Pre-Clade IIb Mpox Virus Exposure in Ghana: A Retrospective Serological Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population and Sample Size
2.3. Sample Processing
2.4. Detection of IgG Antibodies Against MPVX
2.5. Data Curation and Analysis
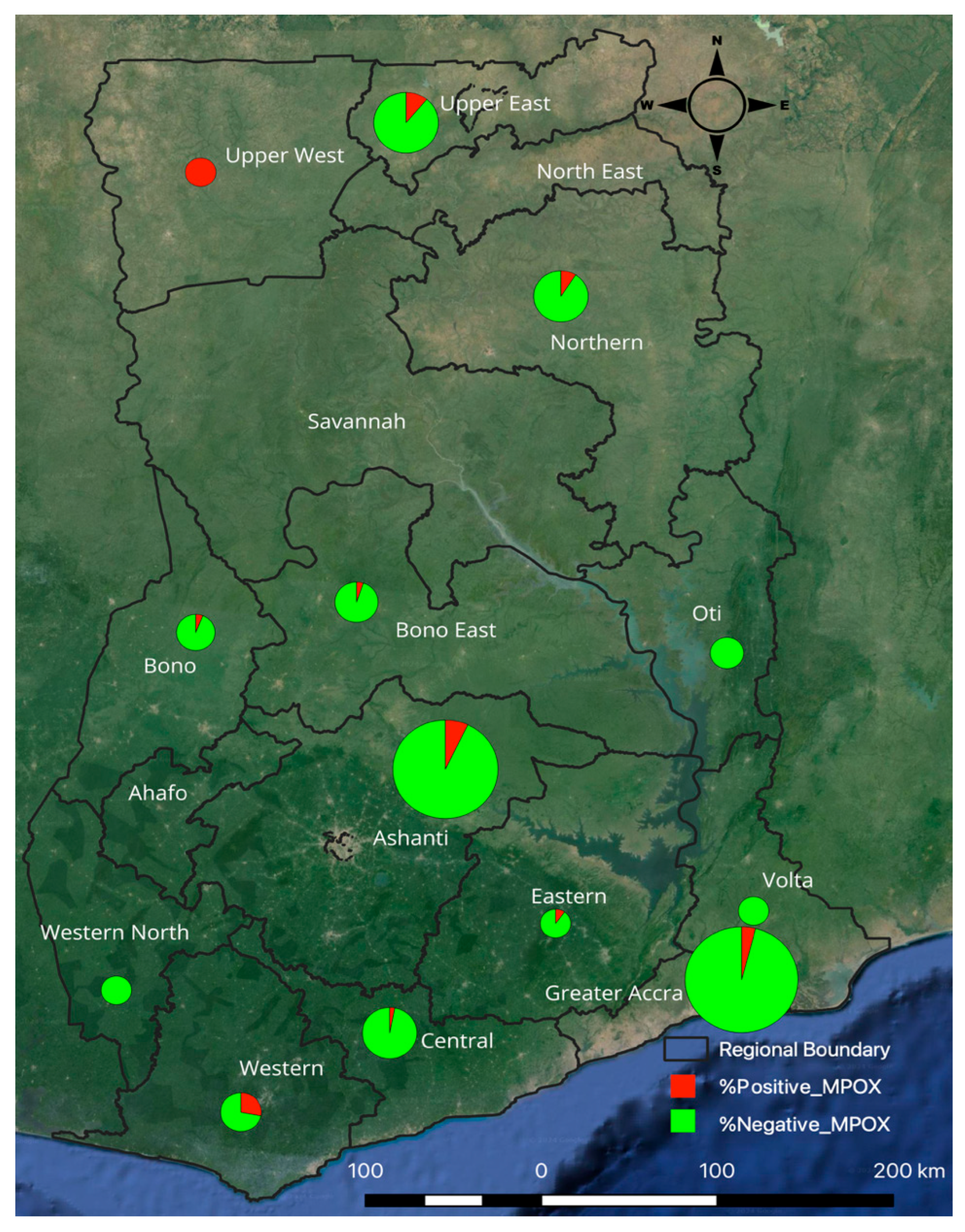
3. Results
Factors Associated with MPXV Seropositivity in the Tested Samples
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arita, I.; Henderson, D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968, 39, 277–283. [Google Scholar]
- Ulaeto, D.; Agafonov, A.; Burchfield, J.; Carter, L.; Happi, C.; Jakob, R.; Krpelanova, E.; Kuppalli, K.; Lefkowitz, E.J.; Mauldin, M.R.; et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect. Dis. 2023, 23, 273–275. [Google Scholar] [CrossRef]
- Wawina-Bokalanga, T.; Akil-Bandali, P.; Kinganda-Lusamaki, E.; Lokilo, E.; Jansen, D.; Amuri-Aziza, A.; Makangara-Cigolo, J.-C.; Pukuta-Simbu, E.; Ola-Mpumbe, R.; Muyembe, M.; et al. Co-circulation of monkeypox virus subclades Ia and Ib in Kinshasa Province, Democratic Republic of the Congo, July to August 2024. Eurosurveillance 2024, 29, 2400592. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; David-Olawade, A.C. Strengthening Africa’s response to Mpox (monkeypox): Insights from historical outbreaks and the present global spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef]
- WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 10 February 2025).
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; de Coul, E.O.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C.; et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Eurosurveillance 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Branda, F.; Ceccarelli, G.; Maruotti, A.; Ciccozzi, M.; Scarpa, F. Global spread of mpox Clade I: Implications for travel and public health. Travel Med. Infect. Dis. 2024, 62, 102781. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Mpox: The need for a coordinated international response. Lancet 2024, 404, 725. [Google Scholar] [CrossRef]
- 2022 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 10 December 2022).
- Beer, E.M.; Bhargavi Rao, V. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLOS Neglected Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Mpox—South Africa. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON525 (accessed on 21 October 2024).
- Ligon, B.L. Monkeypox: A review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 2004, 15, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Adadi, P.; Mensah, E.O.; Abdul-Razak, S. The outbreak of monkeypox (MPX) in Ghana. J. Med. Virol. 2023, 95, e28171. [Google Scholar] [CrossRef]
- Greater Accra Has More Than 50% of Monkeypox Cases—Citinewsroom—Comprehensive News in Ghana. Available online: https://citinewsroom.com/2022/07/greater-accra-has-more-than-50-of-monkeypox-cases/#google_vignette (accessed on 31 August 2025).
- Mpox (Monkeypox)—Africa, C.D.C. Available online: https://africacdc.org/disease/monkeypox/ (accessed on 31 August 2025).
- Owusu Donkor, I.; Mensah, S.K.; Dwomoh, D.; Akorli, J.; Abuaku, B.; Ashong, Y.; Opoku, M.; Andoh, N.E.; Sumboh, J.G.; Ohene, S.-A.; et al. Modeling SARS-CoV-2 antibody seroprevalence and its determinants in Ghana: A nationally representative cross-sectional survey. PLoS Glob. Public Health 2023, 3, e0001851. [Google Scholar] [CrossRef] [PubMed]
- Ghana Health Service—Your Health Our Concern. Available online: https://ghs.gov.gh/disease-outbreaks (accessed on 9 October 2025).
- RayBio ® Monkeypox Virus E8L Protein Human IgG ELISA Kit RayBio® Monkeypox Virus E8L Protein Human IgG ELISA Kit. Available online: www.RayBiotech.com (accessed on 30 September 2025).
- Leendertz, S.A.J.; Stern, D.; Theophil, D.; Anoh, E.; Mossoun, A.; Schubert, G.; Wiersma, L.; Akoua-Koffi, C.; Couacy-Hymann, E.; Muyembe-Tamfum, J.-J.; et al. A cross-sectional serosurvey of anti-orthopoxvirus antibodies in central and Western Africa. Viruses 2017, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 383729. [Google Scholar] [CrossRef] [PubMed]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of Monkeypox—West and Central Africa, 1970–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 306. [Google Scholar] [CrossRef]
- Mpox Crisis: 71% of Cases Concentrated in Western Region—MyJoyOnline. Available online: https://www.myjoyonline.com/mpox-crisis-71-of-cases-concentrated-in-western-region/ (accessed on 1 September 2025).
- Western Regional Co-Ordinating Council. Mpox Outbreak: Western Regional Health Directorate Springs Into Action. Available online: https://wrcc.gov.gh/mpox-outbreak-western-regional-health-directorate-springs-into-action/ (accessed on 1 September 2025).
- Curaudeau, M.; Besombes, C.; Nakouné, E.; Fontanet, A.; Gessain, A.; Hassanin, A. Identifying the Most Probable Mammal Reservoir Hosts for Monkeypox Virus Based on Ecological Niche Comparisons. Viruses 2023, 15, 727. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Karem, K.L.; Reynolds, M.; Hughes, C.; Braden, Z.; Nigam, P.; Crotty, S.; Glidewell, J.; Ahmed, R.; Amara, R.; Damon, I.K. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 2007, 14, 1318–1327. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Damon, I.K. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends Microbiol. 2012, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Adetifa, I.; Muyembe, J.J.; Bausch, D.G.; Heymann, D.L. Mpox neglect and the smallpox niche: A problem for Africa, a problem for the world. Lancet 2023, 401, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Frequency (N = 457) | Percentage (%) |
|---|---|---|
| Area | ||
| Rural | 79 | 17.29 |
| Urban | 286 | 62.58 |
| Age | ||
| <16 | 114 | 24.95 |
| 16–25 | 127 | 27.79 |
| 26–35 | 65 | 14.22 |
| 36–45 | 50 | 10.94 |
| 46–55 | 47 | 10.28 |
| 56–65 | 29 | 6.35 |
| 65+ | 25 | 5.47 |
| Sex | ||
| Female | 253 | 55.36 |
| Male | 204 | 44.64 |
| Region | ||
| Ashanti | 119 | 26.04 |
| Bono East | 20 | 4.38 |
| Brong Ahafo | 19 | 4.16 |
| Central | 32 | 7.00 |
| Eastern | 10 | 2.19 |
| Greater Accra | 137 | 29.98 |
| Northern | 32 | 7.00 |
| Oti | 12 | 2.63 |
| Upper East | 45 | 9.85 |
| Upper West | 1 | 0.22 |
| Volta | 9 | 1.97 |
| Western | 18 | 3.94 |
| Western North | 3 | 0.66 |
| Vaccination Status | ||
| Unvaccinated | 310 | 67.83 |
| Vaccinated | 101 | 22.1 |
| Uncertain vaccination status | 46 | 10.07 |
| Characteristics | Seropositivity | cOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Sex | ||||||
| Female | 238 (94.07) | 15 (5.93) | Ref | Ref | ||
| Male | 189 (92.65) | 15 (7.35) | 1.259 (0.6–2.641) | 0.542 | 1.158 (0.509–2.636) | 0.726 |
| Age (years) | ||||||
| <16 | 102 (89.47) | 12 (10.53) | Ref | Ref | ||
| 16–25 | 121 (95.28) | 6 (4.72) | 0.421 (0.153–1.163) | 0.095 | 0.412 (0.135–1.259) | 0.12 |
| 26–35 | 60 (92.31) | 5 (7.69) | 0.708 (0.238–2.109) | 0.536 | 0.645 (0.203–2.048) | 0.457 |
| 36–45 | 48 (96.00) | 2 (4.00) | 0.354 (0.076–1.645) | 0.185 | 0.367 (0.065–2.087) | 0.259 |
| 46–55 | 45 (95.74) | 2 (4.26) | 0.378 (0.081–1.758) | 0.215 | 0.433 (0.086–2.171) | 0.309 |
| 56–65 | 29 (100.00) | 0 (0.00) | 1 | 1 | ||
| 65+ | 22 (88.00) | 3 (12.00) | 1.159 (0.302–4.455) | 0.83 | 1.755 (0.416–7.406) | 0.444 |
| Location | ||||||
| Rural | 73 (92.41) | 6 (7.59) | Ref | Ref | ||
| Undisclosed | 80 (86.96) | 12 (13.04) | 1.825 (0.652–5.112) | 0.252 | 5.649 (1.524–20.943) | 0.01 |
| Urban | 274 (95.80) | 12 (4.20) | 0.533 (0.193–1.468) | 0.223 | 1.094 (0.337–3.55) | 0.881 |
| Region | ||||||
| Ashanti | 111 (93.28) | 8 (6.72) | Ref | Ref | ||
| Bono East | 19 (95.00) | 1 (5.00) | 0.73 (0.086–6.176) | 0.773 | 0.543 (0.051–5.844) | 0.615 |
| Brong Ahafo | 18 (94.74) | 1 (5.26) | 0.771 (0.091–6.536) | 0.811 | 1.1 (0.105–11.516) | 0.936 |
| Central | 31 (96.88) | 1 (3.13) | 0.448 (0.054–3.716) | 0.457 | 0.341 (0.051–2.304) | 0.27 |
| Eastern | 9 (90.00) | 1 (10.00) | 1.542 (0.173–13.734) | 0.698 | 1.625 (0.123–21.467) | 0.712 |
| Greater Accra | 132 (96.35) | 5 (3.65) | 0.526 (0.167–1.652) | 0.271 | 0.281 (0.088–0.903) | 0.033 |
| Northern | 29 (90.63) | 3 (9.38) | 1.435 (0.358–5.754) | 0.61 | 0.661 (0.132–3.324) | 0.616 |
| Oti | 12 (100.00) | 0 (0.00) | 1 | 1 | ||
| Upper East | 40 (88.89) | 5 (11.11) | 1.734 (0.536–5.613) | 0.358 | 0.527 (0.137–2.026) | 0.351 |
| Upper West | 1 (100.00) | 0 (0.00) | 1 | 1 | ||
| Volta | 9 (100.00) | 0 (0.00) | 1 | 1 | ||
| Western | 13 (72.22) | 5 (27.78) | 5.337 (1.519–18.746) | 0.009 | 6.704 (1.754–25.618) | 0.005 |
| Western North | 3 (100.00) | 0 (0.00) | 1 | 1 | ||
| Vaccination Status | ||||||
| Uncertain | 44 (95.65) | 2 (4.35) | Ref | Ref | ||
| Vaccinated | 96 (95.05) | 5 (4.95) | 1.146 (0.214–6.137) | 0.874 | 1.518 (0.238–9.693) | 0.659 |
| Unvaccinated | 287 (92.58) | 23 (7.42) | 1.763 (0.402–7.74) | 0.452 | 1.788 (0.304–10.52) | 0.521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorcoo, C.; Gyamfi, G.O.; Kaiser, F.; Lomotey, E.S.; Sumboh, J.G.; Fischer, R.J.; Yinda, C.K.; Munster, V.J.; Bonney, J.H.K.; Donkor, I.O. Pre-Clade IIb Mpox Virus Exposure in Ghana: A Retrospective Serological Analysis. Viruses 2025, 17, 1415. https://doi.org/10.3390/v17111415
Dorcoo C, Gyamfi GO, Kaiser F, Lomotey ES, Sumboh JG, Fischer RJ, Yinda CK, Munster VJ, Bonney JHK, Donkor IO. Pre-Clade IIb Mpox Virus Exposure in Ghana: A Retrospective Serological Analysis. Viruses. 2025; 17(11):1415. https://doi.org/10.3390/v17111415
Chicago/Turabian StyleDorcoo, Christopher, Grace Opoku Gyamfi, Franziska Kaiser, Elvis Suatey Lomotey, Jeffrey Gabriel Sumboh, Robert J. Fischer, Claude Kwe Yinda, Vincent J. Munster, Joseph H. K. Bonney, and Irene Owusu Donkor. 2025. "Pre-Clade IIb Mpox Virus Exposure in Ghana: A Retrospective Serological Analysis" Viruses 17, no. 11: 1415. https://doi.org/10.3390/v17111415
APA StyleDorcoo, C., Gyamfi, G. O., Kaiser, F., Lomotey, E. S., Sumboh, J. G., Fischer, R. J., Yinda, C. K., Munster, V. J., Bonney, J. H. K., & Donkor, I. O. (2025). Pre-Clade IIb Mpox Virus Exposure in Ghana: A Retrospective Serological Analysis. Viruses, 17(11), 1415. https://doi.org/10.3390/v17111415








