-Ripa, M.; Penlap, V.; Donkeng, V.; Pefura-Yone, E.; Fonkoua, M.; Eyangoh, S.; Njouom, R. Viral etiology of lower respiratory tract infections in adults in the pre-COVID-19 pandemic era: A cross-sectional study in a single center experience from Cameroon. Health Sci. Rep. 2023, 6, e1234. [Google Scholar] [CrossRef]
- Niang, M.N.; Diop, O.M.; Sarr, F.D.; Goudiaby, D.; Malou-Sompy, H.; Ndiaye, K.; Vabret, A.; Baril, L. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: The EVIRA project. J. Med. Virol. 2010, 82, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.; Prabhakaran, A.O.; Choudhary, M.L.; Biswas, D.; Koul, P.; Kaveri, K.; Dar, L.; Mamta, C.S.; Jadhav, S.; Bhardwaj, S.D.; et al. Multisite surveillance for influenza and other respiratory viruses in India: 2016–2018. PLOS Glob. Public Health 2022, 2, e0001001. [Google Scholar] [CrossRef]
- Bellei, N.; Carraro, E.; Perosa, A.; Watanabe, A.; Arruda, E.; Granato, C. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J. Med. Virol. 2008, 80, 1824–1827. [Google Scholar] [CrossRef]
- Jallow, M.M.; Barry, M.A.; Ndiaye, N.K.; Touré, C.T.; Talla, C.; Kiori, D.; Sagne, S.N.; Sy, S.; Goudiaby, D.; Niang, M.N.; et al. Genetic and antigenic characterization of influenza A (H3N2) virus after 13 consecutive years of influenza surveillance in Senegal, 2010–2022. J. Med. Virol. 2024, 96, e70010. [Google Scholar] [CrossRef]
- Heraud, J.M.; Njouom, R.; Rousset, D.; Kadjo, H.; Caro, V.; Ndiaye, M.N.; Victoir, K.; Collard, J.; Orelle, A.; Yekwa, E.L.; et al. Spatiotemporal circulation of influenza viruses in 5 African countries during 2008–2009: A collaborative study of the Institut Pasteur International Network. J. Infect. Dis. 2012, 206 (Suppl. 1), S5–S13. [Google Scholar] [CrossRef]
- Tallo, V.L.; Kamigaki, T.; Tan, A.G.; Pamaran, R.R.; Alday, P.P.; Mercado, E.S.; Javier, J.B.; Oshitani, H.; Olveda, R.M. Estimating influenza outpatients’ and inpatients’ incidences from 2009 to 2011 in a tropical urban setting in the Philippines. Influenza Other Respir. Viruses 2014, 8, 159–168. [Google Scholar] [CrossRef]
- Boivin, G.; Osterhaus, A.D.; Gaudreau, A.; Jackson, H.C.; Groen, J.; Ward, P. Roleof Picornaviruses in Flu-Like Illnesses of Adults Enrolled in AnOseltamivir Treatment Study Who Had No Evidence of Influenza VirusInfection. J. Clin. Microbiol. 2002, 40, 330–334. [Google Scholar] [CrossRef]
- Vega-Piris, L.; Carretero, S.G.; Mayordomo, J.L.; Zarzuelo, M.B.R.; Río, V.Á.; García, V.G.; Vazquez, M.G.; Rodriguez, M.D.C.G.; Basile, L.; Gonzalez-Coviella, N.L.; et al. Severity of respiratory syncytial virus compared with SARS-CoV-2 and influenza among hospitalised adults ≥ 65 years. J. Infect. 2024, 89, 106292. [Google Scholar] [CrossRef]
- Pierangeli, A.; Gentile, M.; Di Marco, P.; Pagnotti, P.; Scagnolari, C.; Trombetti, S.; Russo, L.L.; Tromba, V.; Moretti, C.; Midulla, F.; et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J. Med. Virol. 2007, 79, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Correia, W.; Dorta-Guerra, R.; Sanches, M.; Valladares, B.; de Pina-Araújo, I.I.M.; Carmelo, E. Epidemiological and clinical profile of viral respiratory infections in children under 5 years at pre-and post-COVID-19 era in Praia, Cabo Verde. Trop. Med. Int. Health 2025, 30, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.M.; Fall, A.; Kiori, D.; Sy, S.; Goudiaby, D.; Barry, M.A.; Fall, M.; Niang, M.N.; Dia, N. Epidemiological, clinical and genotypic features of human Metapneumovirus in patients with influenza-like illness in Senegal, 2012 to 2016. BMC Infect. Dis. 2019, 19, 457. [Google Scholar] [CrossRef]
- Dia, N.; Diene Sarr, F.; Thiam, D.; Faye Sarr, T.; Espié, E.; OmarBa, I.; Coly, M.; Niang, M.; Richard, V.; 4S Network Group. Influenza-like illnesses in Senegal: Not only focus on influenza viruses. PLoS ONE 2014, 9, e93227. [Google Scholar] [CrossRef] [PubMed]
- Faye, M.N.; Barry, M.A.; Jallow, M.M.; Wade, S.F.; Mendy, M.P.; Sy, S.; Fall, A.; Kiori, D.E.; Ndiaye, N.K.; Goudiaby, D.; et al. Epidemiology of non-SARS-CoV2 human coronaviruses (HCoVs) in people presenting with influenza-like illness (ILI) or severe acute respiratory infections (SARI) in Senegal from 2012 to 2020. Viruses 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Masse, S.; Capai, L.; Villechenaud, N.; Blanchon, T.; Charrel, R.; Falchi, A. Epidemiology and clinical symptoms related to seasonal coronavirus identified in patients with acute respiratory infections consulting in primary care over six influenza seasons (2014–2020) in France. Viruses 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Öhrmalm, L.; Malinovschi, A.; Wong, M.; Levinson, P.; Janson, C.; Broliden, K.; Alving, K. Presence of rhinovirus in the respiratory tract of adolescents and young adults with asthma without symptoms of infection. Respir. Med. 2016, 115, 1–6. [Google Scholar] [CrossRef]
- Fall, A.; Dia, N.; Cisse, E.H.A.K.; Kiori, D.E.; Sarr, F.D.; Sy, S.; Goudiaby, D.; Richard, V.; Niang, M.N. Epidemiology and molecular characterization of human respiratory syncytial virus in Senegal after four consecutive years of surveillance, 2012–2015. PLoS ONE 2016, 11, e0157163. [Google Scholar] [CrossRef]
- Abrams, E.M.; Doyon-Plourde, P.; Davis, P.; Brousseau, N.; Irwin, A.; Siu, W.; Killikelly, A. Burden of disease of respiratory syncytial virus in infants, young children and pregnant women and people. Can. Commun. Dis. Rep. 2024, 50, 1–15. [Google Scholar] [CrossRef]
- Cacho, F.; Gebretsadik, T.; Anderson, L.J.; Chappell, J.D.; Rosas-Salazar, C.; Ortiz, J.R.; Hartert, T. Respiratory syncytial virus prevalence and risk factors among healthy term infants, United States. Emerg. Infect. Dis. 2024, 30, 2199. [Google Scholar] [CrossRef]
- Kenmoe, S.; Tchendjou, P.; Vernet, M.A.; Moyo-Tetang, S.; Mossus, T.; Njankouo-Ripa, M.; Kenne, A.; Beng, V.P.; Vabret, A.; Njouom, R. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011–2013. Influenza Other Respir. Viruses 2016, 10, 386–393. [Google Scholar] [CrossRef]
- Jacques, J.; Bouscambert-Duchamp, M.; Moret, H.; Carquin, J.; Brodard, V.; Lina, B.; Motte, J.; Andréoletti, L. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J. Clin. Virol. 2006, 35, 463–466. [Google Scholar] [CrossRef]
| Health Centers | Peltier General Hospital | Cheikho Hospital | CNSS Health Center 2 | PK12 Polyclinic | Khor Bourhan Polyclinic | Farah-Had Polyclinic | Einguella Polyclinic | Total |
|---|---|---|---|---|---|---|---|---|
| Samples Collected | N = 34 | N = 14 | N = 273 | N = 5 | N = 4 | N = 307 | N = 10 | N = 647 |
| Gender, n (%) | ||||||||
| Female | 13 (38.2) | 7 (50) | 108 (39.6) | 3 (60.0) | 2 (50) | 136 (44.3) | 4 (40.0) | 273 (42.2) |
| Male | 21 (61.8) | 7 (50) | 165 (60.4) | 2 (40.0) | 2 (50) | 171 (55.7) | 6 (60.0) | 374 (57.8) |
| Age group, n (%) | ||||||||
| [0–5] | 0 (0.0) | 0 (0.0) | 104 (38.1) | 0 (0.0) | 1 (25.0) | 6 (2.0) | 0 (0.0) | 111 (17.2) |
| [6–14] | 0 (0.0) | 0 (0.0) | 54 (19.8) | 1 (20.0) | 0 (0.0) | 40 (13.0) | 0 (0.0) | 95 (14.7) |
| [15–25] | 11 (32.4) | 7 (50) | 25 (9.1) | 2 (40.0) | 3 (75.0) | 97 (31.6) | 3 (30.0) | 148 (22.9) |
| [26–50] | 17 (50.0) | 7 (50) | 66 (24.2) | 1 (20.0) | 0 (0.0) | 129 (42.0) | 6 (60.0) | 226 (34.9) |
| >50 | 6 (17.6) | 0 (0.0) | 24 (8.8) | 1 (20.0) | 0 (0.0) | 35 (11.4) | 1 (10.0) | 67 (10.3) |
| Diagnostic, n (%) | ||||||||
| URTI | 12 (35.3) | 0 (0.0) | 33 (12.1) | 0 (0.0) | 1 (25) | 21 (6.8) | 0 (0.0) | 67 (10.4) |
| LRTI | 15 (44.1) | 0 (0.0) | 140 (51.3) | 0 (0.0) | 3 (75) | 129 (42) | 0 (0.0) | 287 (44.3) |
| Others | 7 (20.6) | 14 (100) | 100 (36.6) | 5 (100) | 0 (0.0) | 157 (51.1) | 10 (0.0) | 293 (45.3) |
| Symptoms, n (%) | ||||||||
| Fever | 23 (67.6) | 14 (100) | 228 (83.5) | 5 (100) | 3 (75.0) | 276 (89.9) | 10 (100) | 559 (86.4) |
| Cough | 32 (94.1) | 14 (100) | 267 (97.8) | 5 (100) | 3 (75.0) | 300 (97.7) | 10 (100) | 631 (97.5) |
| Headache | 18 (52.9) | 14 (100) | 96 (35.2) | 5 (100) | 2 (50) | 178 (58.0) | 10 (100) | 323 (49.9) |
| Myalgia | 15 (44.1) | 14 (100) | 82 (30.0) | 5 (100) | 2 (50) | 166 (54.1) | 10 (100) | 294 (45.4) |
| Dyspnea | 3 (8.8) | 4 (28.6) | 12 (4.4) | 2 (40.0) | 1 (25.0) | 23 (7.5) | 1 (10.0) | 46 (7.1) |
| Viruses | Total Tested | VRI Cases | Influenza | Rhinovirus | SARS-CoV-2 | PIV | HCoV | RSV | Bocavirus | Enterovirus | HMPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Positives | N = 647 | [N = 133] | [N = 43] | [N = 38] | [N = 22] | [N = 9] | [N = 8] | [N = 7] | [N = 2] | [N = 2] | [N = 2] |
| Gender, n (%) | |||||||||||
| Female | 273 (42.2) | 63 (47.4) | 20 (46.5) | 15 (39.5) | 12 (54.5) | 5 (55.6) | 5 (62.5) | 2 (28.6) | 1 (50.0) | 2 (100) | 1 (50.0) |
| Male | 374 (57.8) | 70 (52.6) | 23 (53.5) | 23 (60.5) | 10 (45.5) | 4 (44.4) | 3 (37.5) | 5 (71.4) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Age group, n (%) | |||||||||||
| [0–5] | 111 (17.1) | 13 (9.8) | 1 (2.3) | 4 (10.5) | 3 (13.6) | 1 (11.1) | 1 (12.5) | 0 (0.0) | 1 (50.0) | 2 (100) | 0 (0.0) |
| [6–14] | 95 (14.7) | 8 (6.0) | 4 (9.3) | 2 (5.3) | 1 (4.5) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [15–25] | 148 (22.9) | 40 (30.1) | 13 (30.2) | 16 (42.1) | 5 (22.7) | 2 (22.2) | 3 (37.5) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [26–50] | 226 (34.9) | 57 (42.8) | 21 (48.8) | 13 (34.2) | 8 (36.4) | 4 (44.4) | 3 (37.5) | 6 (85.7) | 0 (0.0) | 0 (0.0) | 1 (50.0) |
| >50 | 67 (10.4) | 15 (11.3) | 4 (9.3) | 3 (7.9) | 5 (22.7) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Clinical diagnosis, n (%) | |||||||||||
| URTI | 67 (10.4) | 10 (7.5) | 2 (4.6) | 3 (7.9) | 3 (13.6) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) |
| LRTI | 287 (44.3) | 67 (50.4) | 23 (53.5) | 20 (52.6) | 10 (45.4) | 6 (66.7) | 4 (50.0) | 5 (71.4) | 0 (0.0) | 2 (100) | 1 (50.0) |
| Others | 293 (45.3) | 56 (42.1) | 18 (41.9) | 15 (39.5) | 9 (41.0) | 2 (22.2) | 4 (50.0) | 2 (28.6) | 2 (100) | 0 (0.0) | 0 (0.0) |
| Symptoms, n (%) | |||||||||||
| Fever | 559 (86.4) | 123 (92.5) | 39 (90.7) | 36 (94.7) | 19 (86.4) | 9 (100) | 7 (87.5) | 7 (100) | 2 (100) | 2 (100) | 2 (100) |
| Cough | 631 (97.5) | 132 (99.2) | 43 (100) | 38 (100) | 21 (95.5) | 9 (100) | 8 (100) | 7 (100) | 2 (100) | 2 (100) | 2 (100) |
| Headache | 323 (49.9) | 68 (51.1) | 20 (46.5) | 19 (50.0) | 13 (59.1) | 4 (44.4) | 4 (50.0) | 6 (85.7) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Myalgia | 294 (45.4) | 56 (42.1) | 18 (41.9) | 16 (42.1) | 12 (54.5) | 2 (22.2) | 4 (50.0) | 3 (42.8) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 46 (7.1) | 2 (1.5) | 1 (2.3) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assoweh, H.O.; Aboubaker, M.H.; Jallow, M.M.; Dirir, S.D.; Gnasse, I.; Aouled, F.D.; Diallo, D.; Ibrahim, A.A.; Diack, N.; Ahmed, M.N.; et al. Viral Respiratory Infections in Djibouti: Insights from Two Years of Pilot Surveillance. Viruses 2025, 17, 1525. https://doi.org/10.3390/v17121525
Assoweh HO, Aboubaker MH, Jallow MM, Dirir SD, Gnasse I, Aouled FD, Diallo D, Ibrahim AA, Diack N, Ahmed MN, et al. Viral Respiratory Infections in Djibouti: Insights from Two Years of Pilot Surveillance. Viruses. 2025; 17(12):1525. https://doi.org/10.3390/v17121525
Chicago/Turabian StyleAssoweh, Hamda Omar, Mohamed Houmed Aboubaker, Mamadou Malado Jallow, Sahal Darar Dirir, Issa Gnasse, Filsan Daher Aouled, Daouda Diallo, Amina Ahmed Ibrahim, Ndaraw Diack, Mouchtak Nabil Ahmed, and et al. 2025. "Viral Respiratory Infections in Djibouti: Insights from Two Years of Pilot Surveillance" Viruses 17, no. 12: 1525. https://doi.org/10.3390/v17121525
APA StyleAssoweh, H. O., Aboubaker, M. H., Jallow, M. M., Dirir, S. D., Gnasse, I., Aouled, F. D., Diallo, D., Ibrahim, A. A., Diack, N., Ahmed, M. N., Dia, N., & Camara, M. (2025). Viral Respiratory Infections in Djibouti: Insights from Two Years of Pilot Surveillance. Viruses, 17(12), 1525. https://doi.org/10.3390/v17121525






