Oncolytic Vaccinia Virus Expressing HSP70 shRNA Exerts Anti-Tumor Effects in Human Ovarian Cancer via Triggering the Autophagy–ROS Feedback Loop and Immune Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Construction and Preparation of Recombinant oncoVV-shHSP70
2.4. Virus Replication Assay
2.5. Xenograft Assays in Nude Mice
2.6. Xenograft Assays in Humanized Mice
2.7. Western Blot
2.8. Flow Cytometric Analysis
2.9. Histological Examination
2.10. Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
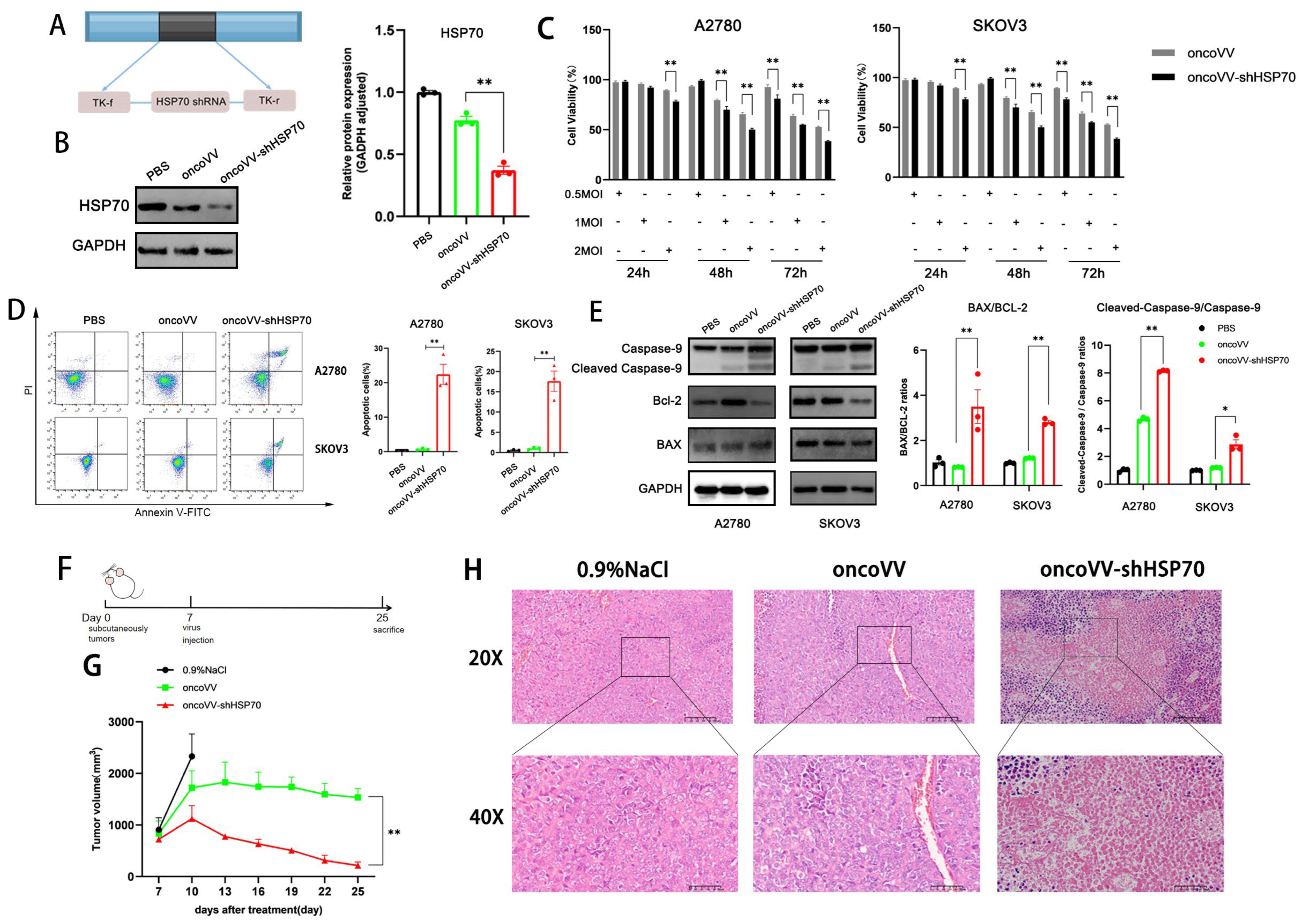
3.1. The Anti-Tumor Effect of oncoVV-shHSP70 Both In Vivo and In Vitro
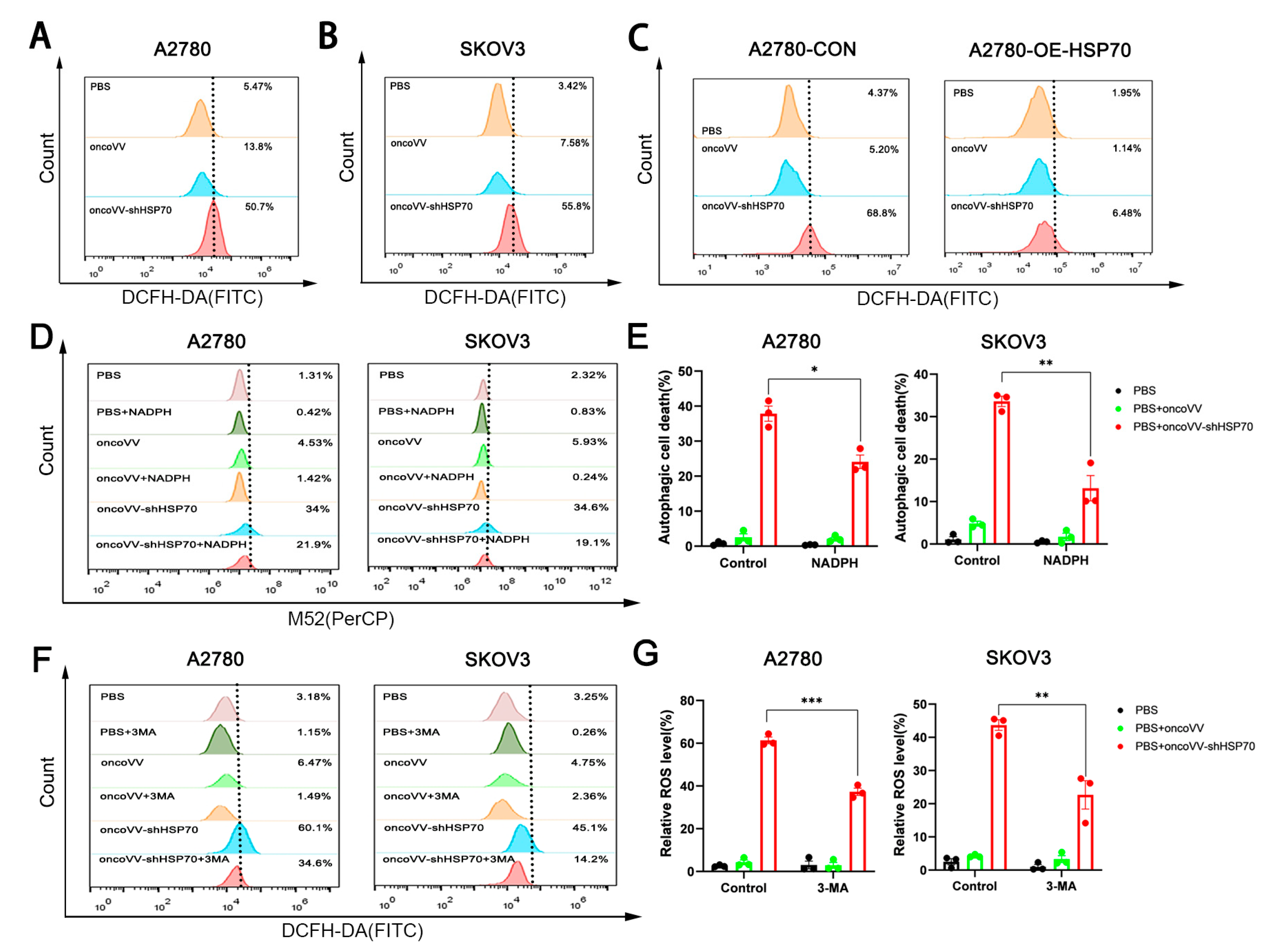
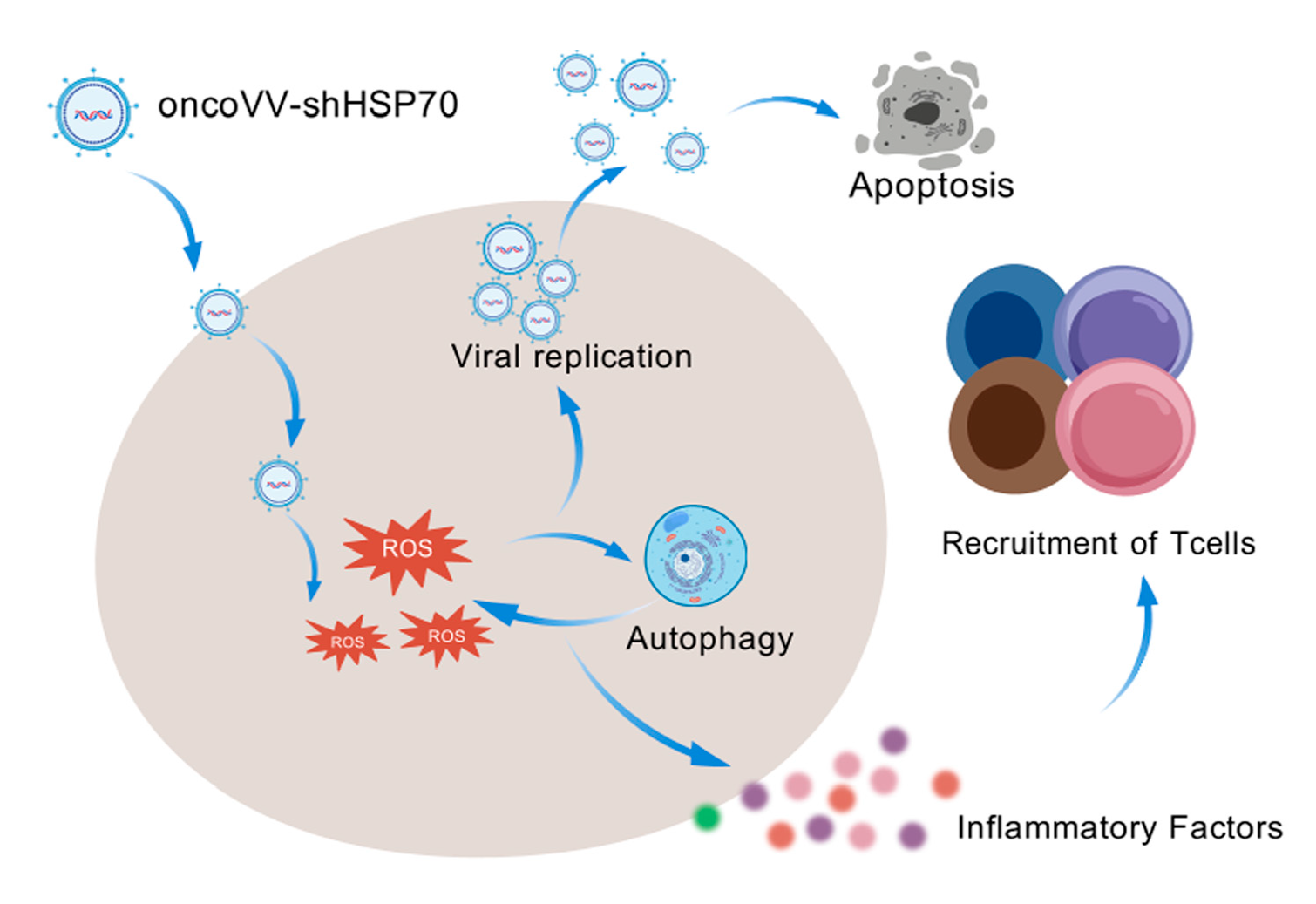
3.2. OncoVV-shHSP70 Orchestrates an ROS–Autophagy Feedback Loop
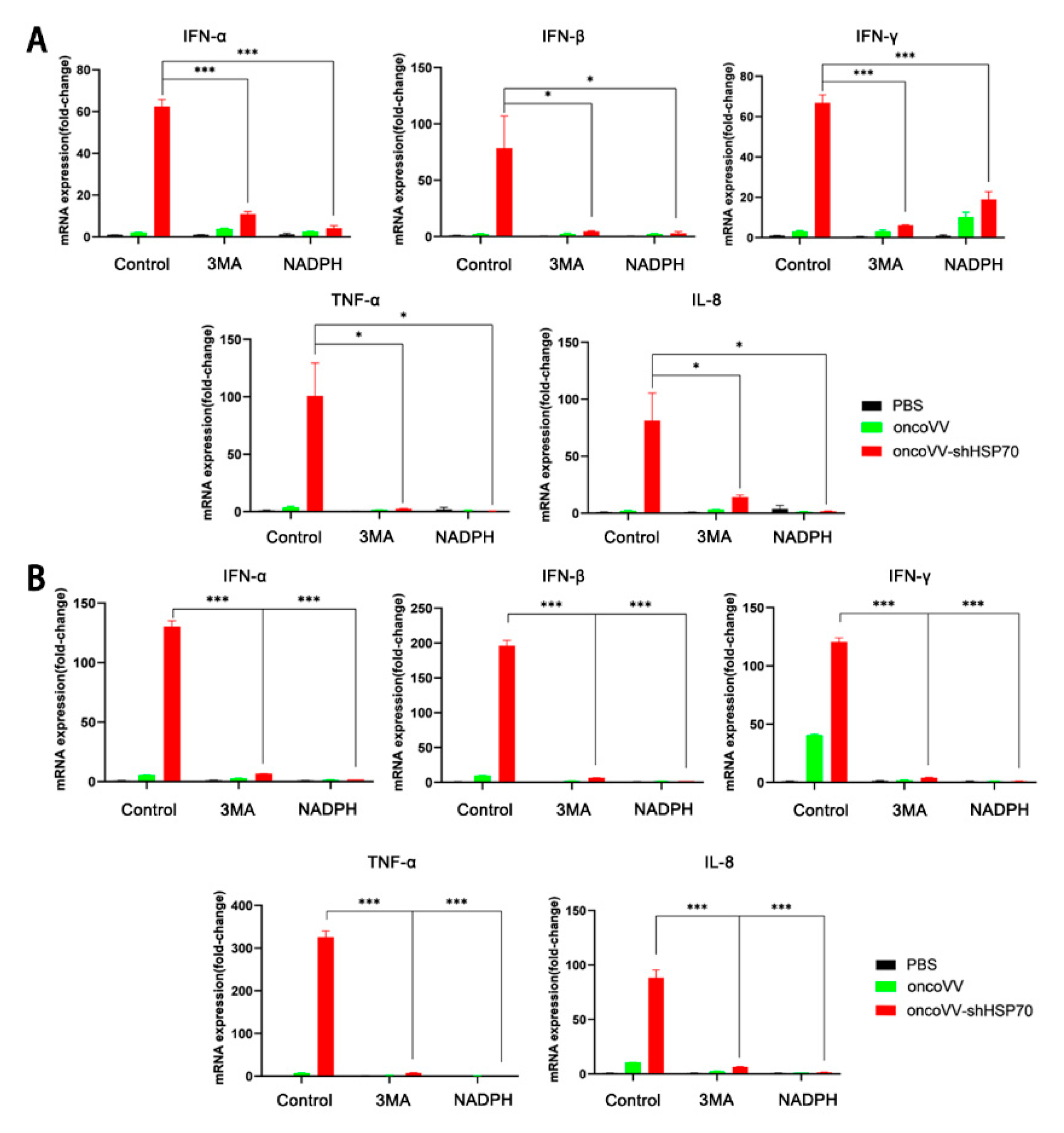
3.3. OncoVV-shHSP70 Activates Antitumor Inflammation
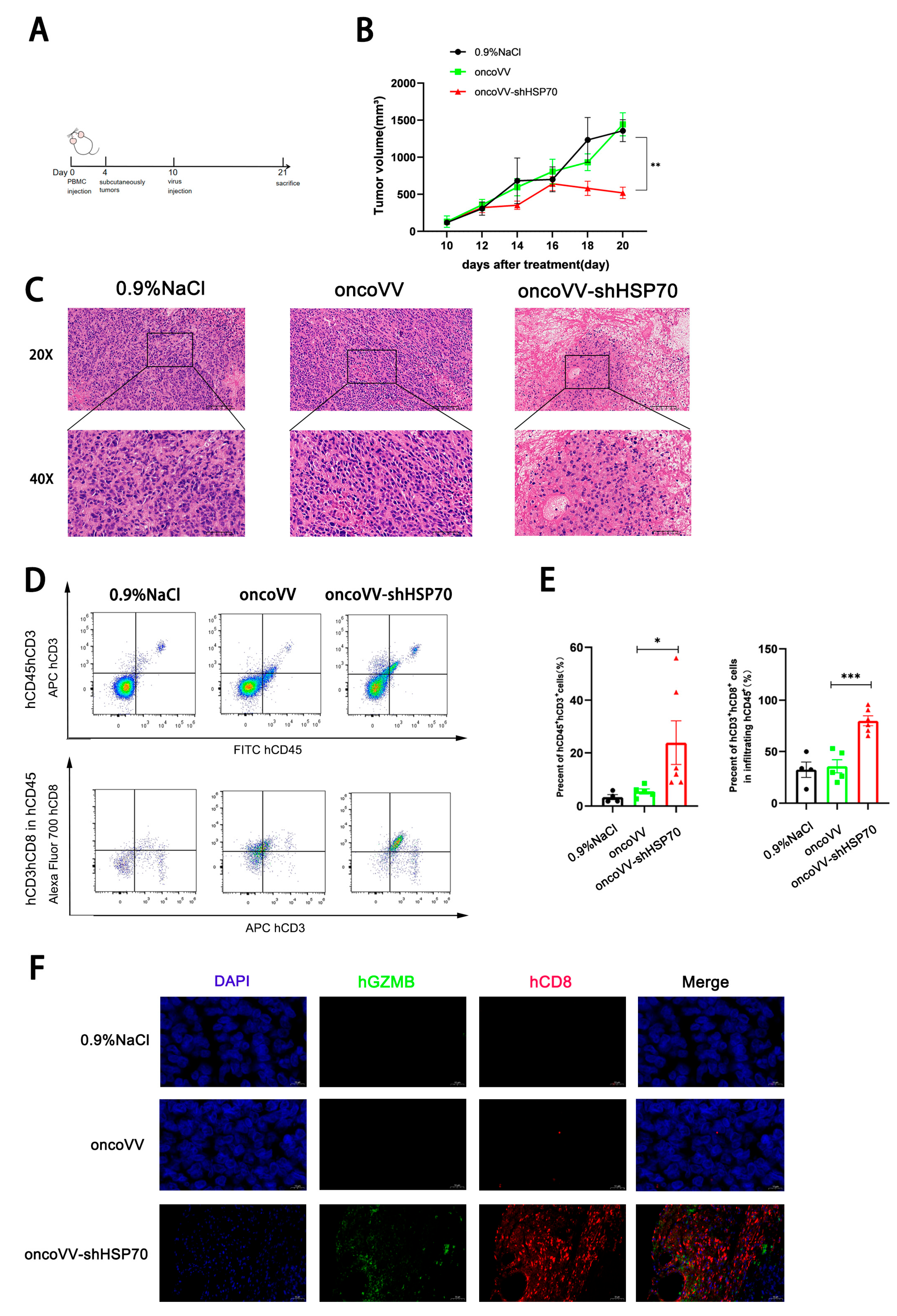
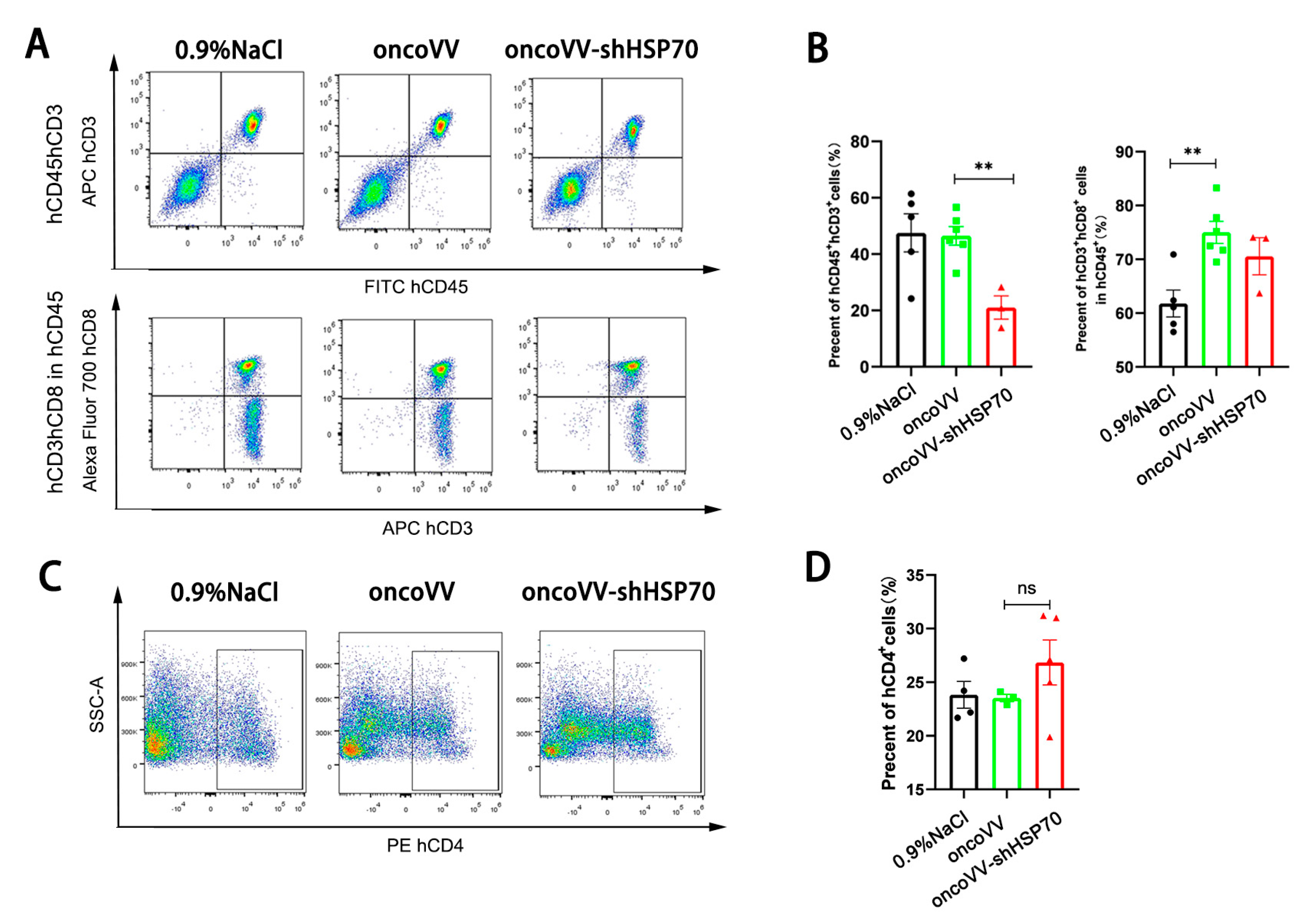
3.4. OncoVV-shHSP70 Remodels TIME via Cytotoxic CD8+ T−Cell Recruitment in Humanized Mice
3.5. OncoVV-shHSP70 Modulates Systemic T Cell Distribution in Humanized Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.N.; Li, C.; Li, H.; Liu, X.S.; Yang, Z.M. OsZIP11 is a trans-Golgi-residing transporter required for rice iron accumulation and development. Gene 2022, 836, 146678. [Google Scholar] [CrossRef] [PubMed]
- Chandfra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Rössler, R.; Wagner, J.; Knaier, R.; Rommers, N.; Kressig, R.W.; Schmidt-Trucksäss, A.; Hinrichs, T. Spatiotemporal gait characteristics across the adult lifespan: Reference values from a healthy population—Analysis of the complete cohort study. Gait Posture 2024, 109, 101–108. [Google Scholar] [CrossRef]
- Wang, G.H.; Lin, Q.M.; Lin, J.F.; Deng, Y.J.; Jiang, Y.R.; Wang, H.W.; Su, R.X.; Qiu, X.C.; Li, C.B.; Jiang, F. Protocol for the development of Chinese guideline for the treatment of bedtime problems and night wakings in children under 6 years of age. Chin. J. Pediatr. 2023, 61, 122–125. [Google Scholar]
- Huang, Z.; Guo, H.; Lin, L.; Li, S.; Yang, Y.; Han, Y.; Huang, W.; Yang, J. Application of oncolytic virus in tumor therapy, J. Med. Virol. 2023, 95, e28729. [Google Scholar] [CrossRef]
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D., Jr.; Silk, A.W.; et al. Challenges and opportunities in cancer immunotherapy: A society for immunotherapy of cancer (SITC) strategic vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef]
- Volovat, S.R.; Scripcariu, D.V.; Vasilache, I.A.; Stolniceanu, C.R.; Volovat, C.; Augustin, I.G.; Volovat, C.C.; Ostafe, M.R.; Andreea-Voichița, S.G.; Bejusca-Vieriu, T.; et al. Oncolytic virotherapy: A new paradigm in cancer immunotherapy. Int. J. Mol. Sci. 2024, 25, 1180. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef]
- Shirbhate, E.; Veerasamy, R.; Boddu, S.H.S.; Tiwari, A.K.; Rajak, H. Histone deacetylase inhibitor-based oncolytic virotherapy: A promising strategy for cancer treatment. DDT 2022, 27, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006, 98, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, H.L.; Zhang, X.R.; Xu, J.P.; Hu, W.K.; Liang, M.; Chen, S.Y.; Hu, F.; Chu, D.T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, Y.L.; Lin, H.W.; Chang, C.F.; Huang, B.S.; Sun, W.Z.; Cheng, W.F. Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and DAMPs. Cancer Lett. 2021, 523, 149–161. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. Phocus: A phase 3, randomized, open-label study of sequential treatment with pexa-vec (JX-594) and sorafenib in patients with advanced hepatocellular carcinoma. Liver Cancer 2024, 13, 248–264. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, W.; Ying, Q.; Ni, J.; Jia, X.; Zhou, Y.; Ye, T.; Li, G.; Chen, K. Oncolytic vaccinia virus carrying aphrocallistes vastus lectin (oncoVV-AVL) enhances inflammatory response in hepatocellular carcinoma cells. Mar. Drugs 2022, 20, 667. [Google Scholar] [CrossRef]
- Xuan, Y.; Yan, W.; Wang, R.; Wang, X.; Guo, Y.; Dun, H.; Huan, Z.; Xu, L.; Han, R.; Sun, X.; et al. GM-CSF and IL-21-armed oncolytic vaccinia virus significantly enhances anti-tumor activity and synergizes with anti-PD1 immunotherapy in pancreatic cancer. Front. Immunol. 2025, 15, 1506632. [Google Scholar] [CrossRef]
- Zhu, B.; Hong, Z.; Zhu, J.; Yu, J.; Zhou, Y.; Chen, K.; Ye, T.; Li, G. Oncolytic vaccinia virus encoding aphrocallistes vastus lectin suppresses the proliferation of gastric cancer cells. Hum. Gene Ther. 2024, 35, 938–950. [Google Scholar] [CrossRef]
- Davola, M.E.; Mossman, K.L. Oncolytic viruses: How “lytic” must they be for therapeutic efficacy? Oncoimmunology 2019, 8, e1581528. [Google Scholar] [CrossRef]
- Neven, L.G.; Wakie, T.; Yee, W.L. Low temperature duration and adult rearing regimes affect eclosion of rhagoletis indifferens (tephritidae: Diptera). Environ. Entomol. 2020, 49, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Q.; Yuan, R.; Zhang, Y.; Chen, K.; Yu, J.; Ye, T.; Jia, X.; Zhou, Y.; Li, G.; et al. Oncolytic vaccinia virus harboring aphrocallistes vastus lectin exerts anti-tumor effects by directly oncolysis and inducing immune response through enhancing ROS in human ovarian cancer. Biochem. Biophys. Res. Commun. 2024, 730, 150355. [Google Scholar] [CrossRef] [PubMed]
- Farrera-Sal, M.; Moya-Borrego, L.; Bazan-Peregrino, M.; Alemany, R. Evolving status of clinical immunotherapy with oncolytic adenovirus. Clin. Cancer Res. 2021, 27, 2979–2988. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 multi-functionality in cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Benes, J.; Kotrc, M.; Kroupova, K.; Wohlfahrt, P.; Kovar, J.; Franekova, J.; Hegarova, M.; Hoskova, L.; Hoskova, E.; Pelikanova, T.; et al. Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF). Sci. Rep. 2022, 12, 13038. [Google Scholar] [CrossRef]
- Hunt, C.; Morimoto, R.I. Conserved features of eukaryotic HSP70 genes revealed by comparison with the nucleotide sequence of human HSP70. Proc. Natl. Acad. Sci. USA 1985, 82, 6455–6459. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, G.B.; Penteado, L.; Miranda, M.M.; Filassi, J.R.; Baracat, E.C.; Linhares, I.M. The circulating 70 kDa heat shock protein (HSPA1A) level is a potential biomarker for breast carcinoma and its progression. Sci. Rep. 2022, 12, 13012. [Google Scholar] [CrossRef] [PubMed]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef]
- Cunningham, S.; Graham, C.; MacLean, M.; Aurora, P.; Ashworth, M.; Barbato, A.; Calder, A.; Carlens, J.; Clement, A.; Hengst, M.; et al. One-year outcomes in a multicentre cohort study of incident rare diffuse parenchymal lung disease in children (ChILD). Thorax 2020, 75, 172–175. [Google Scholar] [CrossRef]
- Choi, J.Y.; Seok, H.J.; Kim, R.K.; Choi, M.Y.; Lee, S.J.; Bae, I.H. Mir-519d-3p suppresses tumorigenicity metastasis by inhibiting Bcl-w HIF-1α in, N.S.C.L.C. Mol. Ther. Oncolytics 2021, 22, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Geslain, E.; Belcour, A.; Frioux, C.; Aïte, M.; Siegel, A.; Corre, E.; Dittami, S.M. Robustness analysis of metabolic predictions in algal microbial communities based on different annotation pipelines. PeerJ 2021, 9, e11344. [Google Scholar] [CrossRef]
- Wang, W.; Ji, W.; Hu, H.; Ma, J.; Li, X.; Mei, W.; Xu, Y.; Hu, H.; Yan, Y.; Song, Q.; et al. Survivin promoter-regulated oncolytic adenovirus with Hsp70 gene exerts effective antitumor efficacy in gastric cancer immunotherapy. Oncotarget 2014, 5, e1430. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Tan, J.; Fang, Y.; An, X.; Chen, B. Autophagy and hippocampal neuronal injury. Sleep Breath. 2014, 18, 243–249. [Google Scholar] [CrossRef]
- Ureshino, R.P.; Rocha, K.K.; Lopes, G.S.; Bincoletto, C.; Smaili, S.S. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid. Redox Signal 2014, 21, 123–137. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, Y.; Liu, T.; Wang, X.; Ren, X.; Ye, T.; Li, G. Oncolytic Vaccinia Virus Expressing Aphrocallistes vastus Lectin as a Cancer Therapeutic Agent. Mar. Drugs 2019, 17, 363. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, P.; Hu, Y.; Guo, K.; Jia, X.; Huang, B.; Liu, X.; He, X.; Huang, F. An oncolytic adenovirus delivering TSLC1 inhibits Wnt signaling pathway and tumor growth in SMMC-7721 xenograft mice model. Acta Biochim. Biophys. Sin. 2021, 53, 766–774. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef]
- Peker, N.; Gozuacik, D. Autophagy as a cellular stress response mechanism in the nervous system. J. Mol. Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.; Huang, P.; Zhao, H.; Zhang, R.; Ma, B.; Chen, K.; Huang, F.; Zhou, X.; Cui, C.; et al. A novel golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma. Oncotarget 2015, 6, 13564–13578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lai, W.; Li, Q.; Yu, Y.; Jin, J.; Guo, W.; Zhou, X.; Liu, X.; Wang, Y. A novel oncolytic adenovirus targeting wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem. Biophys. Res. Commun. 2017, 491, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, T.; Altomonte, J. Fusogenic viruses in oncolytic immunotherapy. Cancers 2018, 10, 216. [Google Scholar] [CrossRef]
- Kirn, D.; Martuza, R.L.; Zwiebel, J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 2001, 7, 781–787. [Google Scholar] [CrossRef]
- Qi, C.; Hao, H.; Zhang, W.; Fu, Y.; Han, Y.; Li, J.; Chen, L.; Cui, G.; Liu, Q.; Li, Y.; et al. Targeting formyl peptide receptor 2 to suppress neuroinflammation in neuromyelitis optica spectrum disorder. Theranostics 2025, 15, 4495–4506. [Google Scholar] [CrossRef]
- Fu, L.; Han, L.; Xie, C.; Li, W.; Lin, L.; Pan, S.; Zhou, Y.; Li, Z.; Jin, M.; Zhang, A. Identification of extracellular actin as a ligand for triggering receptor expressed on myeloid cells-1 signaling. Front. Immunol. 2017, 8, 917. [Google Scholar] [CrossRef]
- Salah, A.; Wang, H.; Li, Y.; Ji, M.; Ou, W.B.; Qi, N.; Wu, Y. Insights into dendritic cells in cancer immunotherapy: From bench to clinical applications. Front. Cell Dev. Biol. 2021, 9, 686544. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.L.; Yang, W.Y.; Qian, Y.C.; Pan, J.Y.; Zhu, C.N.; Liu, L.; Ou, W.B.; Zhao, H.X.; Zhang, D.P. Biosynthesis of silver nanoparticles with antimicrobial and anticancer properties using two novel yeasts. Sci. Rep. 2021, 11, 15795. [Google Scholar] [CrossRef]
- Gao, C.; Ying, Q.; Qiu, Y.; Ren, N.; Chen, K.; Zhou, Y.; Ye, T.; Li, G. Oncolytic vaccinia virus harboring CLEC2A gene enhances viral replication and antitumor efficacy. Mol. Ther. Oncolytics 2024, 32, 200823. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.D.; Morimoto, R.I. Molecular chaperones and the stress of oncogenesis. Oncogene 2004, 23, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress. Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Huryn, D.M.; Brodsky, J.L.; Brummond, K.M.; Chambers, P.G.; Eyer, B.; Ireland, A.W.; Kawasumi, M.; Laporte, M.G.; Lloyd, K.; Manteau, B.; et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc. Natl. Acad. Sci. USA 2011, 108, 6757–6762. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an allosteric small-molecule inhibitor selective for the inducible form of heat shock protein 70. Chem. Biol. 2014, 21, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Yoshimura, K.; Matsui, H.; Shindo, Y.; Tamesa, T.; Tokumitsu, Y.; Hashimoto, N.; Tokuhisa, Y.; Sakamoto, K.; Sakai, K.; et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol. Immun. 2015, 64, 1047–1056. [Google Scholar] [CrossRef]
- Kokowski, K.; Stangl, S.; Seier, S.; Hildebrandt, M.; Vaupel, P.; Multhoff, G. Radiochemotherapy combined with NK cell transfer followed by second-line PD-1 inhibition in a patient with NSCLC stage IIIb inducing long-term tumor control: A case study. Strahlenther. Onkol. 2019, 195, 352–361. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning cold into hot: Firing up the tumor microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Liu, Z.; Han, C.; Fu, Y.X. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol. Immunol. 2020, 17, 13–26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Hong, Z.; Zhang, G.; Zhu, T.; Zhou, Y.; Ye, T.; Li, G.; Chen, K. Oncolytic Vaccinia Virus Expressing HSP70 shRNA Exerts Anti-Tumor Effects in Human Ovarian Cancer via Triggering the Autophagy–ROS Feedback Loop and Immune Activation. Viruses 2025, 17, 1423. https://doi.org/10.3390/v17111423
Cai Z, Hong Z, Zhang G, Zhu T, Zhou Y, Ye T, Li G, Chen K. Oncolytic Vaccinia Virus Expressing HSP70 shRNA Exerts Anti-Tumor Effects in Human Ovarian Cancer via Triggering the Autophagy–ROS Feedback Loop and Immune Activation. Viruses. 2025; 17(11):1423. https://doi.org/10.3390/v17111423
Chicago/Turabian StyleCai, Zheqi, Zhiyun Hong, Guohui Zhang, Tinwei Zhu, Yanrong Zhou, Ting Ye, Gongchu Li, and Kan Chen. 2025. "Oncolytic Vaccinia Virus Expressing HSP70 shRNA Exerts Anti-Tumor Effects in Human Ovarian Cancer via Triggering the Autophagy–ROS Feedback Loop and Immune Activation" Viruses 17, no. 11: 1423. https://doi.org/10.3390/v17111423
APA StyleCai, Z., Hong, Z., Zhang, G., Zhu, T., Zhou, Y., Ye, T., Li, G., & Chen, K. (2025). Oncolytic Vaccinia Virus Expressing HSP70 shRNA Exerts Anti-Tumor Effects in Human Ovarian Cancer via Triggering the Autophagy–ROS Feedback Loop and Immune Activation. Viruses, 17(11), 1423. https://doi.org/10.3390/v17111423





