Analysis of Colored Lesions of Chilli Yellow Ringspot Orthotospovirus Infection in Tomato Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Virus Strains and Inoculation
2.3. Virus Identification
2.4. Transmission Electron Microscopy (TEM)
2.5. Chromoplast Ultrastructural Analysis
2.6. Virus Accumulation Tests
2.7. Reverse Transcription–Real-Time Quantitative PCR (RT–qPCR)
2.8. Detection of Carotenoid Metabolites
2.9. Detection of Flavonoid Metabolites
2.10. Transcriptome Analysis
2.11. CYRSV Greenhouse Isolation Inoculation Experiment
2.12. Statistical Analysis
3. Results
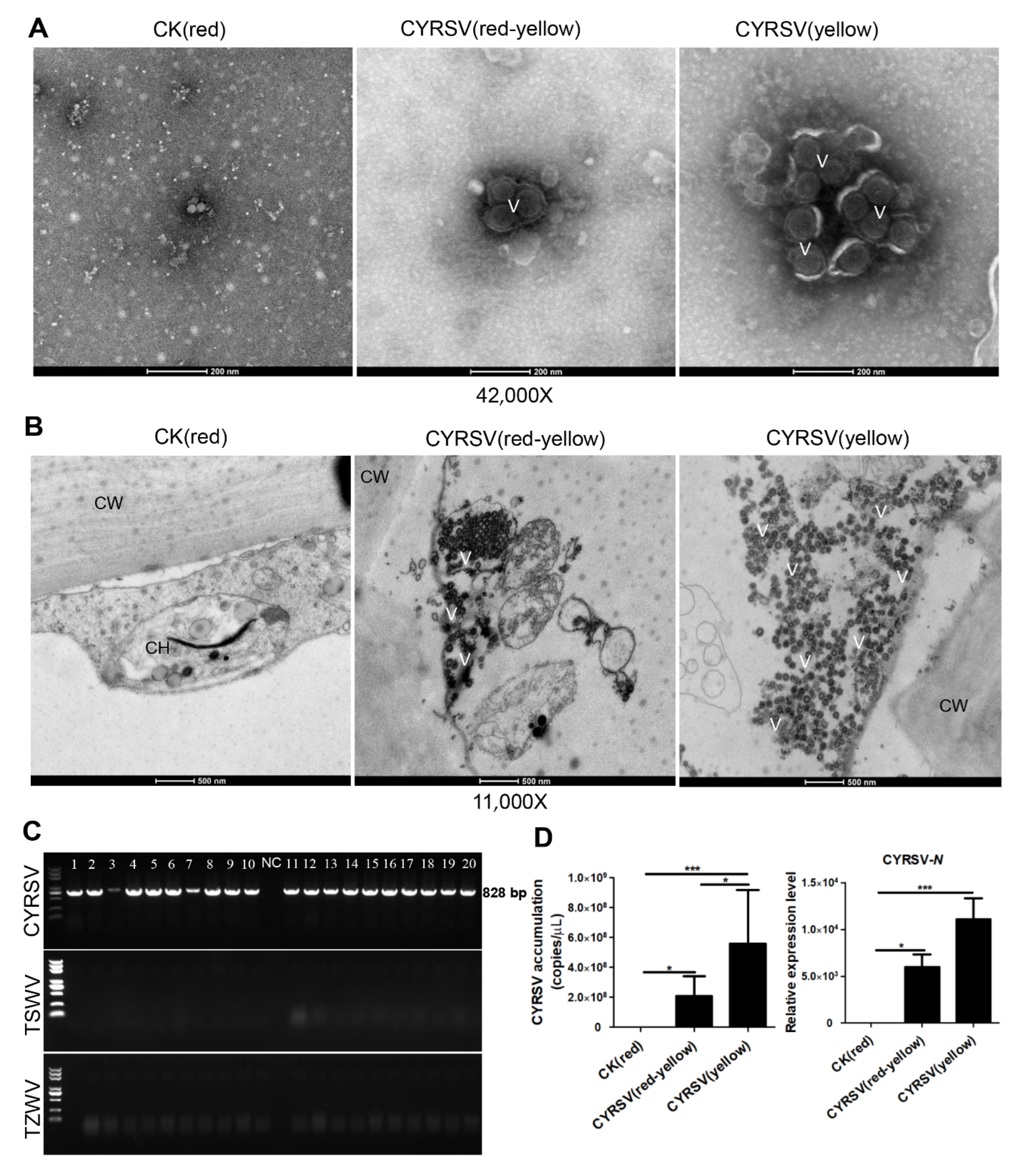
3.1. Color Lesions in CYRSV-Infected Tomato Fruits
3.2. Colored Lesions Were Associated with the Presence of CYRSV in Tomato Fruits
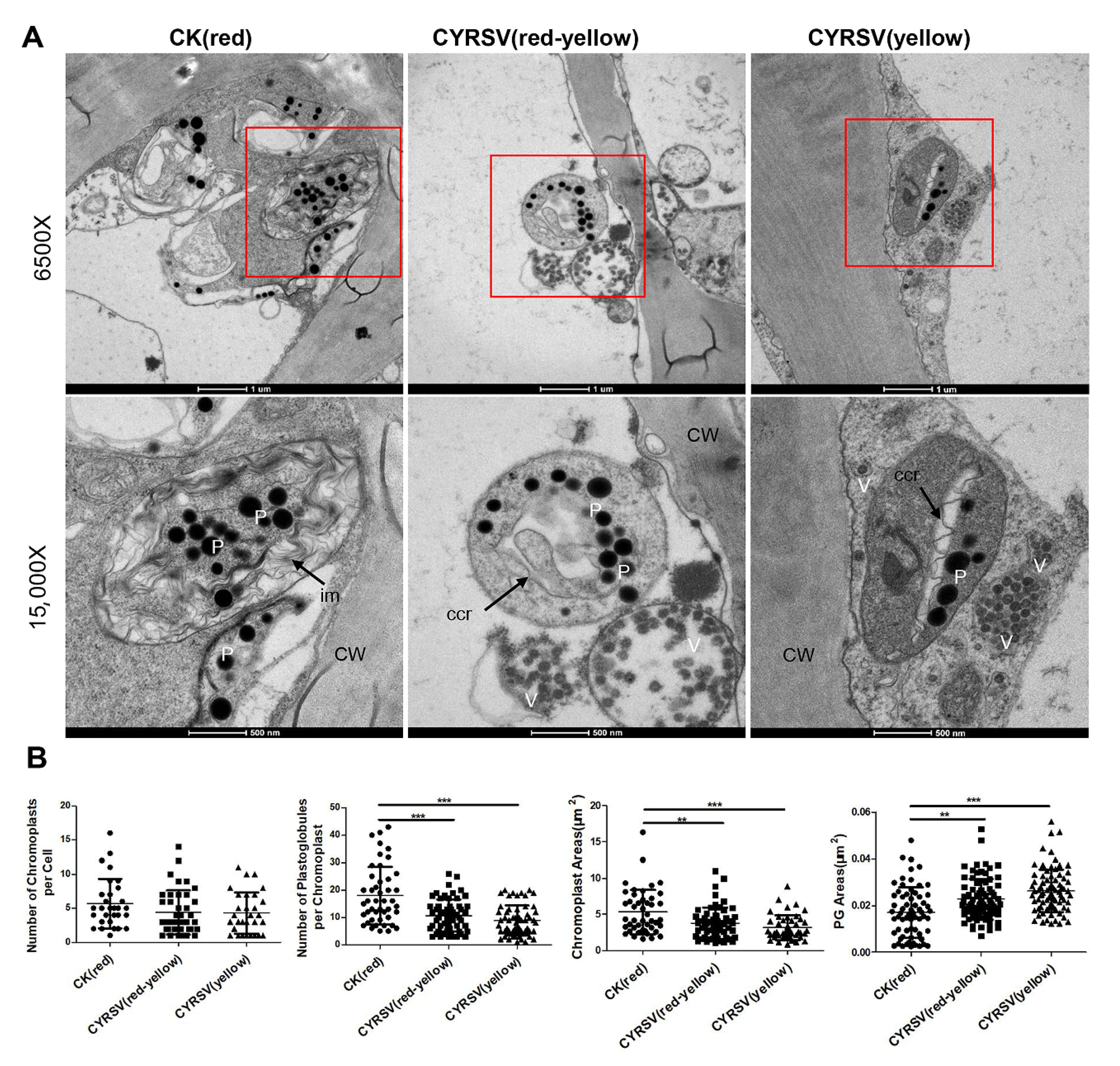
3.3. Ultrastructural Analysis of Chromoplasts in Tomato Fruits with or Without Color Lesions
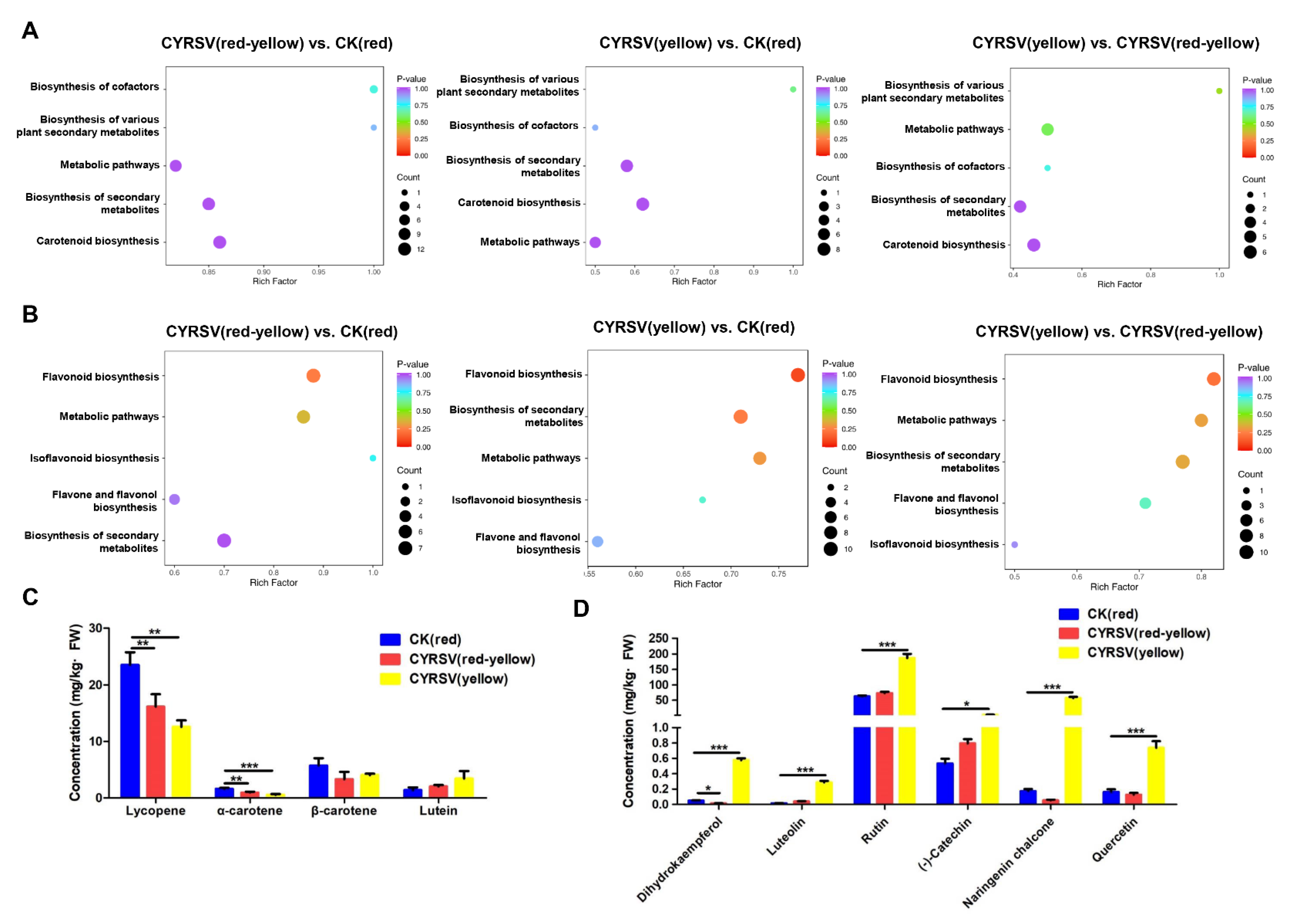
3.4. Metabolome Analysis
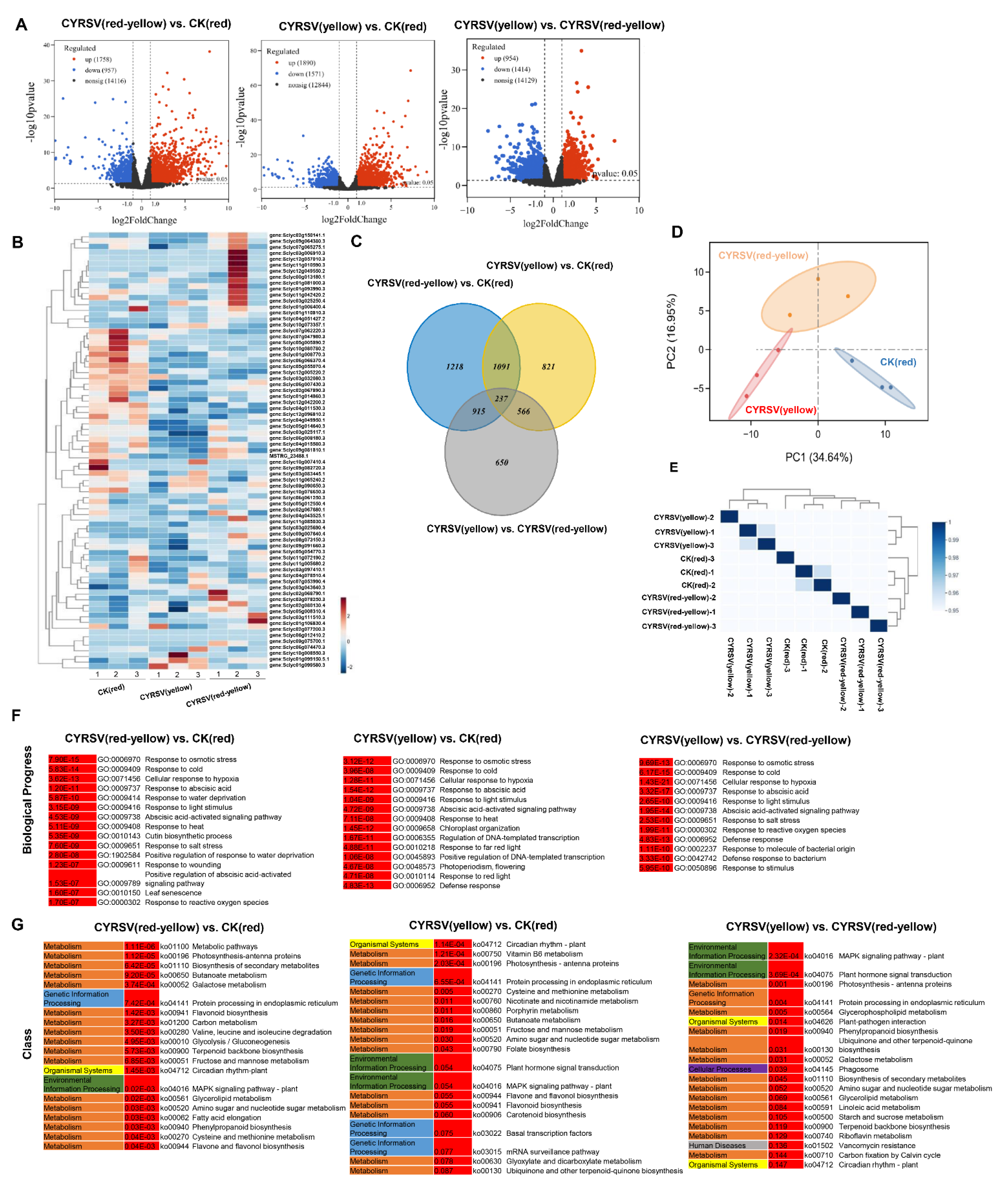
3.5. Transcriptome Analysis
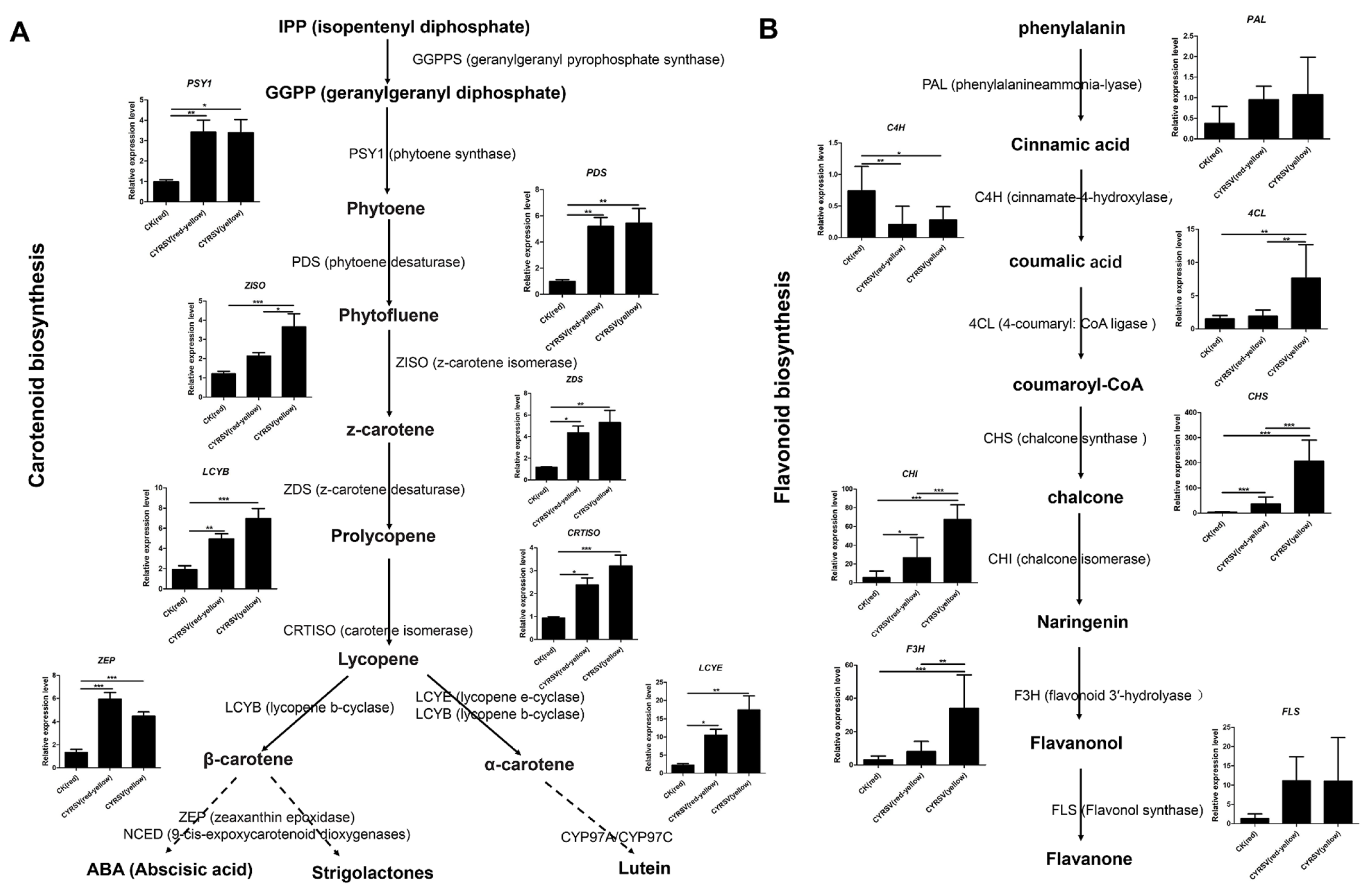
3.6. Expression Analysis of Carotenoid and Flavonoid Metabolic Pathway Genes in Tomato Fruits with and Without Color Lesions
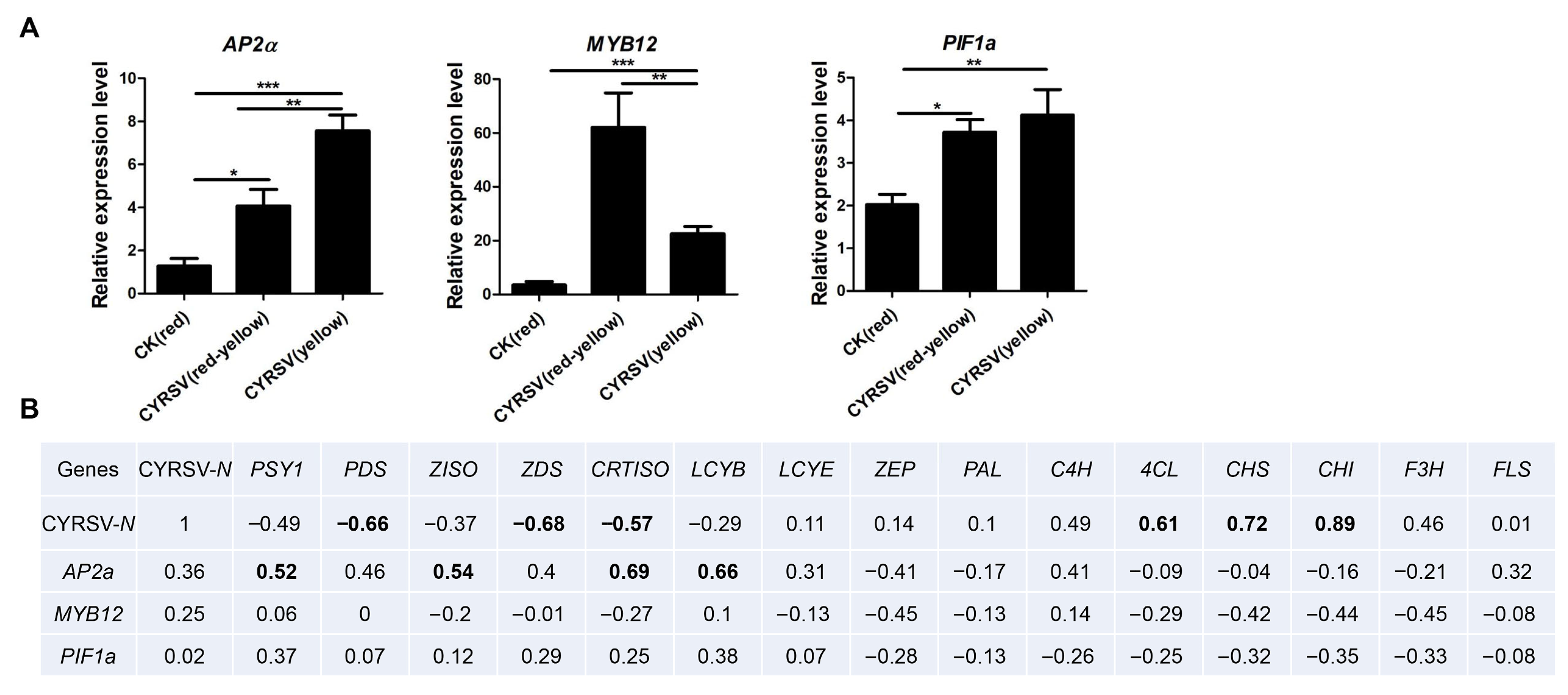
3.7. CYRSV Accumulation Is Associated with the Expression of Genes Encoding the Key Enzyme in Carotenoid and Flavonoid Pathways
3.8. Effects of CYRSV Infection on Tomato Fruit Color, Chromoplast Ultrastructure, and Carotenoid and Flavonoid Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, T.; Zhou, C.J.; Wang, Q.; Chen, X.R.; Sun, Q.; Zhao, T.Y.; Ye, J.C.; Wang, Y.; Zhang, Z.Y.; Zhang, Y.L.; et al. Rice black streaked dwarf virus P7-2 forms a SCF complex through binding to Oryza sativa SKP1-like proteins, and interacts with GID2 involved in the gibberellin pathway. PLoS ONE 2017, 12, e0177518. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Chen, C.; Huang, H.; Tan, X.; Wei, Z.; Li, J.; Yan, F.; Zhang, C.; Chen, J.; et al. A class of independently evolved transcriptional repressors in plant RNA viruses facilitates viral infection and vector feeding. Proc. Natl. Acad. Sci. USA 2021, 118, e2016673118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Chen, T.C.; Wu, K.; Kang, Y.C.; Yeh, S.D.; Zhang, Z.; Dong, J. Characterization of a New Orthotospovirus from Chilli Pepper in Yunnan Province, China. Plant Dis. 2020, 104, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, K.; Zhao, L.; Su, X.; Zheng, X.; Wang, T. Occurrence, Distribution, Evolutionary Relationships, Epidemiology, and Management of Orthotospoviruses in China. Front. Microbiol. 2021, 12, 686025. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Ding, B.Y.; Niu, J.; Shang, F.; Yang, L.; Chang, T.Y.; Wang, J.J. Characterization of the Geranylgeranyl Diphosphate Synthase Gene in Acyrthosiphon pisum (Hemiptera: Aphididae) and Its Association with Carotenoid Biosynthesis. Front. Physiol. 2019, 10, 1398. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H.; Zeng, Y.; Xu, Q. Plastids and Carotenoid Accumulation. Sub-Cell. Biochem. 2016, 79, 273–293. [Google Scholar]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Yin, L.; Liu, J.X.; Tao, J.P.; Xing, G.M.; Tan, G.F.; Li, S.; Duan, A.Q.; Ding, X.; Xu, Z.S.; Xiong, A.S. The gene encoding lycopene epsilon cyclase of celery enhanced lutein and β-carotene contents and confers increased salt tolerance in Arabidopsis. Plant Physiol. Biochem. PPB 2020, 157, 339–347. [Google Scholar] [CrossRef]
- Li, F.; Vallabhaneni, R.; Wurtzel, E.T. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008, 146, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhai, H.; Xue, L.; Zhao, N.; He, S.; Liu, Q. A lycopene β-cyclase gene, IbLCYB2, enhances carotenoid contents and abiotic stress tolerance in transgenic sweetpotato. Plant Sci. Int. J. Exp. Plant Biol. 2018, 272, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Iturra, L.F.; Simpson, K.; Arias, D.; Silva, C.; González-Calquin, C.; Amaza, L.; Handford, M.; Stange, C. Carrot DcALFIN4 and DcALFIN7 Transcription Factors Boost Carotenoid Levels and Participate Differentially in Salt Stress Tolerance When Expressed in Arabidopsis thaliana and Actinidia deliciosa. Int. J. Mol. Sci. 2022, 23, 12157. [Google Scholar] [CrossRef] [PubMed]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Wang, T.T.; Zhang, Z.K.; Li, Y. First Report of Chilli Yellow Ringspot Virus Infecting Tomato (Solanum lycopersicum) in China. Plant Disease. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar]
- Geyer, R.; Peacock, A.D.; White, D.C.; Lytle, C.; Van Berkel, G.J. Atmospheric pressure chemical ionization and atmospheric pressure photoionization for simultaneous mass spectrometric analysis of microbial respiratory ubiquinones and menaquinones. J. Mass Spectrom. JMS 2004, 39, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int. 2020, 138 Pt A, 109711. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Alvi, A.M.; Khan, K.A.; Rehmani, M.I.A.; Ansari, M.J.; Atta, S.; Ghramh, H.A.; Batool, T.; Tariq, M. Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum). Saudi J. Biol. Sci. 2018, 25, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Bréhélin, C.; Kessler, F. The plastoglobule: A bag full of lipid biochemistry tricks. Photochem. Photobiol. 2008, 84, 1388–1394. [Google Scholar] [CrossRef]
- Ytterberg, A.J.; Peltier, J.B.; van Wijk, K.J. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol. 2006, 140, 984–997. [Google Scholar] [CrossRef]
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.C. Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 2010, 51, 1601–1611. [Google Scholar] [CrossRef]
- Chayut, N.; Yuan, H.; Saar, Y.; Zheng, Y.; Sun, T.; Zhou, X.; Hermanns, A.; Oren, E.; Faigenboim, A.; Hui, M.; et al. Comparative transcriptome analyses shed light on carotenoid production and plastid development in melon fruit. Hortic. Res. 2021, 8, 112. [Google Scholar] [CrossRef]
- Moreno, J.C.; Mi, J.; Alagoz, Y.; Al-Babili, S. Plant apocarotenoids: From retrograde signaling to interspecific communication. Plant J. Cell Mol. Biol. 2021, 105, 351–375. [Google Scholar] [CrossRef]
- Huo, M.M.; Liu, W.L.; Zheng, Z.R.; Zhang, W.; Li, A.H.; Xu, D.P. Effect of end groups on the raman spectra of lycopene and β-carotene under high pressure. Molecules 2011, 16, 1973–1980. [Google Scholar] [CrossRef]
- Lin, S.F.; Chen, Y.C.; Chen, R.N.; Chen, L.C.; Ho, H.O.; Tsung, Y.H.; Sheu, M.T.; Liu, D.Z. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Ding, G.; Quan, W. Artificial Intelligence Assisted Ultrasonic Extraction of Total Flavonoids from Rosa sterilis. Molecules 2021, 26, 3835. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Pao, L.H.; Hsiong, C.H.; Shih, T.Y.; Lee, M.S.; Hu, O.Y. Dietary flavonoids modulate CYP2C to improve drug oral bioavailability and their qualitative/quantitative structure-activity relationship. AAPS J. 2014, 16, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Hwang, I.; Goswami, G.; Jung, H.J.; Nath, U.K.; Yoo, H.J.; Lee, J.M.; Nou, I.S. Molecular Insights Reveal Psy1, SGR, and SlMYB12 Genes are Associated with Diverse Fruit Color Pigments in Tomato (Solanum lycopersicum L.). Molecules 2017, 22, 2180. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Liu, C.; Wei, F. Luteolin attenuates doxorubicin-induced cardiotoxicity by modulating the PHLPP1/AKT/Bcl-2 signalling pathway. PeerJ 2020, 8, e8845. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of Methoxylated Flavonoids in the Cultures of Isaria fumosorosea KCH J2. Molecules 2018, 23, 2578. [Google Scholar] [CrossRef]
- Liang, M.H.; Li, X.Y. Involvement of Transcription Factors and Regulatory Proteins in the Regulation of Carotenoid Accumulation in Plants and Algae. J. Agric. Food Chem. 2023, 71, 18660–18673. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Wang, X.C.; Wu, J.; Guan, M.L.; Zhao, C.H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. Cell Mol. Biol. 2020, 101, 637–652. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Tian, S.; Hao, W.; Chen, K.; Du, L. The R2R3MYB transcription factors MaMYBF and MaMYB1 regulate flavonoid biosynthesis in grape hyacinth. Plant Physiol. Biochem. PPB 2023, 194, 85–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, G.; Tang, Q.; Song, W.; Gao, Q.; Xiang, G.; Li, X.; Liu, G.; Fan, W.; Li, X.; et al. EbMYBP1, a R2R3-MYB transcription factor, promotes flavonoid biosynthesis in Erigeron breviscapus. Front. Plant Sci. 2022, 13, 946827. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. Cell Mol. Biol. 2016, 85, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef]
- Sang, K.; Li, J.; Qian, X.; Yu, J.; Zhou, Y.; Xia, X. The APETALA2a/DWARF/BRASSINAZOLE-RESISTANT 1 module contributes to carotenoid synthesis in tomato fruits. Plant J. Cell Mol. Biol. 2022, 112, 1238–1251. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Pan, X.; Li, B.; Yang, Q.; Yang, C.; Zhang, J.; Wu, F.; Yang, A.; Li, Y. PIF1, a phytochrome-interacting factor negatively regulates drought tolerance and carotenoids biosynthesis in tobacco. Int. J. Biol. Macromol. 2023, 247, 125693. [Google Scholar] [CrossRef]
- Zhuge, Y.; Sheng, H.; Zhang, M.; Fang, J.; Lu, S. Grape phytochrome-interacting factor VvPIF1 negatively regulates carotenoid biosynthesis by repressing VvPSY expression. Plant Sci. Int. J. Exp. Plant Biol. 2023, 331, 111693. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef]
- Ballester, A.R.; Molthoff, J.; de Vos, R.; Hekkert, B.; Orzaez, D.; Fernández-Moreno, J.P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J.; et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010, 152, 71–84. [Google Scholar] [CrossRef]
- Luo, J.; Butelli, E.; Hill, L.; Parr, A.; Niggeweg, R.; Bailey, P.; Weisshaar, B.; Martin, C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. Cell Mol. Biol. 2008, 56, 316–326. [Google Scholar] [CrossRef]


| Gene ID | Gene Description | Gene Name | CYRSV (Red-Yellow) vs. CK (Red) | CYRSV (Yellow) vs. CK (Red) | CYRSV (Yellow) vs. CYRSV (Red-Yellow) |
|---|---|---|---|---|---|
| Solyc02g085710.4 | geranylgeranyl pyrophosphate synthase | GGPS | up | up | / |
| Solyc03g031860.3 | phytoene synthase 1 | PSY1 | ns | up | / |
| Solyc03g123760.3 | 15-cis-phytoene desaturase | PDS | ns | ns | / |
| Solyc12g098710.2 | 15-cis-zeta-carotene isomerase | ZISO | ns | ns | / |
| Solyc01g097810.3 | zeta-carotene desaturase | ZDS | ns | up | / |
| Solyc04g040190.1 | lycopene beta cyclase | LCYB | ns | up | / |
| Solyc02g090890.4 | zeaxanthin epoxidase | ZEP | ns | ns | / |
| Solyc07g056570.1 | 9-cis-epoxycarotenoid dioxygenase | NCED | up | up | / |
| Solyc08g076300.3 | 4-coumarate-CoA ligase-like 6 | 4CL | ns | ns | / |
| Solyc09g091510.3 | chalcone synthase 1 | CHS | up | ns | / |
| Solyc05g052240.3 | probable chalcone-flavonone isomerase 3 | CHI | up | up | / |
| Solyc11g013110.2 | flavonol synthase/flavanone 3-hydroxylase | FLS/F3H | up | up | down |
| Gene ID | Gene Description | Gene Name | CYRSV (Red-Yellow) vs. CK (Red) | CYRSV (Yellow) vs. CK (Red) | CYRSV (Yellow) vs. CYRSV (Red-Yellow) |
|---|---|---|---|---|---|
| Solyc02g093130.3 | AP2/ethylene-responsive transcription factor | RAP2-10 | up | up | / |
| Solyc07g054220.1 | AP2/ethylene-responsive transcription factor | ERF054 | ns | up | up |
| Solyc01g079620.4 | Myb12 transcription factor | MYB12 | up | up | ns |
| Solyc09g063010.4 | transcription factor PIF1 | PIF1 | up | up | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, J.; Wu, K.; Chen, Y.; Wang, T.; Zhang, Z. Analysis of Colored Lesions of Chilli Yellow Ringspot Orthotospovirus Infection in Tomato Fruits. Viruses 2025, 17, 1426. https://doi.org/10.3390/v17111426
Li Y, Zhang J, Wu K, Chen Y, Wang T, Zhang Z. Analysis of Colored Lesions of Chilli Yellow Ringspot Orthotospovirus Infection in Tomato Fruits. Viruses. 2025; 17(11):1426. https://doi.org/10.3390/v17111426
Chicago/Turabian StyleLi, Yu, Jie Zhang, Kuo Wu, Yongdui Chen, Tiantian Wang, and Zhongkai Zhang. 2025. "Analysis of Colored Lesions of Chilli Yellow Ringspot Orthotospovirus Infection in Tomato Fruits" Viruses 17, no. 11: 1426. https://doi.org/10.3390/v17111426
APA StyleLi, Y., Zhang, J., Wu, K., Chen, Y., Wang, T., & Zhang, Z. (2025). Analysis of Colored Lesions of Chilli Yellow Ringspot Orthotospovirus Infection in Tomato Fruits. Viruses, 17(11), 1426. https://doi.org/10.3390/v17111426






