Fast-Track to Protection? A Review of Encepur’s Express Dosing Schedule for Tick-Borne Encephalitis
Abstract
1. Introduction
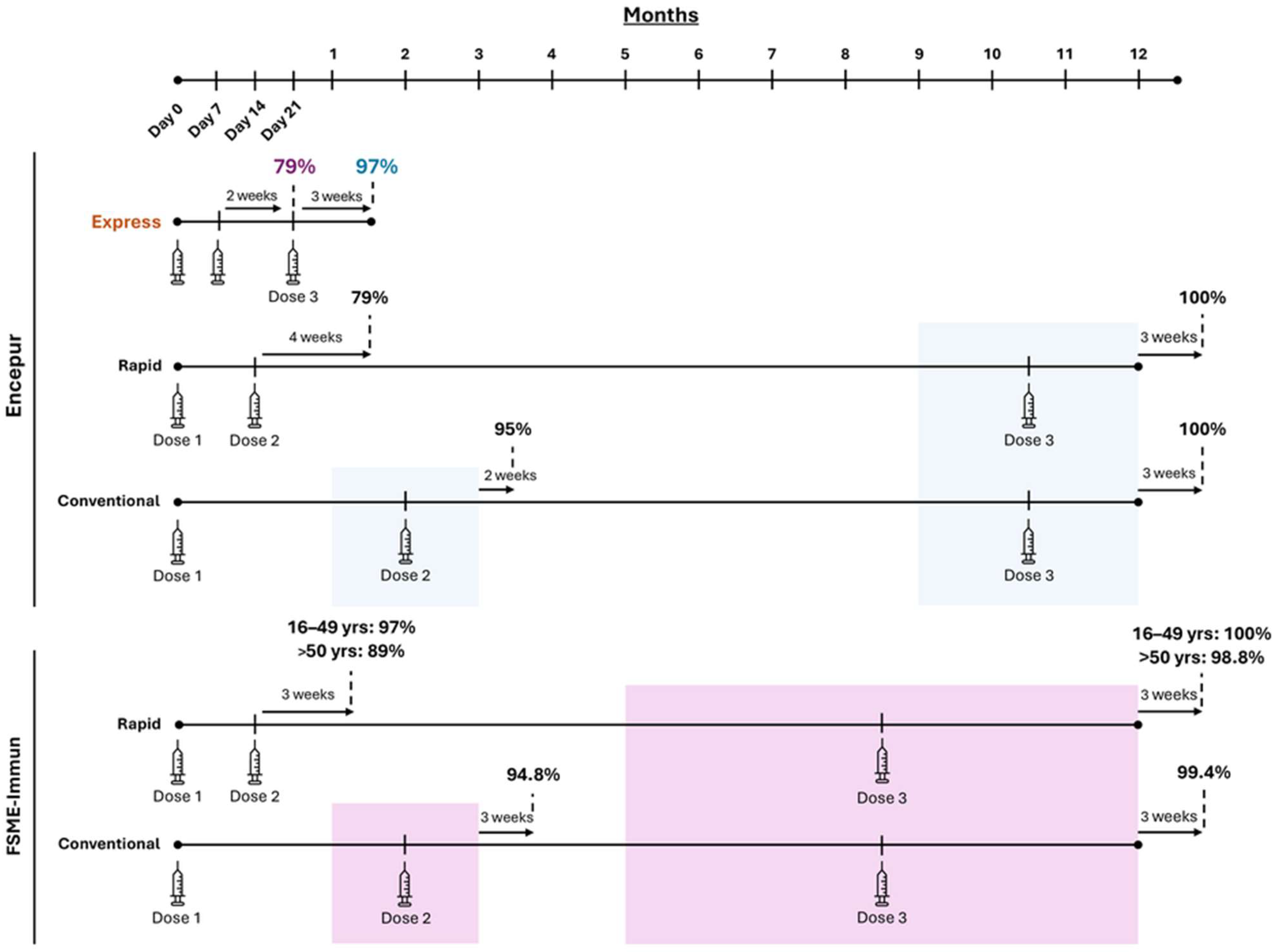
2. TBE Vaccine Dosing Schedules
| Encepur | FSME-Immun | |||||
|---|---|---|---|---|---|---|
| Primary Dosing Schedule | 1st Dose | 2nd Dose | 3rd Dose | 1st Dose | 2nd Dose | 3rd Dose |
| Conventional | Day 1 | 1–3 months a | 9–12 months b | Day 1 | 1–3 months a | 5–12 months b |
| Rapid c | Day 1 | Day 14 a | 9–12 months b | Day 1 | Day 14 a | 5–12 months b |
| Express d | Day 1 | Day 7 a | Day 21 b | - | - | |
3. Safety Profile with Encepur Express
4. Immunogenicity of Encepur Express
Clinical Evidence to Support the Immunogenicity of Encepur Express
5. Booster Response with Encepur’s Express Schedule
6. Real-World Estimates of VE for TBE Vaccines
7. Future Considerations for Broader Use of the Express Schedule
7.1. Travel-Specific Needs
7.2. Residents of TBE-Endemic Regions
7.3. Opportunistic Vaccination Among Low-Adherence Populations
8. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | enzyme-linked immunosorbent assay |
| GMT | geometric mean titre |
| HCP | healthcare professional |
| NT | neutralizing antibody titres |
| TBE | tick-borne encephalitis |
| TBEV | tick-borne encephalitis virus |
| VE | vaccine effectiveness |
References
- Yoshii, K. Epidemiology and pathological mechanisms of tick-borne encephalitis. J. Vet. Med. Sci. 2019, 81, 343–347. [Google Scholar] [CrossRef]
- Kunze, M.; Banovic, P.; Bogovic, P.; Briciu, V.; Civljak, R.; Dobler, G.; Hristea, A.; Kerlik, J.; Kuivanen, S.; Kyncl, J.; et al. Recommendations to improve tick-borne encephalitis surveillance and vaccine uptake in Europe. Microorganisms 2022, 10, 1283. [Google Scholar] [CrossRef]
- Zimmermann, H.; Koch, D. Epidemiology of tick-borne encephalitis (TBE) in Switzerland 1984 to 2004. Ther. Umsch. 2005, 62, 719–725. [Google Scholar] [CrossRef]
- Schuler, M.; Zimmermann, H.; Altpeter, E.; Heininger, U. Epidemiology of tick-borne encephalitis in Switzerland, 2005 to 2011. Surveill. Outbreak Rep. 2014, 19, 20756. [Google Scholar] [CrossRef]
- European Centre of Disease Prevention and Control. Factsheet About Tick-Borne Encephalitis (TBE). Available online: https://www.ecdc.europa.eu/en/tick-borne-encephalitis/facts/factsheet (accessed on 1 October 2025).
- Jong, N.B.; Blanford, J. Mapping the risk of tick-borne encephalitis in Europe for informed vaccination decisions. J. Travel Med. 2024, 32, taae153. [Google Scholar] [CrossRef]
- Sparagano, O.; Földvári, G.; Derdáková, M.; Kazimírová, M. New challenges posed by ticks and tick-borne diseases. Biologia 2022, 77, 1497–1501. [Google Scholar] [CrossRef]
- Riccardi, N.; Antonello, R.M.; Luzzati, R.; Zajkowska, J.; Di Bella, S.; Giacobbe, D.R. Tick-borne encephalitis in Europe: A brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Areas at Risk for Tick-Borne Encephalitis. 2024. Available online: https://www.cdc.gov/tick-borne-encephalitis/data-maps/index.html (accessed on 1 September 2025).
- European Centre of Disease Prevention and Control. Tick-Borne Encephalitis-Annual Epidemiological Report for 2022. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2022 (accessed on 5 September 2025).
- European Centre of Disease Prevention and Control. Tick-Borne Encephalitis in Europe. 2024. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/healthtopics/vectors/world-health-day-2014/Documents/factsheet-tick-borne-encephalitis.pdf#:~:text=Approximately%205000-12%20000%20cases%20of%20TBE,are%20reported%20in%20Europe%20each%20year (accessed on 30 September 2025).
- Albinsson, B.; Hoffman, T.; Kolstad, L.; Bergstrom, T.; Bogdanovic, G.; Heydecke, A.; Hagg, M.; Kjerstadius, T.; Lindroth, Y.; Petersson, A.; et al. Seroprevalence of tick-borne encephalitis virus and vaccination coverage of tick-borne encephalitis, Sweden, 2018 to 2019. Eurosurveillance 2024, 29, 2300221. [Google Scholar] [CrossRef] [PubMed]
- Brechet, A.; Kohler, P.; Dorr, T.; Grassli, F.; Vock, M.; Salat, J.; Ruzek, D.; Friedl, A.; Vuichard-Gysin, D.; Croxatto, A.; et al. Tick-borne encephalitis virus seroprevalence and infection incidence in Switzerland, 2020–2021. Sci. Rep. 2025, 15, 8346. [Google Scholar] [CrossRef]
- Domnich, A.; Panatto, D.; Arbuzova, E.K.; Signori, A.; Avio, U.; Gasparini, R.; Amicizia, D. Immunogenicity against Far Eastern and Siberian subtypes of tick-borne encephalitis (TBE) virus elicited by the currently available vaccines based on the European subtype: Systematic review and meta-analysis. Hum. Vaccine Immunother. 2014, 10, 2819–2833. [Google Scholar] [CrossRef]
- European Medicines Agency. List of Nationally Authorised Medicinal Products, Active Substance: Tick-Borne Encephalitis Vaccine (Inactivated). 2017. Available online: https://www.ema.europa.eu/en/documents/psusa/tick-borne-encephalitis-vaccine-inactivated-list-nationally-authorised-medicinal-products-psusa/00002951/201701_en.pdf (accessed on 1 October 2025).
- Bavarian Nordic. Summary of Product Characteristics. Encepur Children. 2002. Available online: https://docetp.mpa.se/LMF/Encepur%20Barn%20%20%20Suspension%20for%20injection%20ENG%20SmPC_09001bee8294355c.pdf (accessed on 7 September 2025).
- Bavarian Nordic. Summary of Product Characteristics. Encepur. 1998. Available online: https://docetp.mpa.se/LMF/Encepur%20%20%20Suspension%20for%20injection%20ENG%20SmPC_09001bee82943560.pdf (accessed on 7 September 2025).
- Pfizer Limited. TicoVac SmPC. 2021. Available online: https://www.medicines.org.uk/emc/product/1923/smpc#about-medicine (accessed on 1 October 2025).
- Pfizer Limited. TicoVac Junior SmPC. 2021. Available online: https://www.medicines.org.uk/emc/product/1927/smpc (accessed on 1 October 2025).
- Rampa, J.E.; Askling, H.H.; Lang, P.; Zens, K.D.; Gültekin, N.; Stanga, Z.; Schlagenhauf, P. Immunogenicity and safety of the tick-borne encephalitis vaccination (2009–2019): A systematic review. Travel Med. Infect. Dis. 2020, 37, 101876. [Google Scholar] [CrossRef] [PubMed]
- Erber, W.; Khan, F.; Zavadska, D.; Freimane, Z.; Dobler, G.; Bohmer, M.M.; Jodar, L.; Schmitt, H.J. Effectiveness of TBE vaccination in southern Germany and Latvia. Vaccine 2022, 40, 819–825. [Google Scholar] [CrossRef]
- Nygren, T.M.; Pilic, A.; Böhmer, M.M.; Wagner-Wiening, C.; Wichmann, O.; Harder, T.; Hellenbrand, W. Tick-borne encephalitis vaccine effectiveness and barriers to vaccination in Germany. Sci. Rep. 2022, 12, 11706. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Haile, S.R.; Schmidt, A.J.; Altpeter, E.S.; Fehr, J.S.; Lang, P. Retrospective, matched case-control analysis of tickborne encephalitis vaccine effectiveness by booster interval, Switzerland 2006–2020. BMJ Open 2022, 12, e061228. [Google Scholar] [CrossRef] [PubMed]
- Miazga, W.; Wnuk, K.; Tatara, T.; Switalski, J.; Matera, A.; Religioni, U.; Gujski, M. The long-term efficacy of tick-borne encephalitis vaccines available in Europe—A systematic review. BMC Infect. Dis. 2023, 23, 621. [Google Scholar] [CrossRef]
- Zavadska, D.; Freimane, Z.; Karelis, G.; Ermina, I.; Harper, L.R.; Bender, C.; Zhang, P.; Angulo, F.J.; Erber, W.; Bormane, A.; et al. Effectiveness of Tick-borne Encephalitis Vaccines in Children, Latvia, 2018–2020. Pediatr. Infect. Dis. J. 2023, 42, 927–931. [Google Scholar] [CrossRef]
- Zavadska, D.; Freimane, Z.; Karelis, G.; Ermina, I.; Harper, L.R.; Bender, C.; Zhang, P.; Angulo, F.J.; Erber, W.; Bormane, A.; et al. Effectiveness of tick-borne encephalitis vaccination in Latvia, 2018–2020: An observational study. Clin. Microbiol. Infect. 2023, 29, 1443–1448. [Google Scholar] [CrossRef]
- Palmborg, A.; Angulo, F.J.; Zhang, P.; Pilz, A.; Stark, J.; Moisi, J.C.; Jodar, L. Tick-borne encephalitis vaccine uptake, effectiveness, and impact in Sweden from 2018 to 2022. Sci. Rep. 2025, 15, 2927. [Google Scholar] [CrossRef]
- Zens, K.D.; Altpeter, E.; Wymann, M.N.; Mack, A.; Baer, N.B.; Haile, S.R.; Steffen, R.; Fehr, J.S.; Lang, P. A combined cross-sectional analysis and case-control study evaluating tick-borne encephalitis vaccination coverage, disease and vaccine effectiveness in children and adolescents, Switzerland, 2005 to 2022. Eurosurveillance 2024, 29, 2300558. [Google Scholar] [CrossRef]
- Jacob, L.; Kostev, K. Compliance with vaccination against tick-borne encephalitis virus in Germany. Clin. Microbiol. Infect. 2017, 23, 460–463. [Google Scholar] [CrossRef][Green Version]
- Erber, W.; Schmitt, H.J. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick Borne Dis. 2018, 9, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Schley, K.; Malerczyk, C.; Beier, D.; Schiffner-Rohe, J.; von Eiff, C.; Häckl, D.; Süß, J. Vaccination rate and adherence of tick-borne encephalitis vaccination in Germany. Vaccine 2021, 39, 830–838. [Google Scholar] [CrossRef]
- Pilz, A.; Erber, W.; Schmitt, H.J. Vaccine uptake in 20 countries in Europe 2020: Focus on tick-borne encephalitis (TBE). Ticks Tick Borne Dis. 2023, 14, 102059. [Google Scholar] [CrossRef] [PubMed]
- ESCMID Study Group for Tick-Borne Diseases—ESGBOR. European Tick-Borne Disease Information Resource. 2023. Available online: https://www.esgbor.org/other-tick-borne-diseases/tick-borne-encephalitis/ (accessed on 9 September 2025).
- VaccinarSì. Tick-Borne Encephalitis Vaccine (TBE). 2020. Available online: https://www.vaccinarsi.eu/tick-borne-encephalitis-vaccine (accessed on 15 September 2025).
- Patient. Tick-Borne Encephalitis Vaccine. Available online: https://patient.info/travel-and-vaccinations/travel-vaccinations-leaflet/tick-borne-encephalitis-immunisation (accessed on 15 September 2025).
- Pfizer Limited. Highlights of Prescribing Information. TicoVac. 2021. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=15600&format=pdf (accessed on 18 September 2025).
- Zent, O.; Jilg, W.; Plentz, A.; Schwarz, T.F.; Fruhwein, N.; Kuhr, H.B.; Banzhoff, A. Kinetics of the immune response after primary and booster immunization against tick-borne encephalitis (TBE) in adults using the rapid immunization schedule. Vaccine 2003, 21, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Girgsdies, O.E.; Rosenkranz, G. Tick-borne encephalitis: Development of a paediatric vaccine. A controlled, randomized, double-blind and multicentre study. Vaccine 1996, 14, 1421–1428. [Google Scholar] [CrossRef]
- Zent, O.; Beran, J.; Jilg, W.; Mach, T.; Banzhoff, A. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine 2003, 21, 738–741. [Google Scholar] [CrossRef]
- Schöndorf, I.; Beran, J.; Cizkova, D.; Lesna, V.; Banzhoff, A.; Zent, O. Tick-borne encephalitis (TBE) vaccination: Applying the most suitable vaccination schedule. Vaccine 2007, 25, 1470–1475. [Google Scholar] [CrossRef]
- Schoendorf, I.; Ternak, G.; Oroszlan, G.; Nicolay, U.; Banzhoff, A.; Zent, O. Tick-borne encephalitis (TBE) vaccination in children: Advantage of the rapid immunization schedule (i.e., days 0, 7, 21). Hum. Vaccine 2007, 3, 42–47. [Google Scholar] [CrossRef]
- Beran, J.; Douda, P.; Gniel, D.; Zent, O. Long-term immunity after vaccination against tick-borne encephalitis with Encepur using the rapid vaccination schedule. Int. J. Med. Microbiol. 2004, 293 (Suppl. S37), 130–133. [Google Scholar] [CrossRef]
- Beran, J.; Xie, F.; Zent, O. Five year follow-up after a first booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates long-term antibody persistence and safety. Vaccine 2014, 32, 4275–4280. [Google Scholar] [CrossRef]
- Ackermann-Gaumann, R.; Lang, P.; Zens, K.D. Defining the “Correlate(s) of Protection” to tick-borne encephalitis vaccination and infection—Key points and outstanding questions. Front. Immunol. 2024, 15, 1352720. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines Against Tick-Borne Encephalitis (TBE). WHO Position Paper 2011. 2011. Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/tick-borne-encephalitis/tbe-grad-safety.pdf?sfvrsn=16ee9a04_2 (accessed on 7 September 2025).
- Holzmann, H.; Kundi, M.; Stiasny, K.; Clement, J.; McKenna, P.; Kunz, C.; Heinz, F.X. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 1996, 48, 102–107. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Tick-Borne Encephalitis (TBE) Vaccine. 2024. Available online: https://www.cdc.gov/acip/grade/tbe-travel-lab.html?utm_source (accessed on 3 September 2025).
- Hainz, U.; Jenewein, B.; Asch, E.; Pfeiffer, K.P.; Berger, P.; Grubeck-Loebenstein, B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 2005, 23, 3232–3235. [Google Scholar] [CrossRef]
- Lindblom, P.; Wilhelmsson, P.; Fryland, L.; Matussek, A.; Haglund, M.; Sjowall, J.; Vene, S.; Nyman, D.; Forsberg, P.; Lindgren, P.E. Factors determining immunological response to vaccination against tick-borne encephalitis virus in older individuals. PLoS ONE 2014, 9, e100860. [Google Scholar] [CrossRef]
- Allen, J.C.; Toapanta, F.R.; Chen, W.; Tennant, S.M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020, 38, 8264–8272. [Google Scholar] [CrossRef]
- Galgani, I.; Bunge, E.M.; Hendriks, L.; Schludermann, C.; Marano, C.; De Moerlooze, L. Systematic literature review comparing rapid 3-dose administration of the GSK tick-borne encephalitis vaccine with other primary immunization schedules. Expert. Rev. Vaccines 2017, 16, 919–932. [Google Scholar] [CrossRef]
- Global Health Press. Extension of the Booster Interval for the TBE Vaccine, Encepur Adult, Germany. 2025. Available online: https://tbenews.com/tbe/snapshot-week-25-2025-extension-of-the-booster-interval-for-the-tbe-vaccine-encepur-adult-germany (accessed on 5 September 2025).
- Beran, J.; Lattanzi, M.; Costantini, M.; Pammolli, A.; Galgani, I. Sustained antibody persistence for at least 15 years after a booster vaccination against tick-borne encephalitis following different primary vaccination schedules: Third 5-year follow-up. Vaccine 2023, 41, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Lattanzi, M.; Xie, F.; Moraschini, L.; Galgani, I. Second five-year follow-up after a booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates at least 10 years antibody persistence. Vaccine 2019, 37, 4623–4629. [Google Scholar] [CrossRef]
- NHS. Travel Vaccination Advice. Available online: https://www.nhs.uk/vaccinations/travel-vaccinations/travel-vaccination-advice/ (accessed on 1 October 2025).
- Center for Disease Control. Traveler’s Health: Before You Travel. Available online: https://wwwnc.cdc.gov/travel/page/before-travel (accessed on 3 October 2025).
- International Society of Travel Medicine. Pre-Travel Health Advice Fact Sheet. 2024. Available online: https://www.istm.org/wp-content/uploads/general-travel-health-advice.pdf (accessed on 10 September 2025).
- Buhler, S.; Ruegg, R.; Steffen, R.; Hatz, C.; Jaeger, V.K. A profile of travelers—An analysis from a large swiss travel clinic. J. Travel Med. 2014, 21, 324–331. [Google Scholar] [CrossRef]
- Boubaker, R.; Meige, P.; Mialet, C.; Buffat, C.N.; Uwanyiligira, M.; Widmer, F.; Rochat, J.; Fossati, A.H.; Souvannaraj-Blanchant, M.; Payot, S.; et al. Travellers’ profile, travel patterns and vaccine practices—A 10-year prospective study in a Swiss Travel Clinic. J. Travel Med. 2016, 23, tav017. [Google Scholar] [CrossRef]

| Encepur | FSME-Immun | |||
|---|---|---|---|---|
| Primary Dosing Schedule | First Booster | Subsequent Boosters | First Booster | Subsequent Boosters |
| Conventional | 3 years after last primary dose | Every 3–10 years a | 3 years after last primary dose | Every 3–5 years b |
| Rapid c | 3 years after last primary dose | Every 3–10 years a | 3 years after last primary dose | Every 3–5 years b |
| Express d | 12–18 months after last primary dose | Every 3–10 years a | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zens, K.; Torgler, R.; Horn, M.; Larsen, C.S. Fast-Track to Protection? A Review of Encepur’s Express Dosing Schedule for Tick-Borne Encephalitis. Viruses 2025, 17, 1439. https://doi.org/10.3390/v17111439
Zens K, Torgler R, Horn M, Larsen CS. Fast-Track to Protection? A Review of Encepur’s Express Dosing Schedule for Tick-Borne Encephalitis. Viruses. 2025; 17(11):1439. https://doi.org/10.3390/v17111439
Chicago/Turabian StyleZens, Kyra, Ralph Torgler, Michael Horn, and Carsten Schade Larsen. 2025. "Fast-Track to Protection? A Review of Encepur’s Express Dosing Schedule for Tick-Borne Encephalitis" Viruses 17, no. 11: 1439. https://doi.org/10.3390/v17111439
APA StyleZens, K., Torgler, R., Horn, M., & Larsen, C. S. (2025). Fast-Track to Protection? A Review of Encepur’s Express Dosing Schedule for Tick-Borne Encephalitis. Viruses, 17(11), 1439. https://doi.org/10.3390/v17111439





