The Cold Case Files of rAAV Capsid Influence on Transduction: New Leads
Abstract
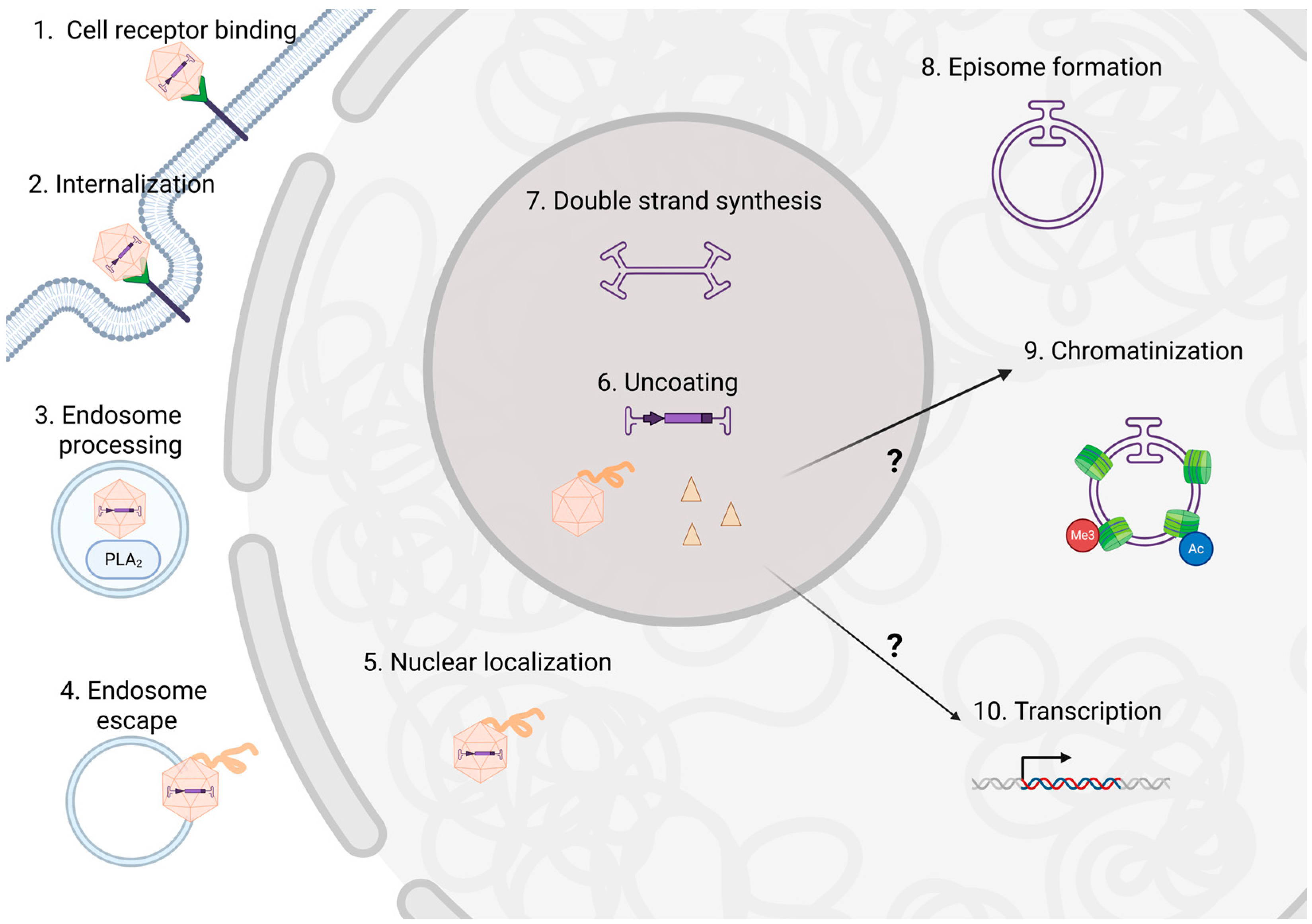
1. Introduction
2. rAAV Capsid in the Nucleus
3. Capsid-Dependent rAAV Transgene Modulation After Nuclear Entry
4. rAAV Capsid-Interacting Proteins That Alter Transduction
5. rAAV Capsid Influence on Transgene Chromatinization
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | Amino acid |
| AAP | Assembly-activating protein |
| AAV | Adeno-associated virus |
| Ad5 | Adenovirus 5 |
| BR3 | Basic region 3 |
| CAG | Chicken β-actin promoter with CMV enhancer and rabbit β-globin slice acceptor site |
| CBA | Chicken β-actin promoter with hybrid CMV enhancer |
| CBh | Chicken β-actin hybrid promoter with MVM intron |
| CDK2 | Cyclin-dependent kinase 2 |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| DNA-PKcs | DNA protein kinases |
| HDAC | Histone deacetylase |
| hnRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 |
| hSyn | Human synapsin 1 promoter |
| HUSH | Human silencing hub |
| JeTI | Synthetic promoter with intron |
| MAAP | Membrane-associated accessory protein |
| MPP8 | M-phase phosphoprotein 8 |
| NHP | Non-human primate |
| NLS | Nuclear localization signal |
| NP220 | Nuclear protein 220 |
| PHF5A | PHD finger protein 5A |
| PHD | Plant homeodomain |
| PLA2 | Phospholipase A2 |
| PPHLN1 | Periphilin 1 |
| RNF121 | Ring finger protein 121 |
| SETDB1 | SET domain bifurcated 1 |
| SH2 | Src homology 2 |
| TASOR | Transgene activation suppressor protein |
| TDP-43 | Transactive response DNA-binding protein 43kDa |
| TSA | Trichostatin A |
| VCP/p97 | Valosin-containing protein |
| VP | Viral protein |
References
- Kerr, J.R. Parvoviruses; Hodder Arnold: London, UK; Distributed in the United States of America by Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Sonntag, F.; Schmidt, K.; Kleinschmidt, J.A. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. USA 2010, 107, 10220–10225. [Google Scholar] [CrossRef] [PubMed]
- Ogden, P.J.; Kelsic, E.D.; Sinai, S.; Church, G.M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 2019, 366, 1139–1143. [Google Scholar] [CrossRef]
- Aksu Kuz, C.; Ning, K.; Hao, S.; Cheng, F.; Qiu, J. Role of the membrane-associated accessory protein (MAAP) in adeno-associated virus (AAV) infection. J. Virol. 2024, 98, e0063324. [Google Scholar] [CrossRef]
- Galibert, L.; Hyvonen, A.; Eriksson, R.A.E.; Mattola, S.; Aho, V.; Salminen, S.; Albers, J.D.; Peltola, S.K.; Weman, S.; Nieminen, T.; et al. Functional roles of the membrane-associated AAV protein MAAP. Sci. Rep. 2021, 11, 21698. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; Padron, E.; McKenna, R.; Muzyczka, N.; Kaludov, N.; Chiorini, J.A.; Agbandje-McKenna, M. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J. Virol. 2006, 80, 11556–11570. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Lane, M.D.; Padron, E.; Gurda, B.; McKenna, R.; Kohlbrenner, E.; Aslanidi, G.; Byrne, B.; Muzyczka, N.; Zolotukhin, S.; et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol. 2007, 81, 12260–12271. [Google Scholar] [CrossRef]
- Xie, Q.; Bu, W.; Bhatia, S.; Hare, J.; Somasundaram, T.; Azzi, A.; Chapman, M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 10405–10410. [Google Scholar] [CrossRef]
- Mietzsch, M.; Penzes, J.J.; Agbandje-McKenna, M. Twenty-Five Years of Structural Parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef]
- Kronenberg, S.; Kleinschmidt, J.A.; Bottcher, B. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. EMBO Rep. 2001, 2, 997–1002. [Google Scholar] [CrossRef]
- Girod, A.; Wobus, C.E.; Zadori, Z.; Ried, M.; Leike, K.; Tijssen, P.; Kleinschmidt, J.A.; Hallek, M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002, 83 Pt. 5, 973–978. [Google Scholar] [CrossRef]
- Sonntag, F.; Bleker, S.; Leuchs, B.; Fischer, R.; Kleinschmidt, J.A. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J. Virol. 2006, 80, 11040–11054. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Snowdy, S.; Samulski, R.J. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J. Virol. 2006, 80, 5199–5210. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016, 21, 75–80. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An essential receptor for adeno-associated virus infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Dhungel, B.P.; Xu, H.; Nagarajah, R.; Vitale, J.; Wong, A.C.; Gokal, D.; Feng, Y.; Tabar, M.S.; Metierre, C.; Parsania, C.; et al. An alternate receptor for adeno-associated viruses. Cell 2025, 188, 4924–4935. [Google Scholar] [CrossRef]
- Dhungel, B.P.; Bailey, C.G.; Rasko, J.E.J. Journey to the Center of the Cell: Tracing the Path of AAV Transduction. Trends Mol. Med. 2021, 27, 172–184. [Google Scholar] [CrossRef]
- Zhong, L.; Li, B.; Jayandharan, G.; Mah, C.S.; Govindasamy, L.; Agbandje-McKenna, M.; Herzog, R.W.; Weigel-Van Aken, K.A.; Hobbs, J.A.; Zolotukhin, S.; et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 2008, 381, 194–202. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, W.; Wu, J.; Li, B.; Zolotukhin, S.; Govindasamy, L.; Agbandje-McKenna, M.; Srivastava, A. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 2007, 15, 1323–1330. [Google Scholar] [CrossRef]
- Miao, C.H.; Nakai, H.; Thompson, A.R.; Storm, T.A.; Chiu, W.; Snyder, R.O.; Kay, M.A. Nonrandom transduction of recombinant adeno-associated virus vectors in mouse hepatocytes in vivo: Cell cycling does not influence hepatocyte transduction. J. Virol. 2000, 74, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.O.; Miao, C.H.; Patijn, G.A.; Spratt, S.K.; Danos, O.; Nagy, D.; Gown, A.M.; Winther, B.; Meuse, L.; Cohen, L.K.; et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 1997, 16, 270–276. [Google Scholar] [CrossRef]
- Johnson, J.S.; Li, C.; DiPrimio, N.; Weinberg, M.S.; McCown, T.J.; Samulski, R.J. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J. Virol. 2010, 84, 8888–8902. [Google Scholar] [CrossRef]
- Salganik, M.; Aydemir, F.; Nam, H.J.; McKenna, R.; Agbandje-McKenna, M.; Muzyczka, N. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J. Virol. 2014, 88, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Samulski, R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009, 83, 2632–2644. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Storm, T.A.; Huang, Z.; Kay, M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004, 78, 3110–3122. [Google Scholar] [CrossRef]
- Sipo, I.; Fechner, H.; Pinkert, S.; Suckau, L.; Wang, X.; Weger, S.; Poller, W. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007, 14, 1319–1329. [Google Scholar] [CrossRef]
- Sutter, S.O.; Lkharrazi, A.; Schraner, E.M.; Michaelsen, K.; Meier, A.F.; Marx, J.; Vogt, B.; Buning, H.; Fraefel, C. Adeno-associated virus type 2 (AAV2) uncoating is a stepwise process and is linked to structural reorganization of the nucleolus. PLoS Pathog. 2022, 18, e1010187. [Google Scholar] [CrossRef]
- Bernaud, J.; Rossi, A.; Fis, A.; Gardette, L.; Aillot, L.; Buning, H.; Castelnovo, M.; Salvetti, A.; Faivre-Moskalenko, C. Characterization of AAV vector particle stability at the single-capsid level. J. Biol. Phys. 2018, 44, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Gliwa, K.; Hull, J.; Kansol, A.; Zembruski, V.; Lakshmanan, R.; Mietzsch, M.; Chipman, P.; Bennett, A.; McKenna, R. Biophysical and structural insights into AAV genome ejection. J. Virol. 2025, 99, e0089924. [Google Scholar] [CrossRef]
- Bennett, A.; Patel, S.; Mietzsch, M.; Jose, A.; Lins-Austin, B.; Yu, J.C.; Bothner, B.; McKenna, R.; Agbandje-McKenna, M. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. Methods Clin. Dev. 2017, 6, 171–182. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Kruse, S.; Kant, R.; Venkatakrishnan, B.; Movahed, N.; Brooke, D.; Lins, B.; Bennett, A.; Potter, T.; McKenna, R.; et al. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013, 87, 13150–13160. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Hafenstein, S.; Tattersall, P. Depletion of virion-associated divalent cations induces parvovirus minute virus of mice to eject its genome in a 3’-to-5’ direction from an otherwise intact viral particle. J. Virol. 2010, 84, 1945–1956. [Google Scholar] [CrossRef]
- Ros, C.; Baltzer, C.; Mani, B.; Kempf, C. Parvovirus uncoating in vitro reveals a mechanism of DNA release without capsid disassembly and striking differences in encapsidated DNA stability. Virology 2006, 345, 137–147. [Google Scholar] [CrossRef]
- Popa-Wagner, R.; Porwal, M.; Kann, M.; Reuss, M.; Weimer, M.; Florin, L.; Kleinschmidt, J.A. Impact of VP1-specific protein sequence motifs on adeno-associated virus type 2 intracellular trafficking and nuclear entry. J. Virol. 2012, 86, 9163–9174. [Google Scholar] [CrossRef]
- Robinson, T.M.; Ho, M.L.; Wahlig, B.; Gough, V.; Banta, A.; Reyes Gamas, K.; Kang, B.; Lee, E.; Chen, W.; Suh, J. An essential N-terminal serine-rich motif in the AAV VP1 and VP2 subunits that may play a role in viral transcription. Virology 2020, 546, 127–132. [Google Scholar] [CrossRef]
- Chen, M.Y.; Chen, W.; Tong, J.; Ho, M.L.; Suh, J. N-terminal serine/threonine motif has diverse and important effects on behavior of multiple AAV serotypes. Virology 2021, 563, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.K.; Samulski, R.J.; McCown, T.J. AAV Capsid-Promoter Interactions Determine CNS Cell-Selective Gene Expression In Vivo. Mol. Ther. 2020, 28, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, M.O.; McCown, T.J.; Powell, S.K.; El-Nahal, H.G.; Daw, T.; Basso, M.A.; Sommer, M.A.; Samulski, R.J. Adeno-Associated Virus Capsid-Promoter Interactions in the Brain Translate from Rat to the Nonhuman Primate. Hum. Gene Ther. 2020, 31, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.K.; McCown, T.J. Adeno-associated virus 9 (AAV9) viral proteins VP1, VP2, and membrane-associated accessory protein (MAAP) differentially influence in vivo transgene expression. J. Virol. 2024, 98, e0168124. [Google Scholar] [CrossRef]
- Elmore, Z.C.; Patrick Havlik, L.; Oh, D.K.; Anderson, L.; Daaboul, G.; Devlin, G.W.; Vincent, H.A.; Asokan, A. The membrane associated accessory protein is an adeno-associated viral egress factor. Nat. Commun. 2021, 12, 6239. [Google Scholar] [CrossRef]
- Nam, H.J.; Gurda, B.L.; McKenna, R.; Potter, M.; Byrne, B.; Salganik, M.; Muzyczka, N.; Agbandje-McKenna, M. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J. Virol. 2011, 85, 11791–11799. [Google Scholar] [CrossRef]
- Aydemir, F.; Salganik, M.; Resztak, J.; Singh, J.; Bennett, A.; Agbandje-McKenna, M.; Muzyczka, N. Mutants at the 2-Fold Interface of Adeno-associated Virus Type 2 (AAV2) Structural Proteins Suggest a Role in Viral Transcription for AAV Capsids. J. Virol. 2016, 90, 7196–7204. [Google Scholar] [CrossRef]
- Warrington, K.H., Jr.; Gorbatyuk, O.S.; Harrison, J.K.; Opie, S.R.; Zolotukhin, S.; Muzyczka, N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J. Virol. 2004, 78, 6595–6609. [Google Scholar] [CrossRef]
- Maurer, A.C.; Weitzman, M.D. Adeno-Associated Virus Genome Interactions Important for Vector Production and Transduction. Hum. Gene Ther. 2020, 31, 499–511. [Google Scholar] [CrossRef]
- Madigan, V.J.; Yuziuk, J.A.; Chiarella, A.M.; Tyson, T.O.; Meganck, R.M.; Elmore, Z.C.; Tse, L.V.; Hathaway, N.A.; Asokan, A. Ring finger protein 121 is a potent regulator of adeno-associated viral genome transcription. PLoS Pathog. 2019, 15, e1007988. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yue, Y.; Yan, Z.; Yang, J.; Engelhardt, J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Investig. 2000, 105, 1573–1587. [Google Scholar] [CrossRef]
- Murphy, S.L.; Bhagwat, A.; Edmonson, S.; Zhou, S.; High, K.A. High-throughput screening and biophysical interrogation of hepatotropic AAV. Mol. Ther. 2008, 16, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Chandran, J.S.; Sharp, P.S.; Karyka, E.; Aves-Cruzeiro, J.; Coldicott, I.; Castelli, L.; Hautbergue, G.; Collins, M.O.; Azzouz, M. Site Specific Modification of Adeno-Associated Virus Enables Both Fluorescent Imaging of Viral Particles and Characterization of the Capsid Interactome. Sci. Rep. 2017, 7, 14766. [Google Scholar] [CrossRef] [PubMed]
- Loeb, E.J.; Havlik, P.L.; Elmore, Z.C.; Rosales, A.; Fergione, S.M.; Gonzalez, T.J.; Smith, T.J.; Benkert, A.R.; Fiflis, D.N.; Asokan, A. Capsid-mediated control of adeno-associated viral transcription determines host range. Cell Rep. 2024, 43, 113902. [Google Scholar] [CrossRef]
- Schreiber, C.A.; Sakuma, T.; Izumiya, Y.; Holditch, S.J.; Hickey, R.D.; Bressin, R.K.; Basu, U.; Koide, K.; Asokan, A.; Ikeda, Y. An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses. PLoS Pathog. 2015, 11, e1005082. [Google Scholar] [CrossRef]
- Brown, M.; Weber, J. Adenoassociated virus has a unique chromatin structure. Can. J. Biochem. 1982, 60, 1001–1005. [Google Scholar] [CrossRef]
- Marcus-Sekura, C.J.; Carter, B.J. Chromatin-like structure of adeno-associated virus DNA in infected cells. J. Virol. 1983, 48, 79–87. [Google Scholar] [CrossRef]
- McCown, T.J.; Xiao, X.; Li, J.; Breese, G.R.; Samulski, R.J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996, 713, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Meyer, E.M.; Peel, A.L.; Zolotukhin, S.; Meyers, C.; Muzyczka, N.; King, M.A. Neuron-specific transduction in the rat septohippocampal or nigrostriatal pathway by recombinant adeno-associated virus vectors. Exp. Neurol. 1998, 150, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Prosch, S.; Stein, J.; Staak, K.; Liebenthal, C.; Volk, H.D.; Kruger, D.H. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol. Chem. Hoppe Seyler 1996, 377, 195–201. [Google Scholar] [CrossRef]
- Chen, W.Y.; Bailey, E.C.; McCune, S.L.; Dong, J.Y.; Townes, T.M. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc. Natl. Acad. Sci. USA 1997, 94, 5798–5803. [Google Scholar] [CrossRef]
- Chen, W.Y.; Townes, T.M. Molecular mechanism for silencing virally transduced genes involves histone deacetylation and chromatin condensation. Proc. Natl. Acad. Sci. USA 2000, 97, 377–382. [Google Scholar] [CrossRef]
- Okada, T.; Uchibori, R.; Iwata-Okada, M.; Takahashi, M.; Nomoto, T.; Nonaka-Sarukawa, M.; Ito, T.; Liu, Y.; Mizukami, H.; Kume, A.; et al. A histone deacetylase inhibitor enhances recombinant adeno-associated virus-mediated gene expression in tumor cells. Mol. Ther. 2006, 13, 738–746. [Google Scholar] [CrossRef]
- Qiu, J.; Brown, K.E. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology 1999, 257, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Vijayan, M.; Walton, E.M.; Stafford, V.G.; Fiflis, D.N.; Asokan, A. Epigenetic Silencing of Recombinant Adeno-associated Virus Genomes by NP220 and the HUSH Complex. J. Virol. 2022, 96, e0203921. [Google Scholar] [CrossRef]
- Muller, I.; Helin, K. Keep quiet: The HUSH complex in transcriptional silencing and disease. Nat. Struct. Mol. Biol. 2024, 31, 11–22. [Google Scholar] [CrossRef]
- Tchasovnikarova, I.A.; Timms, R.T.; Matheson, N.J.; Wals, K.; Antrobus, R.; Gottgens, B.; Dougan, G.; Dawson, M.A.; Lehner, P.J. GENE SILENCING. Epigenetic silencing by the HUSH complex mediates position-effect variegation in human cells. Science 2015, 348, 1481–1485. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, G.Z.; Cingoz, O.; Goff, S.P. NP220 mediates silencing of unintegrated retroviral DNA. Nature 2018, 564, 278–282. [Google Scholar] [CrossRef]
- Gonzalez-Sandoval, A.; Pekrun, K.; Tsuji, S.; Zhang, F.; Hung, K.L.; Chang, H.Y.; Kay, M.A. The AAV capsid can influence the epigenetic marking of rAAV delivered episomal genomes in a species dependent manner. Nat. Commun. 2023, 14, 2448. [Google Scholar] [CrossRef]
- Penaud-Budloo, M.; Le Guiner, C.; Nowrouzi, A.; Toromanoff, A.; Cherel, Y.; Chenuaud, P.; Schmidt, M.; von Kalle, C.; Rolling, F.; Moullier, P.; et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008, 82, 7875–7885. [Google Scholar] [CrossRef] [PubMed]
- Hauck, B.; Zhao, W.; High, K.; Xiao, W. Intracellular viral processing, not single-stranded DNA accumulation, is crucial for recombinant adeno-associated virus transduction. J. Virol. 2004, 78, 13678–13686. [Google Scholar] [CrossRef] [PubMed]
- Stieger, K.; Schroeder, J.; Provost, N.; Mendes-Madeira, A.; Belbellaa, B.; Le Meur, G.; Weber, M.; Deschamps, J.Y.; Lorenz, B.; Moullier, P.; et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol. Ther. 2009, 17, 516–523. [Google Scholar] [CrossRef] [PubMed]
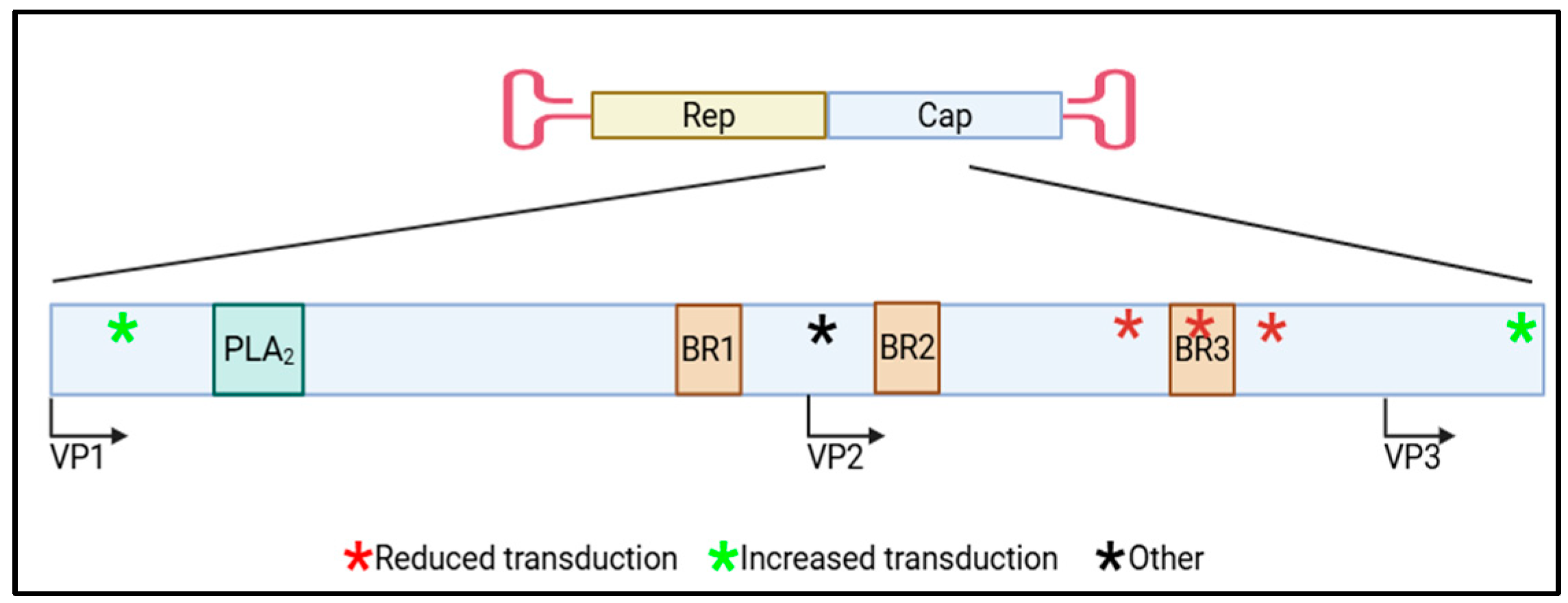
| Capsid | Mutation/Residues(s) | Effect | Ref |
|---|---|---|---|
| rAAV2 | A167K | Reduced rAAV transduction | [23] |
| wtAAV2 | Y704A | Reduced wtAAV transduction | [24] |
| wtAAV2 | D529A, K692A, and E562A individually | Reduced wtAAV transduction | [43] |
| rAAV2 | D154A, S155A, S156A, and G158A individually | Reduced rAAV transduction | [36] |
| rAAV2 | S155A, S156A, and S157A together | Reduced rAAV transduction | [37] |
| rAAV2 | PARKRL (BR3) | Reduced rAAV transduction | [48] |
| rAAV8 | PARKRL (BR3) | Reduced rAAV transduction | [48] |
| rAAV9 | S155A and S156A together | Reduced rAAV transduction | [37] |
| rAAV9 | S155A and S156A together | Reduced rAAV transduction | [37] |
| rAAV9 | 6 glutamate inserts at aa139 | Altered rAAV cellular tropism | [38] |
| rAAV9 | 6 alanine inserts at aa139 | Altered rAAV cellular tropism | [39] |
| rAAV2-retro | Altered rAAV cellular tropism | [39] | |
| Avian rAAV | rAAV8 VP1/2 (avian-8.1) | Increased rAAV transduction | [50] |
| Avian rAAV | N-terminal rAAV8 VP1 (avian-8.3) | Increased rAAV transduction | [50] |
| rAAV-LK03 | G266 = rAAV − AM | Increased rAAV transduction | [65] |
| Capsid(s) | Protein | Function | Ref |
|---|---|---|---|
| rAAV1, rAAV2, rAAV6, and rAAV9 | RNF121 (Ring finger protein 121) | Supports rAAV transduction | [46] |
| rAAV2 and rAAV8 | CDK2/cyclinA | Inhibits rAAV transduction | [48] |
| rAAV9 | TDP-43 (transactive response DNA-binding protein 43kDa) | Binding validated by Western blot | [49] |
| rAAV9 | hnRNPA1 (heterogeneous nuclear ribonucleoprotein A1) | Binding validated by Western blot | [49] |
| rAAV9 | HDAC4 (histone deacetylase 4) | Inhibits rAAV transduction | [49] |
| rAAV2, rAAV6, rAAV8, and rAAV9 | PHF5A (PHD finger protein 5A) | Inhibits rAAV transduction | [51] |
| rAAV2, rAAV5, rAAV6, rAAV8, and rAAV.rh32.33 | NP220 (DNA-binding protein) | Inhibits rAAV transduction (discordant transgene mRNA and protein levels) | [61] |
| rAAV2 | SETDB1 (H3K9 methyltransferase) | Inhibits rAAV transduction (under certain conditions) | [61] |
| rAAV2 | PPHLN1 (periphilin) | Inhibits rAAV transduction (under certain conditions) | [61] |
| rAAV2 | MPP8 (M-phase phosphoprotein 8) | Inhibits rAAV transduction (under certain conditions) | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, S.K. The Cold Case Files of rAAV Capsid Influence on Transduction: New Leads. Viruses 2025, 17, 1476. https://doi.org/10.3390/v17111476
Powell SK. The Cold Case Files of rAAV Capsid Influence on Transduction: New Leads. Viruses. 2025; 17(11):1476. https://doi.org/10.3390/v17111476
Chicago/Turabian StylePowell, Sara K. 2025. "The Cold Case Files of rAAV Capsid Influence on Transduction: New Leads" Viruses 17, no. 11: 1476. https://doi.org/10.3390/v17111476
APA StylePowell, S. K. (2025). The Cold Case Files of rAAV Capsid Influence on Transduction: New Leads. Viruses, 17(11), 1476. https://doi.org/10.3390/v17111476





