Leveraging Classical Virology and High Throughput Sequencing for Viral Discovery Using a Historical Viral Collection
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Viruses from Mosquitoes
2.2. Virus Isolation from Cattle Blood
2.3. Virus Identification: Serogrouping of Virus Isolates
2.4. Virus Identification: Indirect Immunofluorescent Assay
2.5. Sequencing and Bioinformatics Analysis
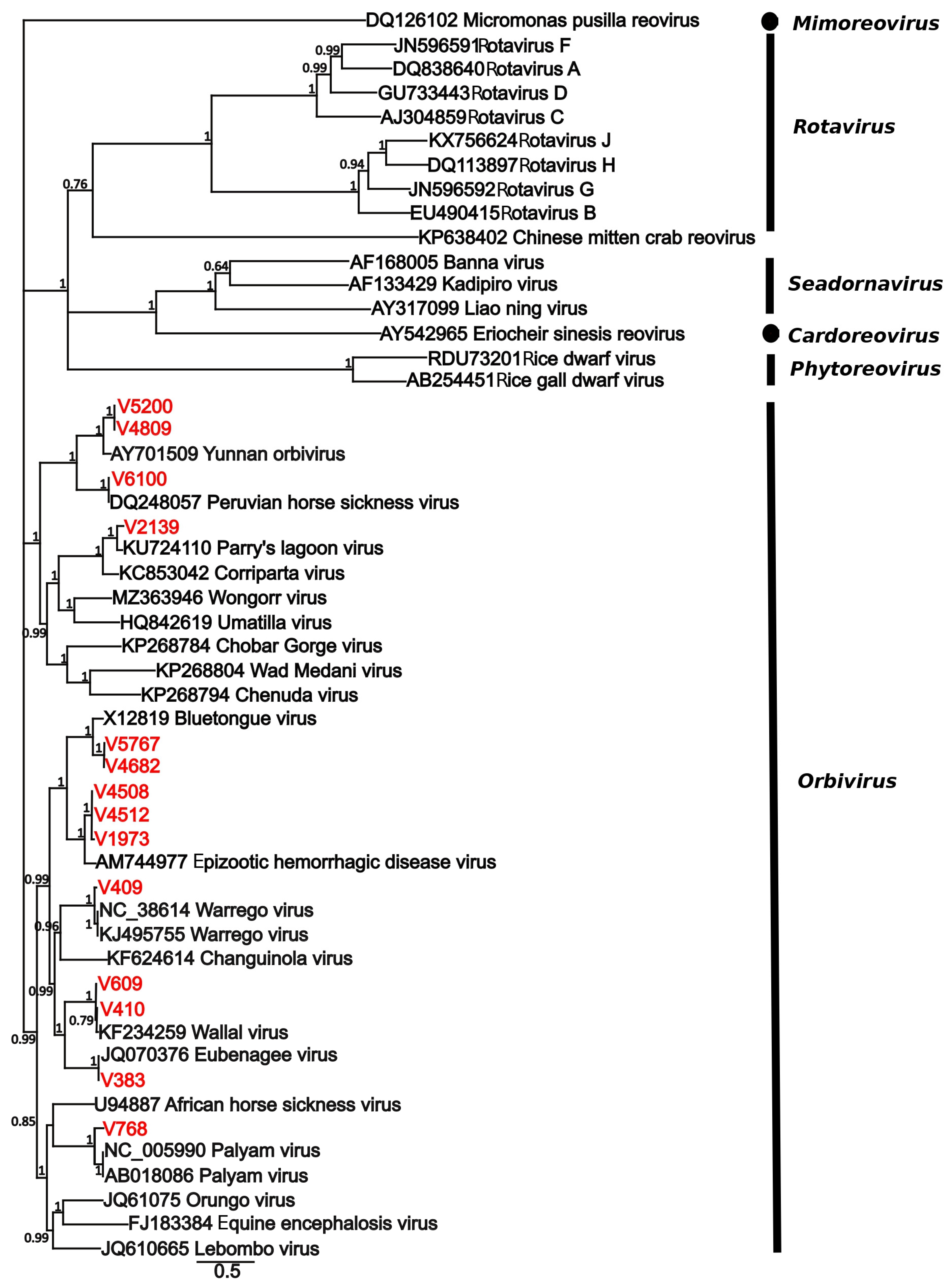
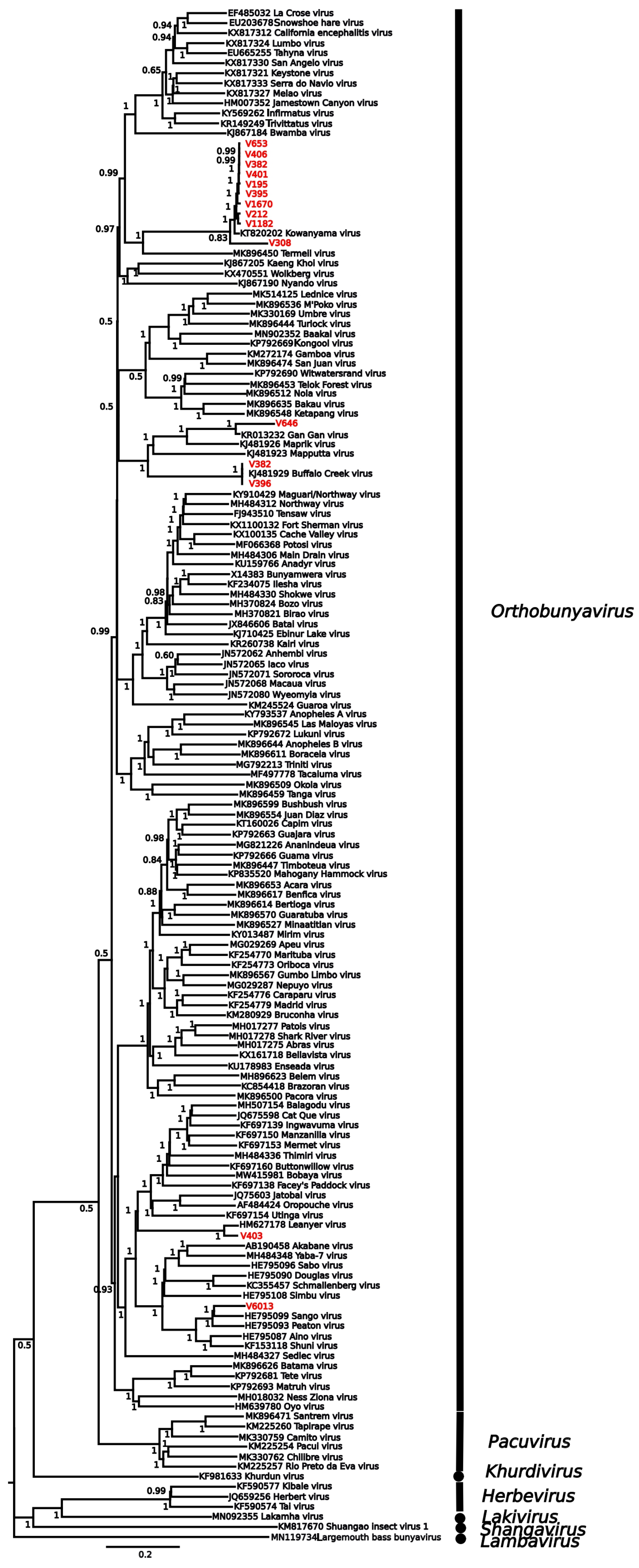
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasilakis, N.; Tesh, R.B.; Popov, V.L.; Widen, S.G.; Wood, T.G.; Forrester, N.L.; Gonzalez, J.P.; Saluzzo, J.F.; Alkhovsky, S.; Lam, S.K.; et al. Exploiting the Legacy of the Arbovirus Hunters. Viruses 2019, 11, 471. [Google Scholar] [CrossRef]
- Next Generation Sequencing Technologies for Insect Virus Discovery—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22069519/ (accessed on 14 July 2025).
- Jones, R.A.C.; Boonham, N.; Adams, I.P.; Fox, A. Historical Virus Isolate Collections: An Invaluable Resource Connecting Plant Virology’s Pre-Sequencing and Post-Sequencing Eras. Plant Pathol. 2021, 70, 235–248. [Google Scholar] [CrossRef]
- Petersen, L.R.; Holcomb, K.; Beard, C.B. Climate Change and Vector-Borne Disease in North America and Europe. J. Health Monit. 2022, 7, 13–15. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and Re-Emergence of Mosquito-Borne Arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, N.; Bradbury, R.S.; Aaskov, J.G.; Taylor-Robinson, A.W. Neglected Australian Arboviruses and Undifferentiated Febrile Illness: Addressing Public Health Challenges Arising From the ‘Developing Northern Australia’ Government Policy. Front. Microbiol. 2017, 8, 2150. [Google Scholar] [CrossRef]
- Gyawali, N.; Bradbury, R.S.; Aaskov, J.G.; Taylor-Robinson, A.W. Neglected Australian Arboviruses: Quam Gravis? Microbes Infect. 2017, 19, 388–401. [Google Scholar] [CrossRef]
- Gould, E.A.; Solomon, T. Pathogenic Flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global Spread of Dengue Virus Types: Mapping the 70 Year History. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Taylor-Robinson, A.W. Complex Transmission Epidemiology of Neglected Australian Arboviruses: Diverse Non-Human Vertebrate Hosts and Competent Arthropod Invertebrate Vectors. Front. Microbiol. 2024, 15, 1469710. [Google Scholar] [CrossRef]
- Huang, B.; Allcock, R.; Warrilow, D. Newly Characterized Arboviruses of Northern Australia. Virol. Rep. 2016, 6, 11–17. [Google Scholar] [CrossRef]
- Hughes, H.R.; Adkins, S.; Alkhovskiy, S.; Beer, M.; Blair, C.; Calisher, C.H.; Drebot, M.; Lambert, A.J.; de Souza, W.M.; Marklewitz, M.; et al. ICTV Virus Taxonomy Profile: Peribunyaviridae. J. Gen. Virol. 2020, 101, 1–2. [Google Scholar] [CrossRef]
- Isolations of Jamestown Canyon Virus (Bunyaviridae: Orthobunyavirus) from Field-Collected Mosquitoes (Diptera: Culicidae) in Connecticut, USA: A Ten-Year Analysis, 1997–2006—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18386967/ (accessed on 16 July 2025).
- Labuda, M. Arthropod Vectors in the Evolution of Bunyaviruses. Acta Virol. 1991, 35, 98–105. [Google Scholar]
- Lasecka, L.; Baron, M.D. The Molecular Biology of Nairoviruses, an Emerging Group of Tick-Borne Arboviruses. Arch. Virol. 2014, 159, 1249–1265. [Google Scholar] [CrossRef]
- Williams, J.E.; Imlarp, S.; Top, F.H.; Cavanaugh, D.C.; Russell, P.K. Kaeng Khoi Virus from Naturally Infected Bedbugs (Cimicidae) and Immature Free-Tailed Bats. Bull. World Health Organ. 1976, 53, 365–369. [Google Scholar]
- Henderson, B.E.; Coleman, P.H. The Growing Importance of California Arboviruses in the Etiology of Human Disease. Prog. Med. Virol. 1971, 13, 404–461. [Google Scholar] [PubMed]
- Dutuze, M.F.; Nzayirambaho, M.; Mores, C.N.; Christofferson, R.C. A Review of Bunyamwera, Batai, and Ngari Viruses: Understudied Orthobunyaviruses with Potential One Health Implications. Front. Vet. Sci. 2018, 5, 69. [Google Scholar] [CrossRef]
- Hayama, Y.; Moriguchi, S.; Yanase, T.; Ishikura, Y.; Abe, S.; Higashi, T.; Ishikawa, H.; Yamamoto, T.; Kobayashi, S.; Murai, K.; et al. Spatial Epidemiological Analysis of Bovine Encephalomyelitis Outbreaks Caused by Akabane Virus Infection in Western Japan in 2011. Trop. Anim. Health Prod. 2016, 48, 843–847. [Google Scholar] [CrossRef]
- Jennings, M.; Mellor, P.S. Culicoides: Biological Vectors of Akabane Virus. Vet. Microbiol. 1989, 21, 125–131. [Google Scholar] [CrossRef]
- Weidmann, M.; Rudaz, V.; Nunes, M.R.T.; Vasconcelos, P.F.C.; Hufert, F.T. Rapid Detection of Human Pathogenic Orthobunyaviruses. J. Clin. Microbiol. 2003, 41, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef] [PubMed]
- Roy, P. Orbivirus Structure and Assembly. Virology 1996, 216, 1–11. [Google Scholar] [CrossRef]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Brownlie, J.; Tesh, R.; Attoui, H.; Mertens, P.P.C. Genetic Characterization of the Tick-Borne Orbiviruses. Viruses 2015, 7, 2185–2209. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Mertens, P.P.C. Taxonomy of African Horse Sickness Viruses. In African Horse Sickness; Mellor, P.S., Baylis, M., Hamblin, C., Mertens, P.P.C., Calisher, C.H., Eds.; Springer: Vienna, Austria, 1998; pp. 3–11. [Google Scholar]
- Liehne, P.F.; Anderson, S.; Stanley, N.F.; Liehne, C.G.; Wright, A.E.; Chan, K.H.; Leivers, S.; Britten, D.K.; Hamilton, N.P. Isolation of Murray Valley Encephalitis Virus and Other Arboviruses in the Ord River Valley 1972-1976. Aust. J. Exp. Biol. Med. Sci. 1981, 59, 347–356. [Google Scholar] [CrossRef]
- Maan, S.; Belaganahalli, M.N.; Maan, N.S.; Attoui, H.; Mertens, P.P.C. Orbiviruses. In Emerging and Transboundary Animal Viruses; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer: Singapore, 2020; pp. 161–214. ISBN 978-981-15-0402-0. [Google Scholar]
- Maclachlan, N.J.; Guthrie, A.J. Re-Emergence of Bluetongue, African Horse Sickness, and Other Orbivirus Diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Tyler, K.L. Orthoreoviruses and Orbiviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1848–1850.e1. [Google Scholar] [CrossRef]
- Rohe, D.L.; Fall, R.P. A Miniature Battery Powered CO2 Baited Light Trap for Mosquito Borne Encephalitis Surveillance. Bull. Soc. Vector Ecol. 1979, 4, 24–27. [Google Scholar]
- Whelan, P.I.; Weir, R. The Isolation of Alpha and Flavi Viruses from Mosquitoes in the Northern Territory 1982–1992; Queensland Institute of Medical Research: Brisbane, Australia, 1993. [Google Scholar]
- Macpherson, I.; Stoker, M. Polyoma Transformation of Hamster Cell Clones—An Investigation of Genetic Factors Affecting Cell Competence. Virology 1962, 16, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A. Isolation of a Singh’s Aedes Albopictus Cell Clone Sensitive to Dengue and Chikungunya Viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Hsu, T.C.; Zenzes, M.T. Mammalian Chromosomes in Vitro. XVII. Idiogram Chin. Hamster. J. Natl. Cancer Inst. 1964, 32, 857–869. [Google Scholar] [CrossRef]
- Gard, G.P.; Shorthose, J.E.; Weir, R.P.; Erasmus, B.J. The Isolation of a Bluetongue Serotype New to Austrlia. Aust. Vet. J. 1987, 64, 87–88. [Google Scholar] [CrossRef]
- Goldsmit, L.; Barzilai, E. An Improved Method for the Isolation and Identification of Bluetongue Virus by Intravenous Inoculation of Embryonating Chicken Eggs. J. Comp. Pathol. 1968, 78, 477–487. [Google Scholar] [CrossRef]
- Lindsay, M.D.; Broom, A.K.; Wright, A.E.; Johansen, C.A.; Mackenzie, J.S. Ross River Virus Isolations from Mosquitoes in Arid Regions of Western Australia: Implication of Vertical Transmission as a Means of Persistence of the Virus. Am. J. Trop. Med. Hyg. 1993, 49, 686–696. [Google Scholar] [CrossRef]
- Sato, M.; Maeda, N.; Yoshida, H.; Urade, M.; Saito, S.; Miyazaki, T.; Shibata, T.; Watanabe, M. Plaque Formation of Herpes Virus Hominis Type 2 and Rubella Virus in Variants Isolated from the Colonies of BHK21/WI-2 Cells Formed in Soft Agar. Arch. Virol. 1977, 53, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, P.J.; Smith, J.R. Expression of Angiotensin-Converting Enzyme Activity in Cultured Pulmonary Artery Endothelial Cells. J. Cell. Physiol. 1981, 108, 337–345. [Google Scholar] [CrossRef]
- Gard, G.P.; Kirkland, P.D. Bluetongue: Virology and Serology. 1993. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19932290397 (accessed on 16 July 2025).
- Zeller, H.G.; Karabatsos, N.; Calisher, C.H.; Digoutte, J.-P.; Cropp, C.B.; Murphy, F.A.; Shope, R.E. Electron microscopic and antigenic studies of uncharacterized viruses. II. Evidence suggesting the placement of viruses in the familyBunyaviridae. Arch. Virol. 1989, 108, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Zeller, H.G.; Karabatsos, N.; Calisher, C.H.; Digoutte, J.P.; Cropp, C.B.; Murphy, F.A.; Shope, R.E. Electron mi-croscopic and antigenic studies of uncharacterized viruses. III. Evidence suggesting the placement of viruses in the family Reoviridae. Arch. Virol. 1989, 109, 253–261. [Google Scholar] [CrossRef]
- Calisher, C.H. Medically important arboviruses of the United States and Canada. Clin. Microbiol. Rev. 1994, 7, 89–116. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Charon, J.; Buchmann, J.P.; Sadiq, S.; Holmes, E.C. RdRp-Scan: A Bioinformatic Resource to Identify and Annotate Divergent RNA Viruses in Metagenomic Sequence Data. Virus Evol. 2022, 8, veac082. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive Protein Alignments at Tree-of-Life Scale Using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Peterson, A.T. Defining Viral Species: Making Taxonomy Useful. Virol. J. 2014, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Bobay, L.-M.; Ochman, H. Biological Species in the Viral World. Proc. Natl. Acad. Sci. USA 2018, 115, 6040–6045. [Google Scholar] [CrossRef]
- Gubala, A.; Davis, S.; Weir, R.; Melville, L.; Cowled, C.; Walker, P.; Boyle, D. Ngaingan Virus, a Macropod-Associated Rhabdovirus, Contains a Second Glycoprotein Gene and Seven Novel Open Reading Frames. Virology 2010, 399, 98–108. [Google Scholar] [CrossRef]
- Doherty, R.L.; Carley, J.G.; Standfast, H.A.; Dyce, A.L.; Kay, B.H.; Snowdon, W.A. Isolation of Arboviruses from Mosquitoes, Biting Midges, Sandflies and Vertebrates Collected in Queensland, 1969 and 1970. Trans. R. Soc. Trop. Med. Hyg. 1973, 67, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Gubala, A.; Walsh, S.; McAllister, J.; Weir, R.; Davis, S.; Melville, L.; Mitchell, I.; Bulach, D.; Gauci, P.; Skvortsov, A.; et al. Identification of Very Small Open Reading Frames in the Genomes of Holmes Jungle Virus, Ord River Virus, and Wongabel Virus of the Genus Hapavirus, Family Rhabdoviridae. Evol. Bioinform. Online 2017, 13, 1176934317713484. [Google Scholar] [CrossRef]
- Amarasinghe, G.K.; Bào, Y.; Basler, C.F.; Bavari, S.; Beer, M.; Bejerman, N.; Blasdell, K.R.; Bochnowski, A.; Briese, T.; Bukreyev, A.; et al. Taxonomy of the Order Mononegavirales: Update 2017. Arch. Virol. 2017, 162, 2493–2504. [Google Scholar] [CrossRef]
- Cowled, C.; Melville, L.; Weir, R.; Walsh, S.; Hyatt, A.; Van Driel, R.; Davis, S.; Gubala, A.; Boyle, D. Genetic and Epidemiological Characterization of Middle Point Orbivirus, a Novel Virus Isolated from Sentinel Cattle in Northern Australia. J. Gen. Virol. 2007, 88, 3413–3422. [Google Scholar] [CrossRef]
- Boughton, C.R.; Hawkes, R.A.; Naim, H.M. Arbovirus Infection in Humans in NSW: Seroprevalence and Pathogenicity of Certain Australian Bunyaviruses. Aust. N. Z. J. Med. 1990, 20, 51–55. [Google Scholar] [CrossRef]
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-Borne Viral Infections of Man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef]
- Tomori, O.; Fabiyi, A. Neutralizing Antibodies to Orungo Virus in Man and Animals in Nigeria. Trop. Geogr. Med. 1976, 28, 233–238. [Google Scholar]
- Attoui, H.; Mendez-lopez, M.R.; Rao, S.; Hurtado-Alendes, A.; Lizaraso-Caparo, F.; Mohd Jaafar, F.; Samuel, A.R.; Belhouchet, M.; Pritchard, L.I.; Melville, L.; et al. Peruvian Horse Sickness Virus and Yunnan Orbivirus, Isolated from Vertebrates and Mosquitoes in Peru and Australia. Virology 2009, 394, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Venter, M. Assessing the Zoonotic Potential of Arboviruses of African Origin. Curr. Opin. Virol. 2018, 28, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kosoy, O.; Rabe, I.; Geissler, A.; Adjemian, J.; Panella, A.; Laven, J.; Basile, A.J.; Velez, J.; Griffith, K.; Wong, D.; et al. Serological Survey for Antibodies to Mosquito-Borne Bunyaviruses Among US National Park Service and US Forest Service Employees. Vector-Borne Zoonotic Dis. 2016, 16, 191–198. [Google Scholar] [CrossRef]
- Reusken, C.; van den Wijngaard, C.; van Beek, P.; Beer, M.; Bouwstra, R.; Godeke, G.-J.; Isken, L.; van den Kerkhof, H.; van Pelt, W.; van der Poel, W.; et al. Lack of Evidence for Zoonotic Transmission of Schmallenberg Virus. Emerg. Infect. Dis. 2012, 18, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Boutzoukas, A.E.; Freedman, D.A.; Koterba, C.; Hunt, G.W.; Mack, K.; Cass, J.; Yildiz, V.O.; de Los Reyes, E.; Twanow, J.; Chung, M.G.; et al. La Crosse Virus Neuroinvasive Disease in Children: A Contemporary Analysis of Clinical/Neurobehavioral Outcomes and Predictors of Disease Severity. Clin. Infect. Dis. 2023, 76, e1114–e1122. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, J.F.T.; de Souza, W.M.; de Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Endy, T.P. 36—Viral Febrile Illnesses and Emerging Pathogens. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; pp. 325–350. ISBN 978-0-323-55512-8. [Google Scholar]
- Cao-Lormeau, V.-M.; Musso, D. Emerging Arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Virus | Location | Date | Host Species | Provisional ID | Best Match | |||
|---|---|---|---|---|---|---|---|---|
| Name | GenBank ID | % Match | % Coverage | |||||
| V1163 | Darwin | 3 February 1987 | C. annulirostris | rhabdovirus-like virus | Hapavirus holmes | ASM90779 | >99 | 93.69 |
| V1182 | Palumpa | 15 April 1987 | A. amictus | Bunyavirus-like virus | Kowanyama virus | AMR73391 | >99 | 100 |
| V1664 | Katherine | 8 February 1989 | A. lineatopennis | Wongorr virus | Wongorr virus | YP009665177 | 95 | 28.8 |
| V1670 | Katherine | 8 February 1989 | C. annulirostris | orbivirus-like virus | Kowanyama virus | AMR73391 | >99 | 99.81 |
| V178 | Darwin | 4 March 1982 | C. annulirostris | Wongorr virus | Wongorr virus | YP009665177 | 93 | 23.07 |
| V193 | Darwin | 28 April 1982 | A. meraukensis | Bunyavirus | Kowanyama virus | AMR73391 | >99 | 100% |
| V197 | Darwin | 16 March 1982 | A. farauti | Wongorr virus | Wongorr virus | YP009665177 | 95 | 16.59 |
| V1973 | Darwin rural | 21 February 1990 | C. annulirostris | Bluetongue virus | Epizootic hemorrhagic disease virus | CAN99549 | >99 | 17.5 |
| V212 | Darwin | 29 March 1982 | A. meraukensis | Bunyavirus-like virus | Kowanyama virus | AMR73391 | >99 | 99.78 |
| V213 | Darwin | 20 March 1982 | A. farauti | Mapputta virus | Mapputta orthobunyavirus | AKO90170 | 98 | 100 |
| V2139 | Kakadu | 8 May 1991 | C. annulirostris | orbivirus-like virus | Parry’s Lagoon virus | ANH10670 | 97 | 99.79 |
| V308 | Unknown | 1 August 1983 | Mosquito pool | Unknown | Kowanyama virus | AMR73391 | >99 | 68.53 |
| V3265 | Western Australia | 19 October 1994 | Unnamed insect | Wongorr virus | Wongorr virus | YP009665177 | 95 | 26.55 |
| V3289 | Western Australia | 21 November 1994 | Unnamed insect | Wongorr virus | Wongorr virus | YP009665177 | 95 | 22.44 |
| V382 | Darwin | 29 March 1983 | A. meraukensis | Bunyavirus-like virus | Buffalo Creek Orthobunyavirus | AJD77610 | >99 | 100 |
| V383 | Katherine | 29 March 1983 | A. lineatopennis | Eubenangee virus | Eubenangee virus | YP009507706 | >99 | 99.8 |
| V3904 | CPRS | 27 June 1996 | B. indicus | Unknown | Yunnan orbivirus | YP443925 | 97.6 | 27.67 |
| V395 | Katherine | 30 April 1983 | A. amictus | Unknown | Kowanyama virus | AMR73391 | >99 | 97.91 |
| V396 | Darwin | 4 May 1983 | A. annulipes | Bunyavirus-like virus | Buffalo Creek Orthobunyavirus | AJD77610 | >99 | 100 |
| V400 | Darwin | 27 April 1983 | A. meraukensis | Bunyavirus-like virus | Kowanyama virus | AMR73391 | >99 | 99.97 |
| V401 | Unknown | 1 August 1983 | Mosquito pool | Unknown | Kowanyama virus | AMR73391 | >99 | 99.8 |
| V403 | Darwin | 17 May 1983 | A. meraukensis | Bunyavirus | Leanyer Orthobunyavirus | AEA02985 | >99 | 99.96% |
| V406 | Darwin | 17 May 1983 | A. meraukensis | Bunyavirus | Kowanyama virus | AMR73391 | >99 | 99.86% |
| V409 | Jabiru | 3 June 1983 | C. annulirostris | Warrego virus | Warrego virus | AGX86082 | 91 | 99.65% |
| V410 | Jabiru | 03 June 1983 | C. annulirostris | Wallal virus | Wallal virus | AGX00987 | >99 | 99.29% |
| V4135 | CPRS | 9 January 1996 | B. indicus | Unknown | Kowanyama virus | AMR73391 | >99 | |
| V4330 | CPRS | 23 June 1997 | B. bubalis | Epizootic hemorrhagic disease virus | Epizootic hemorrhagic disease virus | CAN99549 | >99 | 99.94% |
| V4508 | CPRS | 19 November 1998 | B. indicus | Unknown | Epizootic hemorrhagic disease virus | CAN99549 | >99 | 18.80% |
| V4512 | CPRS | 8 January 1999 | B. indicus | Epizootic hemorrhagic disease virus | Epizootic hemorrhagic disease virus | CAN99549 | >99 | 15.93% |
| V4682 | CPRS | 17 June 1999 | B. indicus | Unknown | Bluetongue virus | AKV60532 | >99 | 34.46% |
| V4809 | CPRS | 10 February 2000 | B. indicus | Unknown | Yunnan orbivirus | YP443925 | 96.1 | 43.65% |
| V4835 | Katherine | N/A | E. caballus | Unknown | Peruvian horse sickness virus | YP460038 | >99 | 60.00% |
| V5200 | CPRS | 5 April 2001 | B. indicus | Unknown | Yunnan orbivirus | YP443925 | 96.5 | 25.22% |
| V5767 | CPRS | 5 December 2002 | B. indicus | Bluetongue virus | Bluetongue virus | AKV60532 | >99 | 57.61% |
| V6013 | CPRS | 28 August 2003 | B. indicus | Unknown | Sango orthobunyavirus | YP009666881 | 95 | 95.05% |
| V609 | Larrimah | 16 February 1984 | A. normanensis | Wallal virus | Wallal virus | AGX00987 | >99 | 99.47% |
| V6100 | Katherine | 5 February 2004 | E. caballus | Unknown | Peruvian horse sickness virus | YP460038 | >99 | 99.30% |
| V6228 | CPRS | 22 April 2004 | B. indicus | Unknown | Yunnan orbivirus | YP443925 | 97.4 | 6.29% |
| V6250 | TWP | 27 May 2004 | O. rufus | Wongorr virus | Wongorr virus | YP009665177 | 95 | 25.65% |
| V646 | Mataranka | 15 March 84 | A. normanensis | Bunyavirus-like virus | Gan Gan orthobunyavirus | ALQ43836 | >99 | 55.73% |
| V653 | Mataranka | 11 April 1984 | A. normanensis | Orbivirus-like virus | Kowanyama virus | AMR73391 | 94 | 99.97 |
| V768 | Darwin | 30 January 1985 | C. annulirostris | Palyam virus | Palyam virus | QCU80060 | 98.5 | 98.02% |
| V771 | Darwin | 12 February 1985 | C. annulirostris | Wongorr virus | Kowanyama virus | AMR73391 | >99 | 99.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sistrom, M.; Neave, M.; Joseph, A.; Newberry, K.; Andrews, H.; Shilton, C.; Bhardwaj, V.; Weir, R. Leveraging Classical Virology and High Throughput Sequencing for Viral Discovery Using a Historical Viral Collection. Viruses 2025, 17, 1513. https://doi.org/10.3390/v17111513
Sistrom M, Neave M, Joseph A, Newberry K, Andrews H, Shilton C, Bhardwaj V, Weir R. Leveraging Classical Virology and High Throughput Sequencing for Viral Discovery Using a Historical Viral Collection. Viruses. 2025; 17(11):1513. https://doi.org/10.3390/v17111513
Chicago/Turabian StyleSistrom, Mark, Matthew Neave, Ancy Joseph, Kim Newberry, Hannah Andrews, Cathy Shilton, Vidya Bhardwaj, and Richard Weir. 2025. "Leveraging Classical Virology and High Throughput Sequencing for Viral Discovery Using a Historical Viral Collection" Viruses 17, no. 11: 1513. https://doi.org/10.3390/v17111513
APA StyleSistrom, M., Neave, M., Joseph, A., Newberry, K., Andrews, H., Shilton, C., Bhardwaj, V., & Weir, R. (2025). Leveraging Classical Virology and High Throughput Sequencing for Viral Discovery Using a Historical Viral Collection. Viruses, 17(11), 1513. https://doi.org/10.3390/v17111513






