Molecular Characterization of Equine-like G3P[8] Rotavirus Strains Detected in South Korean Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and Processing
2.3. RNA Extraction and RT-PCR
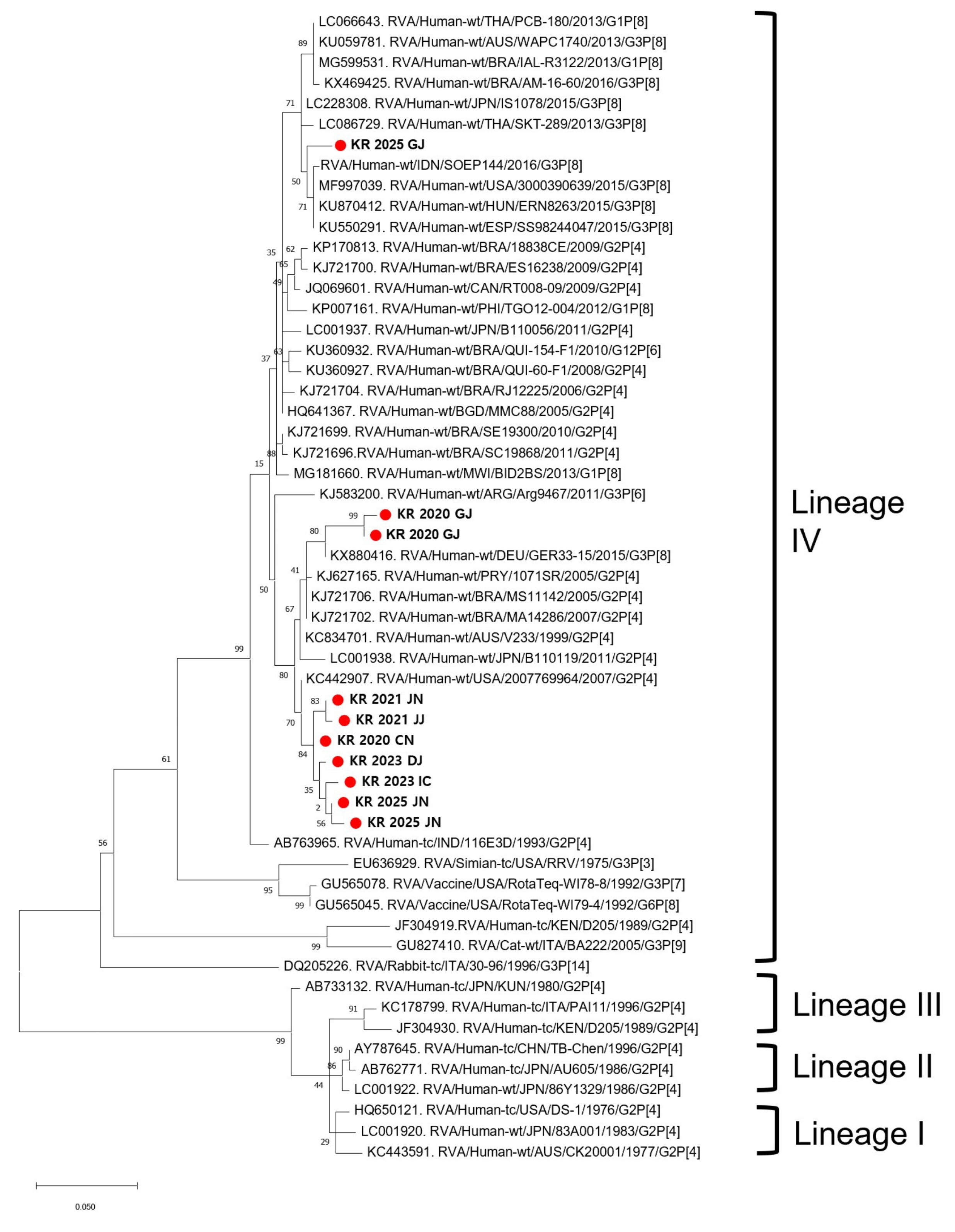
2.4. Phylogenetic Analysis
3. Results
3.1. VP7 and VP4 Gene Analysis
3.2. VP6 Gene Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RT-PCR | Reverse transcription-polymerase chain reaction |
| RVA | Rotavirus A |
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). J. Virol. 2010, 84, 3204–3219. [Google Scholar] [CrossRef] [PubMed]
- Chaiyaem, T.; Chanta, C.; Chan-it, W. An emergence of equine-like G3P[8] rotaviruses associated with acute gastroenteritis in hospitalized children in Thailand, 2016–2018. Microbiol. Biotechnol. Lett. 2021, 49, 120–129. [Google Scholar] [CrossRef]
- Donato, C.M.; Manuelpillai, N.M.; Cowley, D.; Roczo-Farkas, S.; Buttery, J.P.; Crawford, N.W.; Kirkwood, C.D. Genetic characterization of a novel G3P[14] rotavirus strain causing gastroenteritis in a 12-year-old Australian child. Infect. Genet. Evol. 2014, 25, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005, 15, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Bonura, F.; Mangiaracina, L.; Filizzolo, C.; Bonura, C.; Martella, V.; Ciarlet, M.; Giammanco, G.M.; De Grazia, S. Impact of vaccination on rotavirus genotype diversity: A nearly two-decade-long epidemiological study before and after rotavirus vaccine introduction in Sicily, Italy. Pathogens 2022, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Kim, M.S.; Jung, T.W.; Kim, S.J.; Kang, J.H.; Han, S.B.; Kim, S.Y.; Rhim, J.W.; Kim, H.M.; Park, J.H.; et al. Detection of rotavirus genotypes in Korea 5 years after the introduction of rotavirus vaccines. J. Korean Med. Sci. 2015, 30, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, Y.S.; Ha, D.J.; Chun, M.J.; Kwon, Y.S. Epidemiology of Rotavirus Gastroenteritis and Rotavirus-Associated Benign Convulsions with Mild Gastroenteritis after the Introduction of Rotavirus Vaccines in South Korea: Nationwide Data from the Health Insurance Review and Assessment Service. Environ. Res. Public. Health 2020, 17, 8374. [Google Scholar] [CrossRef] [PubMed]
- Komoto, S.; Ide, T.; Negoro, M.; Tanaka, T.; Asada, K.; Umemoto, M.; Kuroki, H.; Ito, H.; Tanaka, S.; Ito, M.; et al. Characterization of unusual DS-1-like G3P[8] rotavirus strains in children with diarrhea in Japan. J. Med. Virol. 2018, 90, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Vizzi, E.; Rosales, R.E.; Piñeros, O.; Fernández, R.; Inaty, D.; López, K.; Peña, L.; De Freitas-Linares, A.; Navarro, D.; Neri, S.; et al. Emergence of equine-like G3P[8] rotavirus strains infecting children in Venezuela. Viruses 2025, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, T.; Wahyuni, R.M.; Doan, Y.H.; Dinana, Z.; Soegijanto, S.; Fujii, Y.; Juniastuti; Yamani, L.N.; Matsui, C.; Deng, L.; et al. Equine-like G3 rotavirus strains as predominant strains among children in Indonesia in 2015–2016. Infect. Genet. Evol. 2018, 61, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Amit, L.N.; Mori, D.; John, J.L.; Chin, A.Z.; Mosiun, A.K.; Jeffree, M.S.; Ahmed, K. Emergence of equine-like G3 strains as the dominant rotavirus among children under five with diarrhea in Sabah, Malaysia during 2018–2019. PLoS ONE 2021, 16, e0254784. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jo, Y.; Park, J.Y.; Han, M.G. Recent trends in the circulation of viruses causing acute gastroenteritis in the Republic of Korea, 2019–2023. Public Health Wkly. Rep. 2024, 17, 1786–1801. [Google Scholar]
- World Health Organization. Manual of Rotavirus Detection and Characterization Methods; Document no. WHO/IVB/08. 17.; WHO: Geneva, Switzerland, 2009. Available online: https://iris.who.int/handle/10665/70122 (accessed on 1 May 2024).
- Lin, Y.P.; Kao, L.C.; Chang, S.Y.; Taniguchi, K.; Hung, P.Y.; Lin, H.C.; Huang, L.M.; Huang, H.H.; Yang, J.Y.; LEE, C.N. Determination of Human Rotavirus VP6 Genogroups I and II by Reverse Transcription-PCR. J. Clin. Microbiol. 2008, 46, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.B.; Arantes, I.; Bello, G.; Berto, L.H.; Dutra, L.H.; Kato, R.B.; Fumian, T.M. Emergence and dissemination of equine-like G3P[8] rotavirus A in Brazil between 2015 and 2021. Microbiol. Spectr. 2024, 12, e03709-23. [Google Scholar] [CrossRef] [PubMed]
- Manjate, F.; João, E.D.; Mwangi, P.; Chirinda, P.; Mogotsi, M.; Messa, A., Jr.; Garrine, M.; Vubil, D.; Nobela, N.; Nhampossa, T.; et al. Genomic characterization of the rotavirus G3P[8] strain in vaccinated children, reveals possible reassortment events between human and animal strains in Manhiça District, Mozambique. Front. Microbiol. 2023, 14, 1193094. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.; Lee, M.; Lee, D.-Y.; Han, M.-G.; Park, S.-W. Molecular Characterization of Equine-like G3P[8] Rotavirus Strains Detected in South Korean Children. Viruses 2025, 17, 1488. https://doi.org/10.3390/v17111488
Jo Y, Lee M, Lee D-Y, Han M-G, Park S-W. Molecular Characterization of Equine-like G3P[8] Rotavirus Strains Detected in South Korean Children. Viruses. 2025; 17(11):1488. https://doi.org/10.3390/v17111488
Chicago/Turabian StyleJo, Yunhee, Minji Lee, Deog-Yong Lee, Myung-Guk Han, and Sun-Whan Park. 2025. "Molecular Characterization of Equine-like G3P[8] Rotavirus Strains Detected in South Korean Children" Viruses 17, no. 11: 1488. https://doi.org/10.3390/v17111488
APA StyleJo, Y., Lee, M., Lee, D.-Y., Han, M.-G., & Park, S.-W. (2025). Molecular Characterization of Equine-like G3P[8] Rotavirus Strains Detected in South Korean Children. Viruses, 17(11), 1488. https://doi.org/10.3390/v17111488







