Baculovirus-Mediated Gene Therapy: Targeting BIRC6 for Lung and Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids Encoding birc6-Specific Short Hairpin RNA
2.2. Generation of Recombinant Baculoviruses
2.3. Tumor Cell Cultures
2.4. BV-Based Gene Transduction
2.5. Expression of BIRC6 by Immunofluorescence
2.6. Expression of BIRC6 by Flow Cytometry
2.7. Microscopic Detection of DNA Fragmentation by TUNEL
2.8. RNA Isolation and RT-qPCR
2.9. Animals
2.10. Breast Cancer Experimental Model
2.11. Lung Cancer Experimental Model
2.12. Tumor Necrosis Assessment
2.13. Statistical Analysis
3. Results
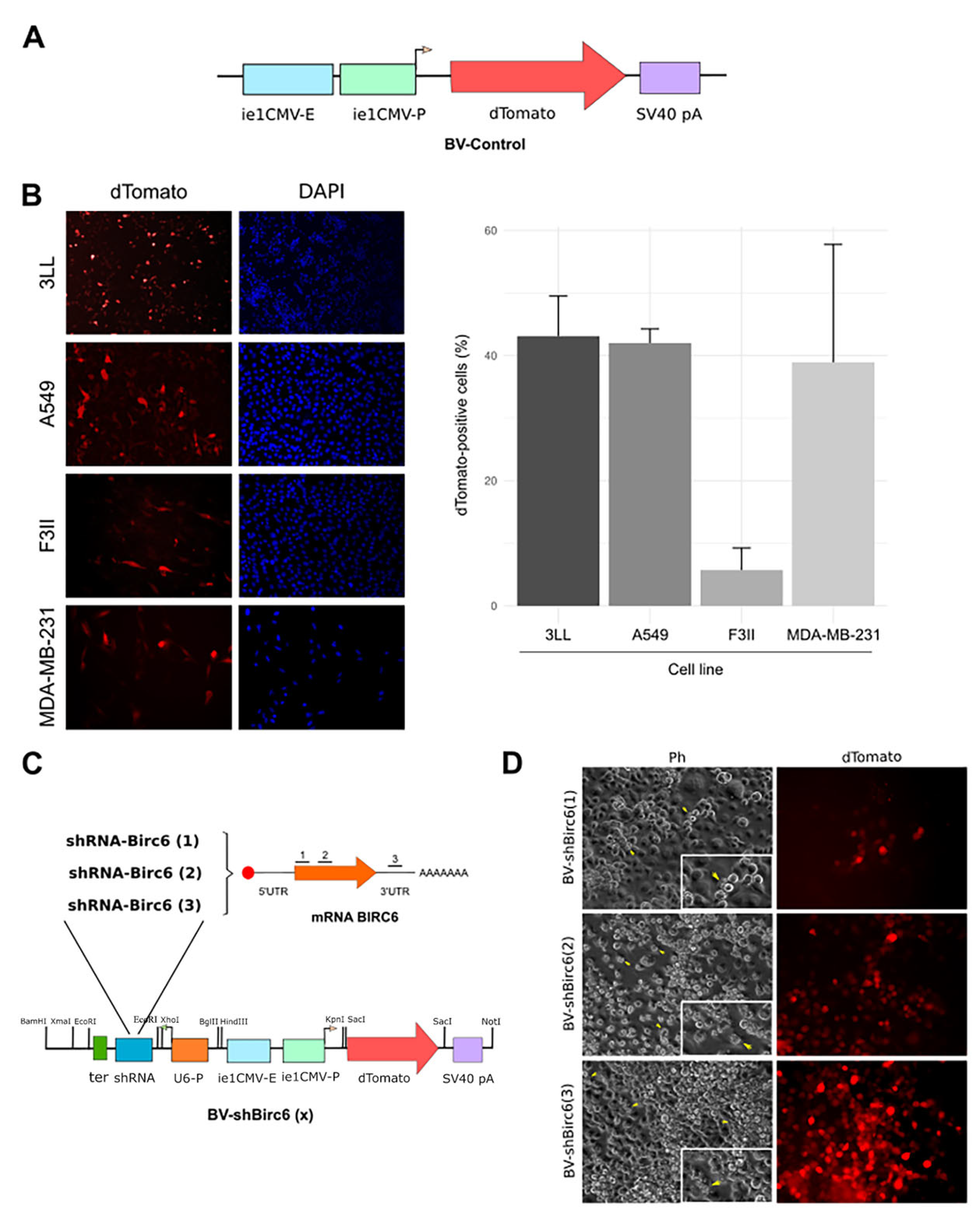
3.1. Baculoviruses Efficiently Transduced Human and Murine Lung and Breast Cancer Cells
3.2. Generation of BIRC6-Silencing Baculoviral Vector
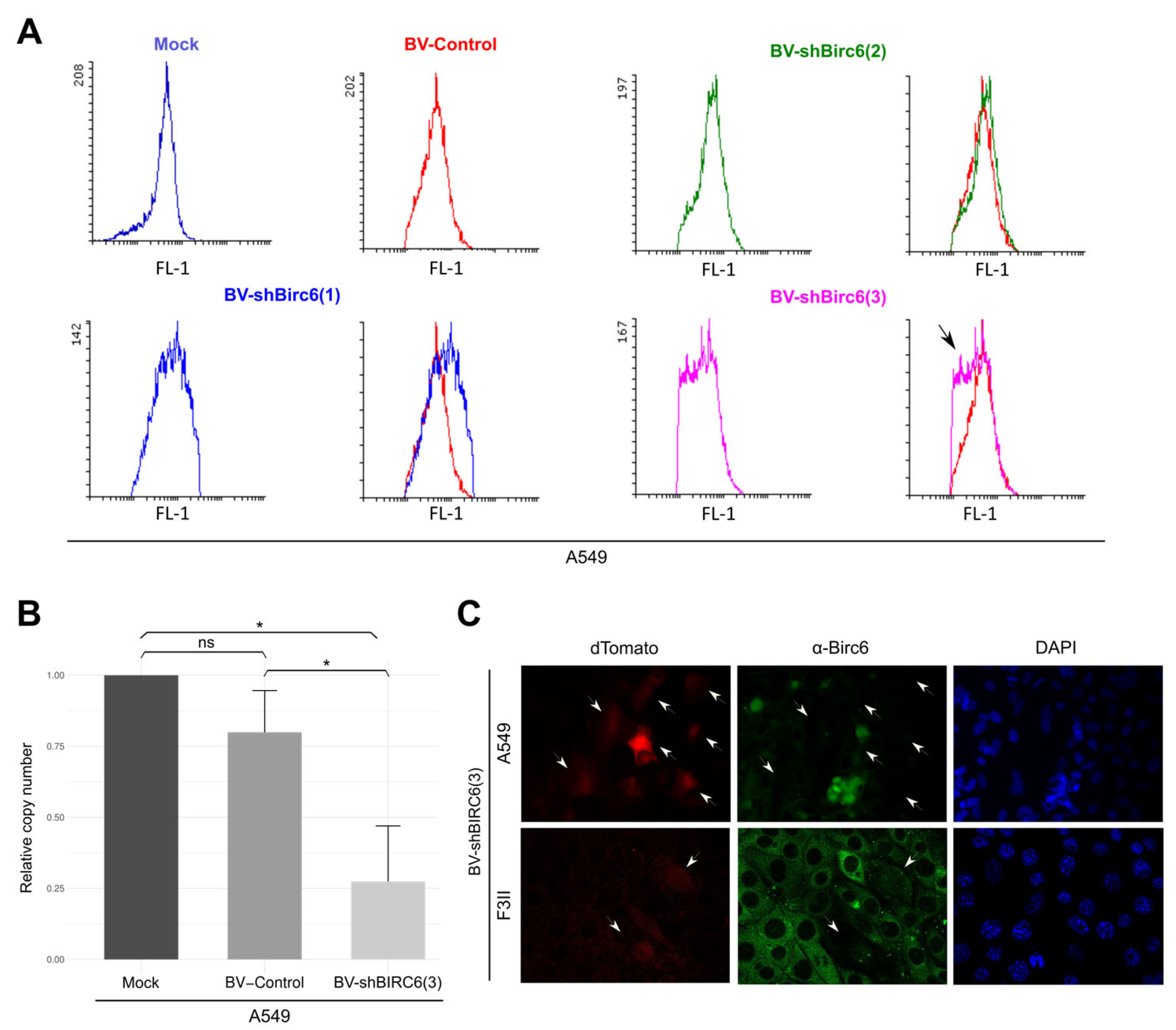
3.3. Effect of BIRC6 Silencing In Vitro
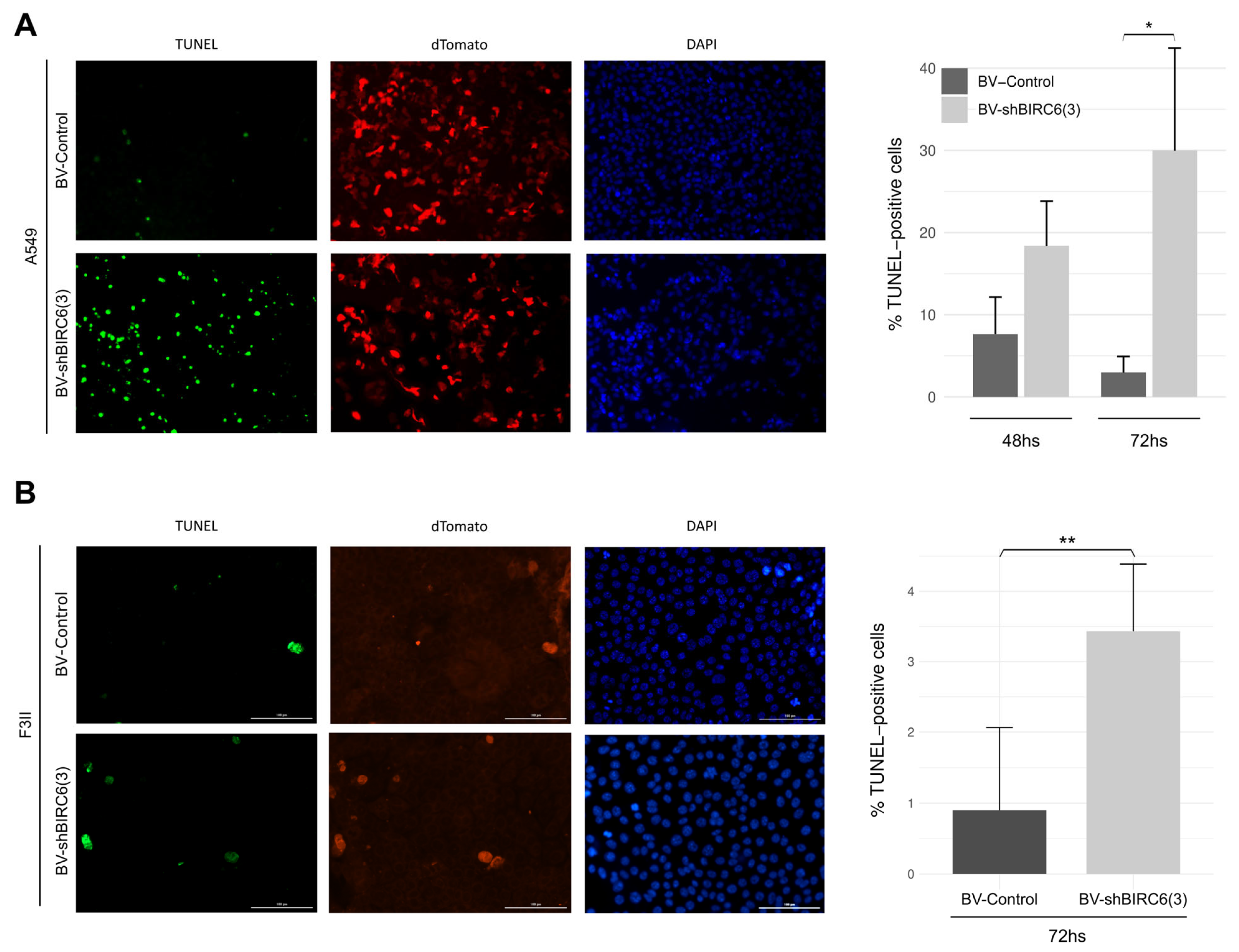
3.4. BIRC6 Silencing Induced Apoptosis In Vitro
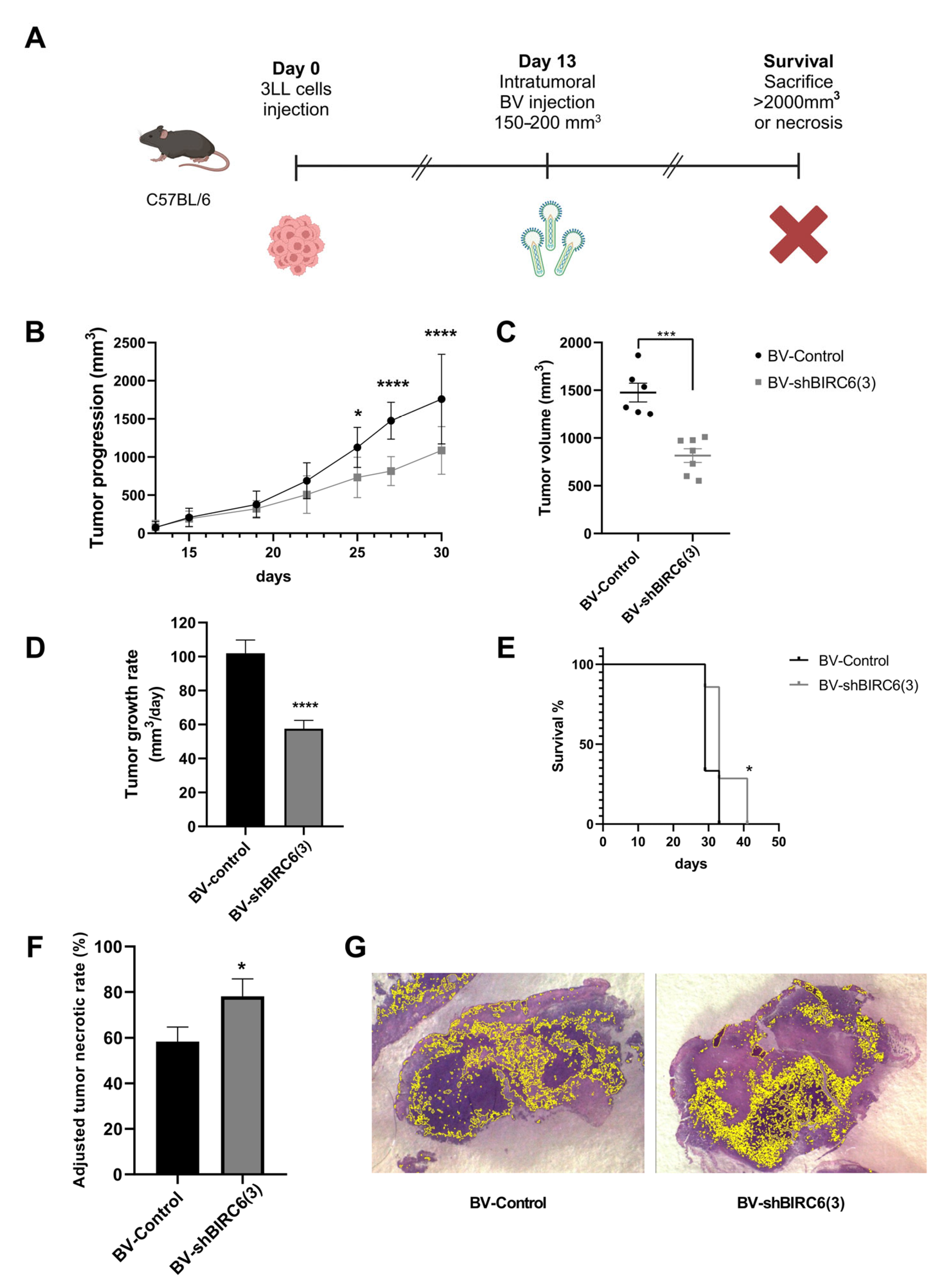
3.5. Baculovirus-Mediated BIRC6 Silencing Reduced Tumor Growth and Increased Survival in Mice Experimental Models of Lung Cancer
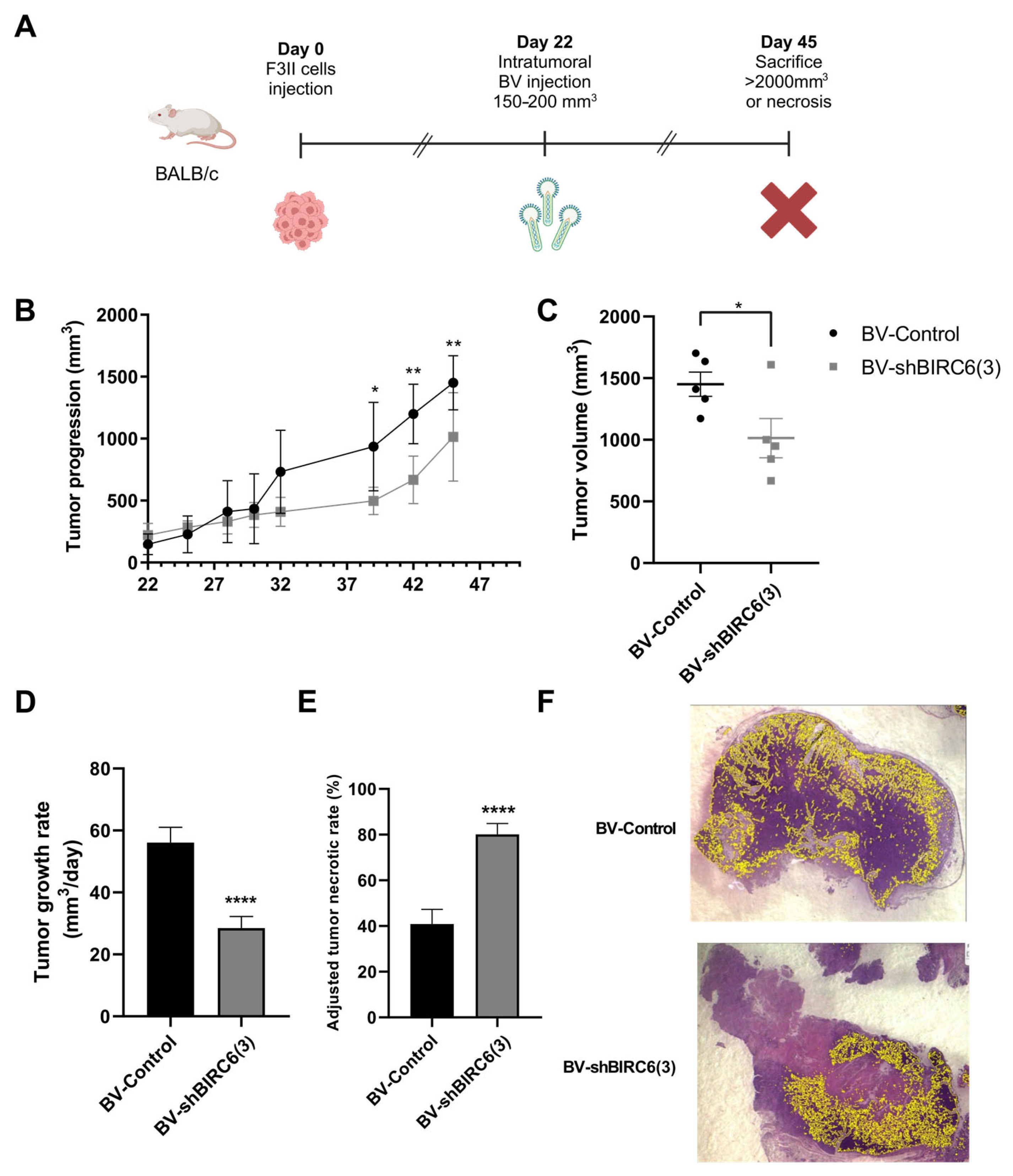
3.6. BIRC6 Silencing Reduced Tumor Growth in Mice Experimental Models of Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIRC6 | Baculoviral IAP repeat containing 6 |
| IAP | Inhibitor of apoptosis protein |
| shRNA | Short hairpin RNA |
| BV | Baculovirus |
| AcMNPV | Autographa californica multiple nucleopolyhedrovirus |
| BSL-1 | Biosafety level 1 |
| PCD | Programmed cell death |
| BIRs | Baculoviral IAP repeats |
| BC | Breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal receptor 2 |
| TNBC | Triple-negative breast cancer |
| SCLC | Small cell lung cancer |
| NSCLC | Non-small cell lung cancer |
| PFUs | Plaque forming units |
| DAPI | 4′,6-diamidino-2-fenilindol |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling |
| ATNR | Adjusted necrotic rate |
| VA | Viable area |
| NA | Necrotic area |
| RTGR | Relative tumor growth rates |
| TGR | Tumor growth rate |
| occ | Occlusion |
| UBC | Ubiquitin-conjugating core domain |
| BEVS | Baculovirus expression vector system |
| IFN | Interferon |
| ATM | Ataxia telagieasia mutated protein |
| ATR | ATM- and Rad3-related |
| CMV | Cytomegalovirus |
| IE | Immediate-early |
| PFA | Paraformaldehyde |
| LUAD | Lung adenocarcinoma |
| LSCC | Lung squamous cell carcinoma |
References
- Ferrelli, M.L.; Salvador, R. Effects of Mixed Baculovirus Infections in Biological Control: A Comprehensive Historical and Technical Analysis. Viruses 2023, 15, 1838. [Google Scholar] [CrossRef]
- Pidre, M.L.; Arrías, P.N.; Amorós Morales, L.C.; Romanowski, V. The Magic Staff: A Comprehensive Overview of Baculovirus-Based Technologies Applied to Human and Animal Health. Viruses 2022, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Targovnik, A.M.; Simonin, J.A.; Mc Callum, G.J.; Smith, I.; Cuccovia Warlet, F.U.; Nugnes, M.V.; Miranda, M.V.; Belaich, M.N. Solutions against Emerging Infectious and Noninfectious Human Diseases through the Application of Baculovirus Technologies. Appl. Microbiol. Biotechnol. 2021, 105, 8195–8226. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Arrías, P.; Massón, T.; Pidre, M.; Romanowski, V. Baculovirus-Derived Vectors for Immunization and Therapeutic Applications. In Emerging and Reemerging Viral Pathogens; Ennaji, M., Ed.; Academic Press: New York, NY, USA, 2020; pp. 197–224. [Google Scholar]
- Pidre, M.L.; Ferrelli, M.L.; Haase, S.; Romanowski, V. Baculovirus Display: A Novel Tool for Vaccination; Intech Open: Rijeka, Croatia, 2013. [Google Scholar]
- Gottardo, M.F.; Pidre, M.L.; Zuccato, C.; Asad, A.S.; Imsen, M.; Jaita, G.; Candolfi, M.; Romanowski, V.; Seilicovich, A. Baculovirus-Based Gene Silencing of Humanin for the Treatment of Pituitary Tumors. Apoptosis 2018, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Marvaldi, C.; Martin, D.; Conte, J.G.; Gottardo, M.F.; Pidre, M.L.; Imsen, M.; Irizarri, M.; Manuel, S.L.; Duncan, F.E.; Romanowski, V.; et al. Mitochondrial Humanin Peptide Acts as a Cytoprotective Factor in Granulosa Cell Survival. Reproduction 2021, 161, 581–591. [Google Scholar] [CrossRef]
- Garcia Fallit, M.; Pidre, M.L.; Asad, A.S.; Peña Agudelo, J.A.; Vera, M.B.; Nicola Candia, A.J.; Sagripanti, S.B.; Pérez Kuper, M.; Amorós Morales, L.C.; Marchesini, A.; et al. Evaluation of Baculoviruses as Gene Therapy Vectors for Brain Cancer. Viruses 2023, 15, 608. [Google Scholar] [CrossRef]
- Peña Agudelo, J.A.; Pidre, M.L.; Garcia Fallit, M.; Pérez Küper, M.; Zuccato, C.; Nicola Candia, A.J.; Marchesini, A.; Vera, M.B.; De Simone, E.; Giampaoli, C.; et al. Mitochondrial Peptide Humanin Facilitates Chemoresistance in Glioblastoma Cells. Cancers 2023, 15, 4061. [Google Scholar] [CrossRef]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.-D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-Negative Breast Cancer: Disease Entity or Title of Convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683–692. [Google Scholar] [CrossRef]
- Qian, J.; Zhao, L.; Xu, L.; Zhao, J.; Tang, Y.; Yu, M.; Lin, J.; Ding, L.; Cui, Q. Cell Death: Mechanisms and Potential Targets in Breast Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 9703. [Google Scholar] [CrossRef] [PubMed]
- Hoy, H.; Lynch, T.; Beck, M. Surgical Treatment of Lung Cancer. Crit. Care Nurs. Clin. N. Am. 2019, 31, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, J.-Q.; Li, X.-P. Differences between Lung Adenocarcinoma and Lung Squamous Cell Carcinoma: Driver Genes, Therapeutic Targets, and Clinical Efficacy. Genes Dis. 2025, 12, 101374. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.P.; Bardroff, M.; Pyrowolakis, G.; Jentsch, S. A Giant Ubiquitin-Conjugating Enzyme Related to IAP Apoptosis Inhibitors. J. Cell. Biol. 1998, 141, 1415–1422. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Bartke, T.; Pohl, C.; Pyrowolakis, G.; Jentsch, S. Dual Role of BRUCE as an Antiapoptotic IAP and a Chimeric E2/E3 Ubiquitin Ligase. Mol. Cell. 2004, 14, 801–811. [Google Scholar] [CrossRef]
- Sekine, K.; Hao, Y.; Suzuki, Y.; Takahashi, R.; Tsuruo, T.; Naito, M. HtrA2 Cleaves Apollon and Induces Cell Death by IAP-Binding Motif in Apollon-Deficient Cells. Biochem. Biophys. Res. Commun. 2005, 330, 279–285. [Google Scholar] [CrossRef]
- Hao, Y.; Sekine, K.; Kawabata, A.; Nakamura, H.; Ishioka, T.; Ohata, H.; Katayama, R.; Hashimoto, C.; Zhang, X.; Noda, T.; et al. Apollon Ubiquitinates SMAC and Caspase-9, and Has an Essential Cytoprotection Function. Nat. Cell. Biol. 2004, 6, 849–860. [Google Scholar] [CrossRef]
- Qiu, X.-B.; Markant, S.L.; Yuan, J.; Goldberg, A.L. Nrdp1-Mediated Degradation of the Gigantic IAP, BRUCE, Is a Novel Pathway for Triggering Apoptosis. EMBO J. 2004, 23, 800–810. [Google Scholar] [CrossRef]
- Ge, C.; Che, L.; Ren, J.; Pandita, R.K.; Lu, J.; Li, K.; Pandita, T.K.; Du, C. BRUCE Regulates DNA Double-Strand Break Response by Promoting USP8 Deubiquitination of BRIT1. Proc. Natl. Acad. Sci. USA 2015, 112, E1210-9. [Google Scholar] [CrossRef]
- Ge, C.; Che, L.; Du, C. The UBC Domain Is Required for BRUCE to Promote BRIT1/MCPH1 Function in DSB Signaling and Repair Post Formation of BRUCE-USP8-BRIT1 Complex. PLoS ONE 2015, 10, e0144957. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Vilfranc, C.L.; Che, L.; Pandita, R.K.; Hambarde, S.; Andreassen, P.R.; Niu, L.; Olowokure, O.; Shah, S.; Waltz, S.E.; et al. The BRUCE-ATR Signaling Axis Is Required for Accurate DNA Replication and Suppression of Liver Cancer Development. Hepatology 2019, 69, 2608–2622. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Bonifacino, J.S. Negative Regulation of Autophagy by UBA6-BIRC6–Mediated Ubiquitination of LC3. eLife 2019, 8, e50034. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.; Poetsch, I.; Deszcz, L.; Hoffmann, T.; Zuber, J.; Ikeda, F. The IAP Family Member BRUCE Regulates Autophagosome-Lysosome Fusion. Nat. Commun. 2018, 9, 599. [Google Scholar] [CrossRef]
- Che, L.; Yang, X.; Ge, C.; El-Amouri, S.S.; Wang, Q.-E.; Pan, D.; Herzog, T.J.; Du, C. Loss of BRUCE Reduces Cellular Energy Level and Induces Autophagy by Driving Activation of the AMPK-ULK1 Autophagic Initiating Axis. PLoS ONE 2019, 14, e0216553. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Y.; Wen, L.; Xing, Z.; Wang, C.; Zhang, L.; Wu, K.; Sun, H.; Li, Y.; Lei, Q.; et al. Overexpression of BIRC6 Driven by EGF-JNK-HECTD1 Signaling Is a Potential Therapeutic Target for Triple-Negative Breast Cancer. Mol. Ther. Nucleic Acids 2021, 26, 798–812. [Google Scholar] [CrossRef]
- Luk, I.S.U.; Shrestha, R.; Xue, H.; Wang, Y.; Zhang, F.; Lin, D.; Haegert, A.; Wu, R.; Dong, X.; Collins, C.C.; et al. BIRC6 Targeting as Potential Therapy for Advanced, Enzalutamide-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 1542–1551. [Google Scholar] [CrossRef]
- Hu, T.; Weng, S.; Tang, W.; Xe, R.; Chen, S.; Cai, G.; Cai, Y.; Shen, X.; Zhang, S.; Dong, L. Overexpression of BIRC6 Is a Predictor of Prognosis for Colorectal Cancer. PLoS ONE 2015, 10, e0125281. [Google Scholar] [CrossRef]
- Sung, K.W.; Choi, J.; Hwang, Y.K.; Lee, S.J.; Kim, H.-J.; Lee, S.H.; Yoo, K.H.; Jung, H.L.; Koo, H.H. Overexpression of Apollon, an Antiapoptotic Protein, Is Associated with Poor Prognosis in Childhood de Novo Acute Myeloid Leukemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 5109–5114. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.J.; Hou, J.; Wang, Y.Y.; Tang, W.Q.; Shen, X.Z.; Tu, R.Q. Expression and Clinical Significance of BIRC6 in Human Epithelial Ovarian Cancer. Tumor Biol. 2014, 35, 4891–4896. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.; Weng, S.; Liu, X.; Rao, B.; Gu, J.; Chen, S.; Wang, Q.; Shen, X.; Xue, R.; et al. Apollon Modulates Chemosensitivity in Human Esophageal Squamous Cell Carcinoma. Oncotarget 2014, 5, 7183–7197. [Google Scholar] [CrossRef] [PubMed]
- Gharabaghi, M.A. Diagnostic Investigation of BIRC6 and SIRT1 Protein Expression Level as Potential Prognostic Biomarkers in Patients with Non-Small Cell Lung Cancer. Clin. Respir. J. 2018, 12, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lin, D.; Low, C.; Vucic, E.A.; English, J.C.; Yee, J.; Murray, N.; Lam, W.L.; Ling, V.; Lam, S.; et al. Elevated Expression of Birc6 Protein in Non-Small-Cell Lung Cancers Is Associated with Cancer Recurrence and Chemoresistance. J. Thorac. Oncol. 2013, 8, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gómez Bergna, S.M.; Marchesini, A.; Amorós Morales, L.C.; Arrías, P.N.; Farina, H.G.; Romanowski, V.; Gottardo, M.F.; Pidre, M.L. Exploring the Role of the Inhibitor of Apoptosis BIRC6 in Breast Cancer: A Database Analysis. JCO Clin. Cancer Inform. 2022, 6, e2200093. [Google Scholar] [CrossRef]
- Bofill-De Ros, X.; Gu, S. Guidelines for the Optimal Design of miRNA-Based shRNAs; Academic Press Inc.: New York, NY, USA, 2016; Volume 103. [Google Scholar]
- Je, H.Y.; Hee Chang, J.; Young Choi, J.; Yul Roh, J.; Rae Jin, B.; O’Reilly, D.R.; Kwon Kang, S. A Defective Viral Genome Maintained in Escherichia coli for the Generation of Baculovirus Expression Vectors. Biotechnol. Lett. 2001, 23, 575–582. [Google Scholar] [CrossRef]
- Je, Y.H.; Chang, J.H.; Roh, J.; Jin, B.R. Generation of Baculovirus Expression Vector Using Defective Autographa Californica Nuclear Polyhedrosis Virus Genome Maintained in Escherichia coli for Occ+ Virus Production. Int. J. Indust. Entomol. 2001, 2, 155–160. [Google Scholar]
- Je, Y.H.; Jin, B.R.; Park, H.W.; Roh, J.Y.; Chang, J.H.; Seo, S.J.; Olszewski, J.A.; O’Reilly, D.R.; Kang, S.K. Baculovirus Expression Vectors That Incorporate the Foreign Protein into Viral Occlusion Bodies. Biotechniques 2003, 34, 81–87. [Google Scholar] [CrossRef]
- Amorós Morales, L.C.; Marchesini, A.; Gómez Bergna, S.M.; García Fallit, M.; Tongiani, S.E.; Vásquez, L.; Ferrelli, M.L.; Videla-Richardson, G.A.; Candolfi, M.; Romanowski, V.; et al. PluriBAC: A Versatile Baculovirus-Based Modular System to Express Heterologous Genes in Different Biotechnological Platforms. Viruses 2023, 15, 1984. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.H.; Hu, Y.; Watson, P.H.; Liu, H.; Geiger, T.R.; Anver, M.R.; Haines, D.C.; Martin, P.; Green, J.E.; et al. Immunocompetent Mouse Allograft Models for Development of Therapies to Target Breast Cancer Metastasis. Oncotarget 2017, 8, 30621–30643. [Google Scholar] [CrossRef]
- Alonso, D.F.; Farías, E.F.; Urtreger, A.; Ladeda, V.; Vidal, M.C.; Bal De Kier Joffé, E. Characterization of F3II, a Sarcomatoid Mammary Carcinoma Cell Line Originated from a Clonal Subpopulation of a Mouse Adenocarcinoma. J. Surg. Oncol. 1996, 62, 288–297. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, M.F.; Capobianco, C.S.; Sidabra, J.E.; Garona, J.; Perera, Y.; Perea, S.E.; Alonso, D.F.; Farina, H.G. Preclinical Efficacy of CIGB-300, an Anti-CK2 Peptide, on Breast Cancer Metastasic Colonization. Sci. Rep. 2020, 10, 14689. [Google Scholar] [CrossRef] [PubMed]
- Segatori, V.I.; Vazquez, A.M.; Gomez, D.E.; Gabri, M.R.; Alonso, D.F. Preclinical Evaluation of Racotumomab, an Anti-Idiotype Monoclonal Antibody to N-Glycolyl-Containing Gangliosides, with or without Chemotherapy in a Mouse Model of Non-Small Cell Lung Cancer. Front. Oncol. 2012, 2, 160. [Google Scholar] [CrossRef] [PubMed]
- Solernó, L.M.; Sobol, N.T.; Gottardo, M.F.; Capobianco, C.S.; Ferrero, M.R.; Vásquez, L.; Alonso, D.F.; Garona, J. Propranolol Blocks Osteosarcoma Cell Cycle Progression, Inhibits Angiogenesis and Slows Xenograft Growth in Combination with Cisplatin-Based Chemotherapy. Sci. Rep. 2022, 12, 15058. [Google Scholar] [CrossRef]
- Duanmu, J.; Cheng, J.; Xu, J.; Booth, C.J.; Hu, Z. Effective Treatment of Chemoresistant Breast Cancer in Vitro and in Vivo by a Factor VII-Targeted Photodynamic Therapy. Br. J. Cancer 2011, 104, 1401–1409. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, C.; Zhang, D.; Gao, M.; Peng, F.; Huang, D.; Sun, Z.; Ni, Y.; Zhang, J.; Yin, Z. Tumor Necrosis Targeted Radiotherapy of Non-Small Cell Lung Cancer Using Radioiodinated Protohypericin in a Mouse Model. Oncotarget 2015, 6, 26400–26410. [Google Scholar] [CrossRef]
- Zhong, N.S.; Tong, W.L.; Zhang, Y.; Xiao, S.N.; Liu, J.M.; Li, A.A.; Yao, G.L.; Lin, Q.; Liu, Z.L. HELQ Suppresses Migration and Proliferation of Non-small Cell Lung Cancer Cells by Repairing DNA Damage and Inducing Necrosis. Cell. Biol. Int. 2023, 47, 188–200. [Google Scholar] [CrossRef]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of Human Beta Interferon in Insect Cells Infected with a Baculovirus Expression Vector. Biotechnology 1992, 24, 434–443. [Google Scholar]
- Wang, J.; Zhu, L.; Chen, X.; Huang, R.; Wang, S.; Dong, P. Human Bone Marrow Mesenchymal Stem Cells Functionalized by Hybrid Baculovirus-Adeno-Associated Viral Vectors for Targeting Hypopharyngeal Carcinoma. Stem Cells Dev. 2019, 28, 543–553. [Google Scholar] [CrossRef]
- Airenne, K.J.; Makkonen, K.E.; Mähönen, A.J.; Ylä-Herttuala, S. Baculoviruses Mediate Efficient Gene Expression in a Wide Range of Vertebrate Cells. Methods Mol. Biol. 2011, 737, 279–301. [Google Scholar]
- Airenne, K.J.; Hu, Y.-C.; Kost, T.A.; Smith, R.H.; Kotin, R.M.; Ono, C.; Matsuura, Y.; Wang, S.; Ylä-Herttuala, S. Baculovirus: An Insect-Derived Vector for Diverse Gene Transfer Applications. Mol. Ther. 2013, 21, 739–749. [Google Scholar] [CrossRef]
- Ang, W.X.; Zhao, Y.; Kwang, T.; Wu, C.; Chen, C.; Toh, H.C.; Mahendran, R.; Esuvaranathan, K.; Wang, S. Local Immune Stimulation by Intravesical Instillation of Baculovirus to Enable Bladder Cancer Therapy. Sci. Rep. 2016, 6, 27455. [Google Scholar] [CrossRef]
- Aulicino, F.; Pelosse, M.; Toelzer, C.; Capin, J.; Ilegems, E.; Meysami, P.; Rollarson, R.; Berggren, P.-O.; Dillingham, M.S.; Schaffitzel, C.; et al. Highly Efficient CRISPR-Mediated Large DNA Docking and Multiplexed Prime Editing Using a Single Baculovirus. Nucleic Acids Res. 2022, 50, 7783–7799. [Google Scholar] [CrossRef] [PubMed]
- Hindriksen, S.; Bramer, A.J.; Truong, M.A.; Vromans, M.J.M.; Post, J.B.; Verlaan-Klink, I.; Snippert, H.J.; Lens, S.M.A.; Hadders, M.A. Baculoviral Delivery of CRISPR/Cas9 Facilitates Efficient Genome Editing in Human Cells. PLoS ONE 2017, 12, e0179514. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, S. A pH-Sensitive Heparin-Binding Sequence from Baculovirus Gp64 Protein Is Important for Binding to Mammalian Cells but Not to Sf9 Insect Cells. J. Virol. 2012, 86, 484–491. [Google Scholar] [CrossRef] [PubMed]
- van Loo, N.D.; Fortunati, E.; Ehlert, E.; Rabelink, M.; Grosveld, F.; Scholte, B.J. Baculovirus Infection of Nondividing Mammalian Cells: Mechanisms of Entry and Nuclear Transport of Capsids. J. Virol. 2001, 75, 961–970. [Google Scholar] [CrossRef]
- Long, G.; Pan, X.; Kormelink, R.; Vlak, J.M. Functional Entry of Baculovirus into Insect and Mammalian Cells Is Dependent on Clathrin-Mediated Endocytosis. J. Virol. 2006, 80, 8830–8833. [Google Scholar] [CrossRef]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-Based Motility Drives Baculovirus Transit to the Nucleus and Cell Surface. J. Cell Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef]
- Laakkonen, J.P.; Mäkelä, A.R.; Kakkonen, E.; Turkki, P.; Kukkonen, S.; Peränen, J.; Ylä-Herttuala, S.; Airenne, K.J.; Oker-Blom, C.; Vihinen-Ranta, M.; et al. Clathrin-Independent Entry of Baculovirus Triggers Uptake of E. Coli in Non-Phagocytic Human Cells. PLoS ONE 2009, 4, e5093. [Google Scholar] [CrossRef]
- Hunkeler, M.; Jin, C.Y.; Fischer, E.S. Structures of BIRC6-Client Complexes Provide a Mechanism of SMAC-Mediated Release of Caspases. Science 2023, 379, 1105–1111. [Google Scholar] [CrossRef]
- Dietz, L.; Ellison, C.J.; Riechmann, C.; Cassidy, C.K.; Felfoldi, F.D.; Pinto-Fernández, A.; Kessler, B.M.; Elliott, P.R. Structural Basis for SMAC-Mediated Antagonism of Caspase Inhibition by the Giant Ubiquitin Ligase BIRC6. Science 2023, 379, 1112–1117. [Google Scholar] [CrossRef]
- Ehrmann, J.F.; Grabarczyk, D.B.; Heinke, M.; Deszcz, L.; Kurzbauer, R.; Hudecz, O.; Shulkina, A.; Gogova, R.; Meinhart, A.; Versteeg, G.A.; et al. Structural Basis for Regulation of Apoptosis and Autophagy by the BIRC6/SMAC Complex. Science 2023, 379, 1117–1123. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Wang, X.; Zhang, H.; Chen, N.; Mai, Y.; Dai, S.; Yang, L.; Chen, D.; Zhong, W. BIRC6 Modulates the Protein Stability of Axin to Regulate the Growth, Stemness, and Resistance of Renal Cancer Cells via the β-Catenin Pathway. ACS Omega 2024, 9, 7782–7792. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xue, R.; Weng, S.; Wu, J.; Fang, Y.; Wang, Y.; Ji, L.; Hu, T.; Liu, T.; Huang, X.; et al. BIRC6 Promotes Hepatocellular Carcinogenesis: Interaction of BIRC 6 with P53 Facilitating P53 Degradation. Int. J. Cancer 2015, 136, E475–E487. [Google Scholar] [CrossRef] [PubMed]
- Lotz, K.; Pyrowolakis, G.; Jentsch, S. BRUCE, a Giant E2/E3 Ubiquitin Ligase and Inhibitor of Apoptosis Protein of the Trans-Golgi Network, Is Required for Normal Placenta Development and Mouse Survival. Mol. Cell. Biol. 2004, 24, 9339–9350. [Google Scholar] [CrossRef]
- Arunachalam, H.B.; Mishra, R.; Daescu, O.; Cederberg, K.; Rakheja, D.; Sengupta, A.; Leonard, D.; Hallac, R.; Leavey, P. Viable and Necrotic Tumor Assessment from Whole Slide Images of Osteosarcoma Using Machine-Learning and Deep-Learning Models. PLoS ONE 2019, 14, e0210706. [Google Scholar] [CrossRef]
- Ziółkowska-Suchanek, I.; Rozwadowska, N. Advancements in Gene Therapy for Non-Small Cell Lung Cancer: Current Approaches and Future Prospects. Genes 2025, 16, 569. [Google Scholar] [CrossRef]
- Dong, J.; Kong, L.; Wang, S.; Xia, M.; Zhang, Y.; Wu, J.; Yang, F.; Zuo, S.; Wei, J. Oncolytic Adenovirus Encoding Apolipoprotein A1 Suppresses Metastasis of Triple-Negative Breast Cancer in Mice. J. Exp. Clin. Cancer Res. 2024, 43, 102. [Google Scholar] [CrossRef]
- Green-Tripp, G.; Nattress, C.; Halldén, G. Targeting Triple Negative Breast Cancer With Oncolytic Adenoviruses. Front. Mol. Biosci. 2022, 9, 901392. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Zhan, Q.; Jin, H.; Wang, Y.; Wang, H.; Huang, B.; Huang, F.; Jia, X.; Wang, Y.; et al. Oncolytic Adenovirus Encoding LHPP Exerts Potent Antitumor Effect in Lung Cancer. Sci. Rep. 2024, 14, 13108. [Google Scholar] [CrossRef]
- Boyce, F.M.; Bucher, N.L.R. Baculovirus-Mediated Gene Transfer into Mammalian Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2348–2352. [Google Scholar] [CrossRef]
- Paul, A.; Elias, C.B.; Shum-Tim, D.; Prakash, S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci. Rep. 2013, 3, 2366. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.A.; Cuccovia Warlet, F.U.; Bauzá, M.D.R.; Plastine, M.D.P.; Alfonso, V.; Olea, F.D.; Cerrudo, C.S.; Belaich, M.N. Early to Late VSV-G Expression in AcMNPV BV Enhances Transduction in Mammalian Cells but Does Not Affect Virion Yield in Insect Cells. Vaccines 2025, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Ylä-Herttuala, S.; Airenne, K.J. How to Avoid Complement Attack in Baculovirus-Mediated Gene Delivery. J. Invertebr. Pathol. 2011, 107, S71–S79. [Google Scholar] [CrossRef] [PubMed]
- Plastine, M.D.P.; Amalfi, S.; López, M.G.; Gravisaco, M.J.; Taboga, O.; Alfonso, V. Development of a Stable Sf9 Insect Cell Line to Produce VSV-G Pseudotyped Baculoviruses. Gene Ther. 2024, 31, 187–194. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; Von Minckwitz, G. Molecular Alterations in Triple-Negative Breast Cancer—The Road to New Treatment Strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Grodzka, A.; Knopik-Skrocka, A.; Kowalska, K.; Kurzawa, P.; Krzyżaniak, M.; Stencel, K.; Bryl, M. Molecular Alterations of Driver Genes in Non-Small Cell Lung Cancer—From Diagnostics to Targeted Therapy. EXCLI J. 2023, 22, 415–432. [Google Scholar] [CrossRef]
| Primer | Sequence |
|---|---|
| Fw qbirc6 | ATGGGCAGACAAGGCTCTCT |
| Rv qbirc6 | TGCAGTGTTCACAATAGCCCT |
| Fw ywhaz | AGGAGATTACTACCGTTACTTGGC |
| Rv ywhaz | AGCTTCTTGGTATGCTTGTTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesini, A.; Gómez Bergna, S.M.; Amorós Morales, L.C.; López, M.F.; Vásquez, L.; Tongiani, S.E.; González Morán, F.; Romanowski, V.; Gottardo, M.F.; Pidre, M.L. Baculovirus-Mediated Gene Therapy: Targeting BIRC6 for Lung and Breast Cancer. Viruses 2025, 17, 1458. https://doi.org/10.3390/v17111458
Marchesini A, Gómez Bergna SM, Amorós Morales LC, López MF, Vásquez L, Tongiani SE, González Morán F, Romanowski V, Gottardo MF, Pidre ML. Baculovirus-Mediated Gene Therapy: Targeting BIRC6 for Lung and Breast Cancer. Viruses. 2025; 17(11):1458. https://doi.org/10.3390/v17111458
Chicago/Turabian StyleMarchesini, Abril, Santiago M. Gómez Bergna, Leslie C. Amorós Morales, María Florencia López, Larisa Vásquez, Silvana E. Tongiani, Florencia González Morán, Víctor Romanowski, María Florencia Gottardo, and Matias L. Pidre. 2025. "Baculovirus-Mediated Gene Therapy: Targeting BIRC6 for Lung and Breast Cancer" Viruses 17, no. 11: 1458. https://doi.org/10.3390/v17111458
APA StyleMarchesini, A., Gómez Bergna, S. M., Amorós Morales, L. C., López, M. F., Vásquez, L., Tongiani, S. E., González Morán, F., Romanowski, V., Gottardo, M. F., & Pidre, M. L. (2025). Baculovirus-Mediated Gene Therapy: Targeting BIRC6 for Lung and Breast Cancer. Viruses, 17(11), 1458. https://doi.org/10.3390/v17111458






