Seroprevalence of Arboviruses and Genetic Characterization of Orbiviruses in Sloths from Western Panama
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Sample Collection
2.3. Sloth Trapping and Sample Collection
2.4. Serological Testing
2.5. Molecular Testing and Viral Isolation from Sera Samples
2.6. Genetic Characterization of an Orbivirus from Sloths
2.7. Phylogenetic Analysis
3. Results
3.1. Characteristics of Sloths Collected
3.2. Plaque-Reduction Neutralization Test (PRNT)
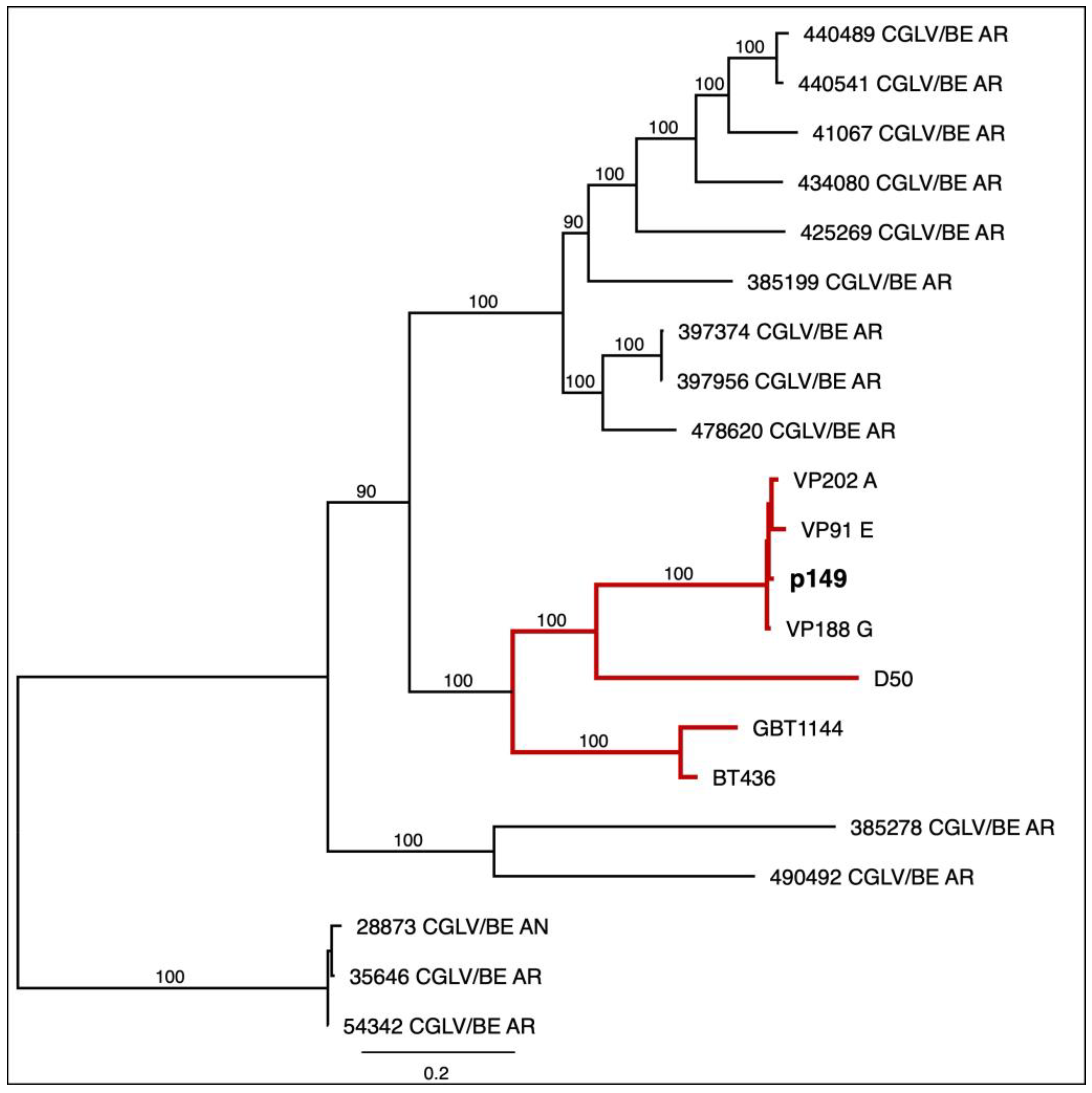
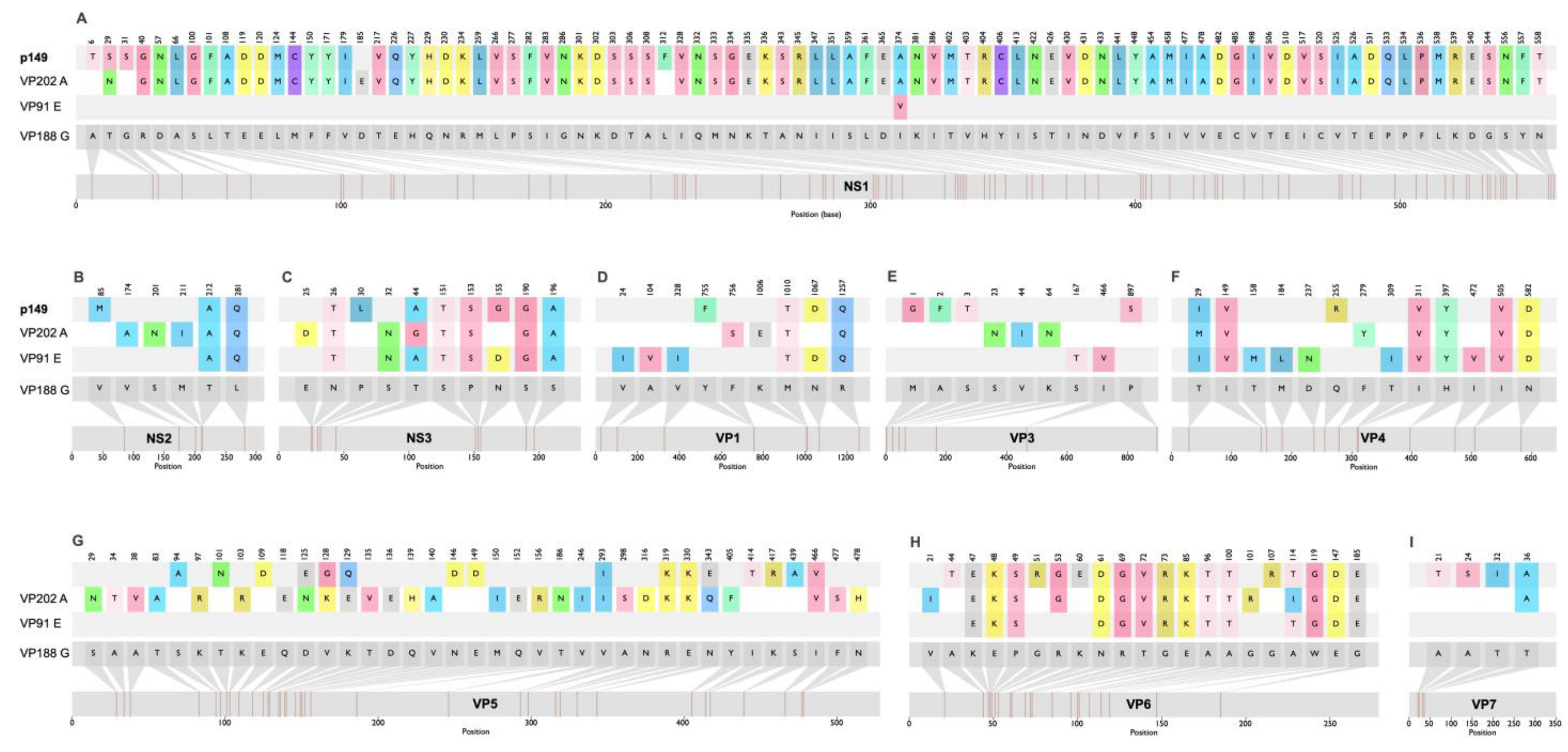
3.3. Pansloth 149 Is a Changuinola-like Virus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| ZIKV | Zika virus |
| VEEV | Venezuelan equine encephalitis |
| CGLV | Changuinola virus |
| PTV | Punta Toro virus |
| EEEV | Eastern equine encephalitis virus |
| MADV | Madariaga virus |
| OROV | Oropouche virus |
| CHIKV | Chikungunya virus |
| MAYV | Mayaro virus |
| GMI | Gorgas Memorial Institute |
| DIDETEC | Research and Technological Development Division |
| UTMB | University of Texas Medical Branch |
| WHO | World Health Organization |
| HI | Hemagglutination Inhibition |
References
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- World Health Organization. Arboviruses and Human Disease: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 1967; pp. 1–84. [Google Scholar]
- World Health Organization Zoonoses. Available online: https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 5 June 2025).
- Johnson, C.K.; Hitchens, P.L.; Pandit, P.S.; Rushmore, J.; Evans, T.S.; Young, C.C.W.; Doyle, M.M. Global Shifts in Mammalian Population Trends Reveal Key Predictors of Virus Spillover Risk. Proc. R. Soc. B 2020, 287, 20192736. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Zika, M.L. Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, R.C. Zika Virus Emergence and Expansion: Lessons Learned from Dengue and Chikungunya May Not Provide All the Answers. Am. Soc. Trop. Med. Hyg. 2016, 95, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Del Valle, S.; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Humboldt-Dachroeden, S.; Rubin, O.; Frid Nielsen, S. The State of One Health Research across Disciplines and Sectors—A Bibliometric Analysis. One Health 2020, 10, 100146. [Google Scholar] [CrossRef]
- Cabello, C.; Cabello, F. Zoonosis Con Reservorios Silvestres: Amenazas a La Salud Pública y a La Economía. Rev. Med. Chil. 2008, 136, 385–393. [Google Scholar] [CrossRef]
- Galindo, P. Los Arbovirus de Panama. Rev. Med. Panama 1978, 3, 1–41. [Google Scholar]
- Arauz, D.; De Urriola, L.; Jones, J.; Castillo, M.; Martinez, A.; Murillo, E.; Lopez-Verges, S. Febrile or Exanthematous Illness Associated with Zika, Dengue, and Chikungunya Viruses, Panama. Emerg. Infect. Dis. 2016, 22, 1516–1517. [Google Scholar] [CrossRef]
- Díaz, Y.; Chen-Germán, M.; Quiroz, E.; Carrera, J.-P.; Cisneros, J.; Moreno, B.; Cerezo, L.; Martinez-Torres, A.O.; Moreno, L.; Barahona De Mosca, I.; et al. Molecular Epidemiology of Dengue in Panama: 25 Years of Circulation. Viruses 2019, 11, 764. [Google Scholar] [CrossRef]
- Carrera, J.-P.; Forrester, N.; Wang, E.; Vittor, A.Y.; Haddow, A.D.; López-Vergès, S.; Abadía, I.; Castaño, E.; Sosa, N.; Báez, C.; et al. Eastern Equine Encephalitis in Latin America. N. Engl. J. Med. 2013, 369, 732–744. [Google Scholar] [CrossRef]
- Gilmore, D.P.; Da Costa, C.P.; Duarte, D.P.F. Sloth Biology: An Update on Their Physiological Ecology, Behavior and Role as Vectors of Arthropods and Arboviruses. Braz. J. Med. Biol. Res. 2001, 34, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Kaup, M.; Trull, S.; Hom, E.F.Y. On the Move: Sloths and Their Epibionts as Model Mobile Ecosystems. Biol. Rev. 2021, 96, 2638–2660. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Tesh, R.B.; Guzman, H.; Delwart, E. Genomic Characterization of Changuinola Viruses from Panama: Evidence for Multiple Genome Segment Reassortment. Virus Genes 2020, 56, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Travassos da Rosa, A.P.A.; Tesh, R.B.; Pinheiro, F.P.; Travassos da Rosa, J.F.S.; Peralta, P.H.; Knudson, D.L. Characterization of the Changuinola Serogroup Viruses. Intervirology 1984, 21, 38–49. [Google Scholar] [CrossRef]
- Attoui, H.; Nomikou, K.; Maan, S.; Belaganahalli, M.; Mertens, P.P.C. Orbiviruses; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801238-3. [Google Scholar]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects 2021, 13, 20. [Google Scholar] [CrossRef]
- Ministry of Environment of Panama. Panama Land Cover and Forest Distribution. 2021. Available online: https://miambiente.gob.pa/miambiente-implementa-sistema-nacional-de-informacion-ambiental-y-presenta-los-resultados-del-diagnostico-de-cobertura-boscosa/ (accessed on 11 June 2025).
- Hanley, C.S.; Siudak-Campfield, J.; Paul-Murphy, J.; Vaughan, C.; Ramirez, O.; Keuler, N.S.; Sladky, K.K. Immobilization of Free-Ranging Hoffmann’s Two-Toed and Brown-Throated Three-Toed Sloths Using Ketamine and Medetomidine: A Comparison of Physiologic Parameters. J. Wildl. Dis. 2008, 44, 938–945. [Google Scholar] [CrossRef]
- Díaz, Y.; Carrera, J.P.; Cerezo, L.; Arauz, D.; Guerra, I.; Cisneros, J.; Armién, B.; Botello, A.M.; Araúz, A.B.; Gonzalez, V.; et al. Chikungunya Virus Infection: First Detection of Imported and Autochthonous Cases in Panama. Am. J. Trop. Med. Hyg. 2015, 92, 482–485. [Google Scholar] [CrossRef]
- Quiroz, E.; Aguilar, P.V.; Cisneros, J.; Tesh, R.B.; Weaver, S.C. Venezuelan Equine Encephalitis in Panama: Fatal Endemic Disease and Genetic Diversity of Etiologic Viral Strains. PLoS Negl. Trop. Dis. 2009, 3, e472. [Google Scholar] [CrossRef]
- Carrera, J.; Araúz, D.; Rojas, A.; Cardozo, F.; Stittleburg, V.; Claro, I.M.; Galue, J.; Lezcano-coba, C.; Romero, F.; Moreira, R.; et al. Real-Time RT-PCR for Venezuelan Equine Encephalitis Complex, Madariaga, and Eastern Equine Encephalitis Viruses: Application in Human and Mosquito Public Health Surveillance in Panama. J. Clin. Microbiol. 2023, 61, e00152-23. [Google Scholar] [CrossRef]
- Peralta, P.H.; Shelokov, A. Isolation and Characterization of Arboviruses from Almirante, Republic of Panama. Am. J. Trop. Med. Hyg. 1966, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Carrera, J.P.; Bagamian, K.H.; Travassos Da Rosa, A.P.; Wang, E.; Beltran, D.; Gundaker, N.D.; Armien, B.; Arroyo, G.; Sosa, N.; Pascale, J.M.; et al. Human and Equine Infection with Alphaviruses and Flaviviruses in Panamá during 2010: A Cross-Sectional Study of Household Contacts during an Encephalitis Outbreak. Am. J. Trop. Med. Hyg. 2018, 98, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, J.L.; Beltrán, D.; Pitti, A.; Saenz, L.; Araúz, A.B.; Vergara, R.; Harris, E.; Lanier, L.L.; Blish, C.A.; López-Vergès, S. HLA Upregulation During Dengue Virus Infection Suppresses the Natural Killer Cell Response. Front. Cell. Infect. Microbiol. 2019, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Wiley, M.R.; Travassos da Rosa, A.P.A.; Guzman, H.; Quiroz, E.; Savji, N.; Carrera, J.P.; Bussetti, A.V.; Ladner, J.T.; Ian Lipkin, W.; et al. Characterization of the Punta Toro Species Complex (Genus Phlebovirus, Family Bunyaviridae). J. Gen. Virol. 2015, 96, 2079–2085. [Google Scholar] [CrossRef]
- Watts, D.M.; Shope, R.E.; Barrett, A.D.T.; Tesh, R.B.; Saeed, M.F.; Nunes, M.; Wang, H.; Weaver, S.C.; Vasconcelos, P.F.C. Nucleotide Sequences and Phylogeny of the Nucleocapsid Gene of Oropouche Virus. J. Gen. Virol. 2000, 81, 743–748. [Google Scholar] [CrossRef]
- Chen-Germán, M.; Araúz, D.; Aguilar, C.; Vega, M.; Gonzalez, C.; Gondola, J.; Moreno, L.; Cerezo, L.; Franco, L.; Mendez-Rico, J.; et al. Detection of Dengue Virus Serotype 4 in Panama After 23 Years Without Circulation. Front. Cell. Infect. Microbiol. 2024, 14, 1467465. [Google Scholar] [CrossRef]
- Gundacker, N.D.; Carrera, J.P.; Castillo, M.; Díaz, Y.; Valenzuela, J.; Tamhane, A.; Moreno, B.; Pascale, J.M.; Tesh, R.B.; López-Vergès, S. Clinical Manifestations of Punta Toro Virus Species Complex Infections, Panama, 2009. Emerg. Infect. Dis. 2017, 23, 872–874. [Google Scholar] [CrossRef]
- Sánchez-Seco, M.P.; Rosario, D.; Quiroz, E.; Guzmán, G.; Tenorio, A. A Generic Nested-RT-PCR Followed by Sequencing for Detection and Identification of Members of the Alphavirus Genus. J. Virol. Methods 2001, 95, 153–161. [Google Scholar] [CrossRef]
- Ayers, M.; Adachi, D.; Johnson, G.; Andonova, M.; Drebot, M.; Tellier, R. A Single Tube RT-PCR Assay for the Detection of Mosquito-Borne Flaviviruses. J. Virol. Methods 2006, 135, 235–239. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, 1–3. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Zhang, X.; Boyce, M.; Bhattacharya, B.; Zhang, X.; Schein, S.; Roy, P.; Zhou, Z.H. Bluetongue Virus Coat Protein VP2 Contains Sialic Acid-Binding Domains, and VP5 Resembles Enveloped Virus Fusion Proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 6292–6297. [Google Scholar] [CrossRef]
- Seymour, C.; Peralta, P.H.; Montgomery, G.G. Viruses Isolated from Panamanian Sloths. Am. J. Trop. Med. Hyg. 1982, 32, 1435–1444. [Google Scholar] [CrossRef]
- Medlin, S.; Deardorff, E.R.; Hanley, C.S.; Vergneau-grosset, C.; Siudak-campfield, A.; Dallwig, R.; Travassos, A.; Tesh, R.B.; Martin, M.P.; Weaver, S.C.; et al. Serosurvey of Selected Arboviral Pathogens in Free-Ranging, Two-Toed Sloths (Choloepus hoffmanni) and Three-Toed Sloths (Bradypus variegatus) in Costa Rica. J. Wildl. Dis. 2016, 52, 883–892. [Google Scholar] [CrossRef]
- Rivera, L.F.; Lezcano-Coba, C.; Galué, J.; Rodriguez, X.; Juarez, Y.; De Souza, W.M.; Capitan-Barrios, Z.; Valderrama, A.; Abrego, L.; Cedeño, H.; et al. Characteristics of Madariaga and Venezuelan Equine Encephalitis Virus Infections, Panama. Emerg. Infect. Dis. 2024, 30, 94–104. [Google Scholar] [CrossRef]
- Carrera, J.-P.; Pittí, Y.; Molares-Martínez, J.C.; Casal, E.; Pereyra-Elias, R.; Saenz, L.; Guerrero, I.; Galué, J.; Rodriguez-Alvarez, F.; Jackman, C.; et al. Clinical and Serological Findings of Madariaga and Venezuelan Equine Encephalitis Viral Infections: A Follow-up Study 5 Years After an Outbreak in Panama. Open Forum Infect. Dis. 2020, 7, ofaa359. [Google Scholar] [CrossRef]
- Vittor, A.Y.; Armien, B.; Gonzalez, P.; Carrera, J.P.; Dominguez, C.; Valderrama, A.; Glass, G.E.; Beltran, D.; Cisneros, J.; Wang, E.; et al. Epidemiology of Emergent Madariaga Encephalitis in a Region with Endemic Venezuelan Equine Encephalitis: Initial Host Studies and Human Cross-Sectional Study in Darien, Panama. PLoS Neglected Trop. Dis. 2016, 10, e0004554. [Google Scholar] [CrossRef]
- Galué, J.; De Souza, W.M.; Torres-Cosme, R.; Lezcano-Coba, C.; Tesh, R.B.; Guzman, H.; Weaver, S.C.; Capitan-Barrios, Z.; Valderraama, A.; Samudio, R.; et al. Contrasting Ecological Patterns of Venezuelan Equine Encephalitis and Madariaga Viruses in Small Mammal and Mosquito Populations from Two Enzootic Regions of Panama. Vector Borne Zoonotic Dis. 2025. ahead of print. [Google Scholar] [CrossRef]
- Deardorff, E.R.; Forrester, N.L.; Travassos Da Rosa, A.P.; Estrada-Franco, J.G.; Navarro-Lopez, R.; Tesh, R.B.; Weaver, S.C. Experimental Infection of Potential Reservoir Hosts with Venezuelan Equine Encephalitis Virus, Mexico. Emerg. Infect. Dis. 2009, 15, 519–525. [Google Scholar] [CrossRef]
- Carrara, A.-S.; Coffey, L.L.; Aguilar, P.V.; Moncayo, A.C.; Da Rosa, A.P.A.T.; Nunes, M.R.T.; Tesh, R.B.; Weaver, S.C. Venezuelan Equine Encephalitis Virus Infection of Cotton Rats. Emerg. Infect. Dis. 2007, 13, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Ferreira, M.; Martins, L.C.; De Vleeschouwer, K.M.; Cassano, C.R.; Oliveira, L.C.; Canale, G.; Deem, S.L.; Tello, J.S.; Parker, P.; et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. EcoHealth 2018, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.T.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo, R.D.S.D.S.; Travassos Da Rosa, A.P.A.; Vasconcelos, P.F.D.C. Oropouche Virus Isolation, Southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Travassos Da Rosa, A.P.A.; Gomes, M.L.C.; LeDuc, J.W.; Hoch, A.L. Transmission of Oropouche Virus from Man to Hamster by the Midge “Culicoides Paraensis”. Science 1982, 215, 1251–1253. [Google Scholar] [CrossRef]
- Travassos Da Rosa, J.F.; De Souza, W.M.; Pinheiro, F.D.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Naveca, F.G.; Almeida, T.A.P.D.; Souza, V.; Nascimento, V.; Silva, D.; Nascimento, F.; Mejía, M.; Oliveira, Y.S.D.; Rocha, L.; Xavier, N.; et al. Human Outbreaks of a Novel Reassortant Oropouche Virus in the Brazilian Amazon Region. Nat. Med. 2024, 30, 3509–3521. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; De Souza, W.M.; Savji, N.; Figueiredo, M.L.; Cardoso, J.F.; Da Silva, S.P.; Da Silva De Lima, C.P.; Vasconcelos, H.B.; Rodrigues, S.G.; Ian Lipkin, W.; et al. Oropouche Orthobunyavirus: Genetic Characterization of Full-Length Genomes and Development of Molecular Methods to Discriminate Natural Reassortments. Infect. Genet. Evol. 2019, 68, 16–22. [Google Scholar] [CrossRef]
- Valderrama, A.; Díaz, Y.; López-Vergès, S. Interaction of Flavivirus with Their Mosquito Vectors and Their Impact on the Human Health in the Americas. Biochem. Biophys. Res. Commun. 2017, 492, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Nuevo, K.M.; Cunha, M.S.; Luchs, A.; Fernandes, A.; Rocco, I.M.; Mucci, L.F.; De Souza, R.P.; Medeiros-Sousa, A.R.; Ceretti-Junior, W.; Marrelli, M.T. Detection of Zika and Dengue Viruses in Wild-Caught Mosquitoes Collected during Field Surveillance in an Environmental Protection Area in São Paulo, Brazil. PLoS ONE 2020, 15, e0227239. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the Forest: Prospects for the Continued Emergence of Sylvatic Dengue Virus and Its Impact on Public Health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus Fever: The Role of Host and Vector Susceptibility and Interspecific Competition in Shaping the Current and Future Distributions of the Sylvatic Cycles of Dengue Virus and Yellow Fever Virus. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef]
- Schwarz, E.R.; Long, M.T. Comparison of West Nile Virus Disease in Humans and Horses: Exploiting Similarities for Enhancing Syndromic Surveillance. Viruses 2023, 15, 1230. [Google Scholar] [CrossRef]
- Tesh, R.; Chaniotis, B.; Peralta, P.; Johnson, K. Ecology of Viruses Isolated from Panamanian Phlebotomine Sandflies. Am. J. Trop. Med. Hyg. 1974, 23, 258–269. [Google Scholar] [CrossRef]
- Romero-Ricardo, L.; Paternina-Tuirán, L.; Bejarano-Martínez, E. First Report of Phleboviruses in Phlebotomine Sandfly Communities from Northern Colombia. Actual. Biol. 2025, 47, e4708. [Google Scholar] [CrossRef]
- Saegerman, C.; Berkvens, D.; Mellor, P.S. Bluetongue Epidemiology in the European Union. Emerg. Infect. Dis. 2008, 14, 539–544. [Google Scholar] [CrossRef]
- Dennis, S.J.; Meyers, A.E.; Hitzeroth, I.I.; Rybicki, E.P. African Horse Sickness: A Review of Current Understanding and Vaccine Development. Viruses 2019, 11, 844. [Google Scholar] [CrossRef]
- Silva, S.P.; Dilcher, M.; Weber, F.; Hufert, F.T.; Weidmann, M.; Cardoso, J.F.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; Lima, C.P.S.; et al. Genetic and Biological Characterization of Selected Changuinola Viruses (Reoviridae, Orbivirus) from Brazil. J. Gen. Virol. 2014, 95, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-H.; Nason, E.; Staeuber, N.; Jiang, W.; Monastryrskaya, K.; Roy, P. RGD Tripeptide of Bluetongue Virus VP7 Protein Is Responsible for Core Attachment to Culicoides Cells. J. Virol. 2001, 75, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Roy, P. Expression and Functional Characterization of Bluetongue Virus VP2 Protein: Role in Cell Entry. J. Virol. 1999, 73, 9832–9842. [Google Scholar] [CrossRef] [PubMed]
- Forzan, M.; Marsh, M.; Roy, P. Bluetongue Virus Entry into Cells. J. Virol. 2007, 81, 4819–4827. [Google Scholar] [CrossRef]
- Huismans, H.; van Dijk, A.A.; Els, H.J. Uncoating of Parental Bluetongue Virus to Core and Subcore Particles in Infected L Cells. Virology 1987, 157, 180–188. [Google Scholar] [CrossRef]
- King, A.M.Q. Virus Taxonomy: Classification and Nomenclature of Viruses Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-384684-6. [Google Scholar]
- Yamaguchi, S.; Fukusho, A.; Roy, P. Complete Sequence of VP2 Gene of the Bluetongue Virus Serotype 1 (BTV-1). Nucl. Acids Res. 1988, 16, 2725. [Google Scholar] [CrossRef][Green Version]
- Bissett, S.L.; Roy, P. Impact of VP2 Structure on Antigenicity: Comparison of BTV1 and the Highly Virulent BTV8 Serotype. J. Virol. 2024, 98, e00953-24. [Google Scholar] [CrossRef]
- Schwartz-Cornil, I.; Mertens, P.P.C.; Contreras, V.; Hemati, B.; Pascale, F.; Bréard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue Virus: Virology, Pathogenesis and Immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef]
- Bonneau, K.R.; Zhang, N.; Zhu, J.; Zhang, F.; Li, Z.; Zhang, K.; Xiao, L.; Xiang, W.; MacLachlan, N.J. Sequence Comparison of the L2 and S10 Genes of Bluetongue Viruses from the United States and the People’s Republic of China. Virus Res. 1999, 61, 153–160. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Tyler, K.L. Orthoreoviruses and Orbiviruses. Mand. Douglas Bennett’s Princ. Pract. Infect. Dis. 2015, 1848–1850.e1. [Google Scholar] [CrossRef]
- Eastwood, G.; Loaiza, J.R.; Pongsiri, M.J.; Sanjur, O.I.; Pecor, J.E.; Auguste, A.J.; Kramer, L.D. Enzootic Arbovirus Surveillance in Forest Habitat and Phylogenetic Characterization of Novel Isolates of Gamboa Virus in Panama. Am. Soc. Trop. Med. Hyg. 2016, 94, 786–793. [Google Scholar] [CrossRef][Green Version]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.D.P.; Pissinatti, A.; Cunha, R.V.D.; Freire, M.; Martins, R.M.; Homma, A. Yellow Fever Outbreak in Brazil: The Puzzle of Rapid Viral Spread and Challenges for Immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Prist, P.R.; Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Marrelli, M.T. The Role of Forest Fragmentation in Yellow Fever Virus Dispersal. Acta Trop. 2023, 245, 106983. [Google Scholar] [CrossRef]
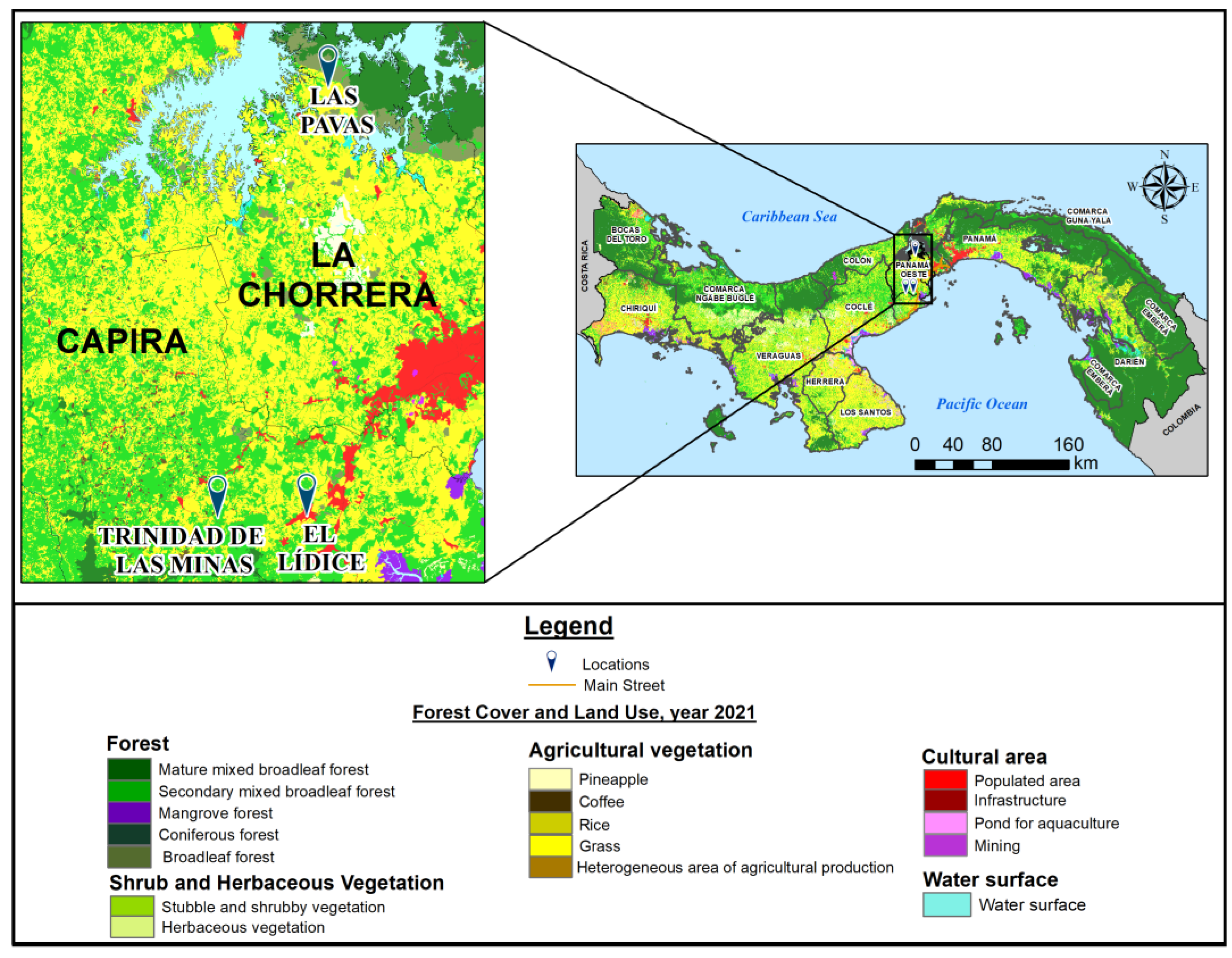
| Community | Subdistrict | District | Population Density (inhab./km2) | Distance from Panama City (km) | Coordinates | Altitude (m.a.s.I.) |
|---|---|---|---|---|---|---|
| Lidice | Lidice | Capira | 149.5 | 60 | 8°45′ N, 79°54′ W | 970 |
| Trinidad de las minas | Cacao | Capira | 31.2 | 85 | 8°46′32″ N, 79°59′45″ W | 230 |
| Las Pavas | Amador | La Chorrera | 25 | 99 | 9°6′15″ N, 79°53′9″ W | 50–156 |
| Viral Genus | Virus Species | Strain ID | Origin | Reference |
|---|---|---|---|---|
| Alphavirus | CHIKV | GMI-256137 | GMI | [23] |
| VEEV | TC-83 | GMI/WRCEVA | [24] | |
| MADV | GMI-267113 | GMI | [25] | |
| UNAV | BT-1495-3 | GMI/WRCEVA | [26] | |
| MAYV | CH1 | WRCEVA | [27] | |
| Orthoflavivirus | DENV-2 | 429557 (NR-12216) | WRCEVA | [28] |
| ZIKV | GMI-259249 | GMI | [12] | |
| Phlebovirus | PTV | GMI-483391 | GMI | [29] |
| Orthobunyavirus | OROV | TVP-14297 | WRCEVA | [30] |
| Orbivirus | D50 | - | GMI/WRCEVA | [17] |
| Pansloth149 | GMI-p149 | GMI/WRCEVA | — |
| Characteristics | N (%) |
|---|---|
| Year of collection | |
| 2013 | 23 (38.3%) |
| 2014 | 13 (21.7%) |
| 2015 | 6 (10.0%) |
| 2018 | 18 (30.0%) |
| Collection site | |
| Lidice | 27 (45.0%) |
| Las Pavas | 10 (16.7%) |
| Trinidad de Las Minas | 23 (37.3%) |
| Species | |
| Choloepus hoffmanni | 55 (91.7%) |
| Bradypus variegatus | 5 (8.3%) |
| Sex | |
| Female | 34 (56.7%) |
| Male | 26 (43.3%) |
| Virus | Positive | Titers (PRNT) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:20 | 1:40 | 1:80 | 1:160 | 1:320 | 1:640 | 1:1280 | 1:2560 | ||
| 4/60 | - | 3 | 1 | - | - | - | - | - |
| 4/60 | 4 | - | - | - | - | - | - | - |
| 2/42 | 2 | - | - | - | - | - | - | - |
| 14/60 | 1 | 7 | - | 2 | 3 | 1 | - | - |
| 32/60 | 4 | 9 | 2 | 5 | 7 | 4 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales, R.; Díaz, Y.; Pineda, V.; Pitti, Y.; Saenz, L.; Carrera, J.-P.; Aguilar, C.; Martínez, A.; Chen-Germán, M.; Hanley, K.A.; et al. Seroprevalence of Arboviruses and Genetic Characterization of Orbiviruses in Sloths from Western Panama. Viruses 2025, 17, 1507. https://doi.org/10.3390/v17111507
Corrales R, Díaz Y, Pineda V, Pitti Y, Saenz L, Carrera J-P, Aguilar C, Martínez A, Chen-Germán M, Hanley KA, et al. Seroprevalence of Arboviruses and Genetic Characterization of Orbiviruses in Sloths from Western Panama. Viruses. 2025; 17(11):1507. https://doi.org/10.3390/v17111507
Chicago/Turabian StyleCorrales, Rita, Yamilka Díaz, Vanessa Pineda, Yaneth Pitti, Lisseth Saenz, Jean-Paul Carrera, Celestino Aguilar, Alexander Martínez, Maria Chen-Germán, Kathryn A. Hanley, and et al. 2025. "Seroprevalence of Arboviruses and Genetic Characterization of Orbiviruses in Sloths from Western Panama" Viruses 17, no. 11: 1507. https://doi.org/10.3390/v17111507
APA StyleCorrales, R., Díaz, Y., Pineda, V., Pitti, Y., Saenz, L., Carrera, J.-P., Aguilar, C., Martínez, A., Chen-Germán, M., Hanley, K. A., Vasilakis, N., Tesh, R. B., Saldaña, A., & López-Vergès, S. (2025). Seroprevalence of Arboviruses and Genetic Characterization of Orbiviruses in Sloths from Western Panama. Viruses, 17(11), 1507. https://doi.org/10.3390/v17111507








