First Detection of an Alphaherpesvirus Gene in Humpback Whale Blow Samples Collected Noninvasively Using Unmanned Aerial Vehicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Survey
2.2. UAV Operation
2.3. Sample Processing
2.4. RNA Extraction, DNA Library Preparation, and Sequencing
2.5. Virome Analysis
2.6. Phylogenetic Analysis
2.7. Ethics Approval
3. Results
3.1. Sample Collection
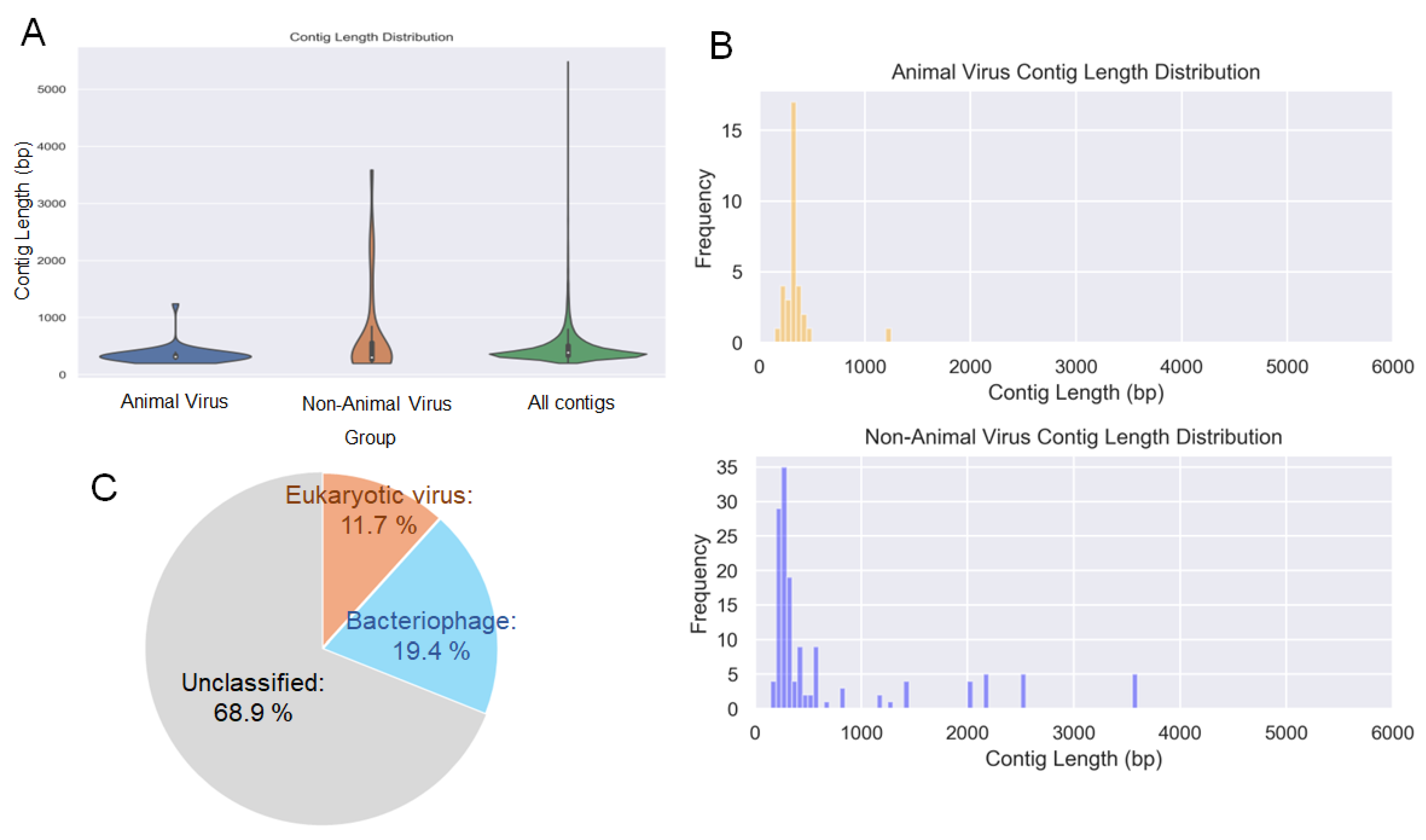
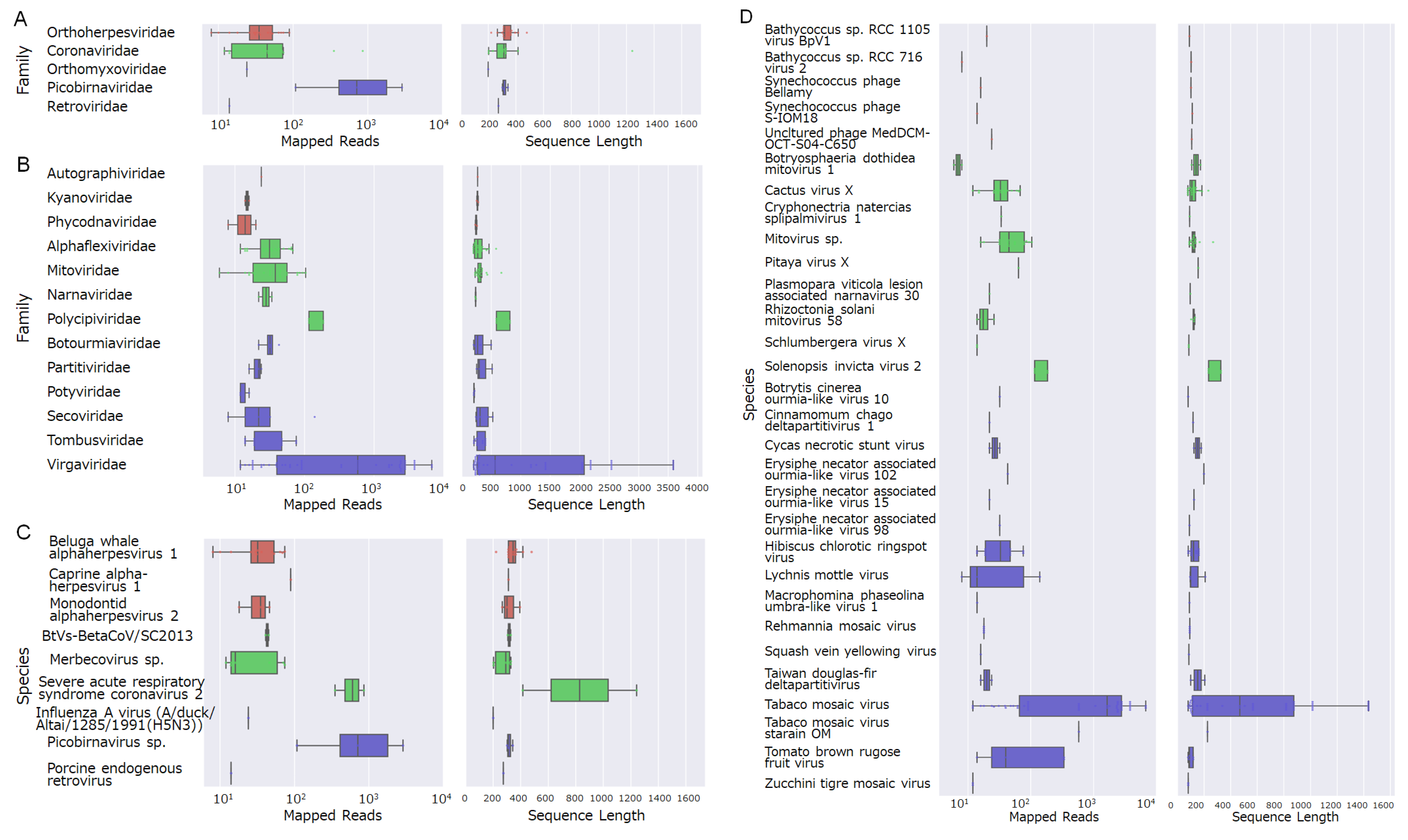
3.2. Virome Analysis
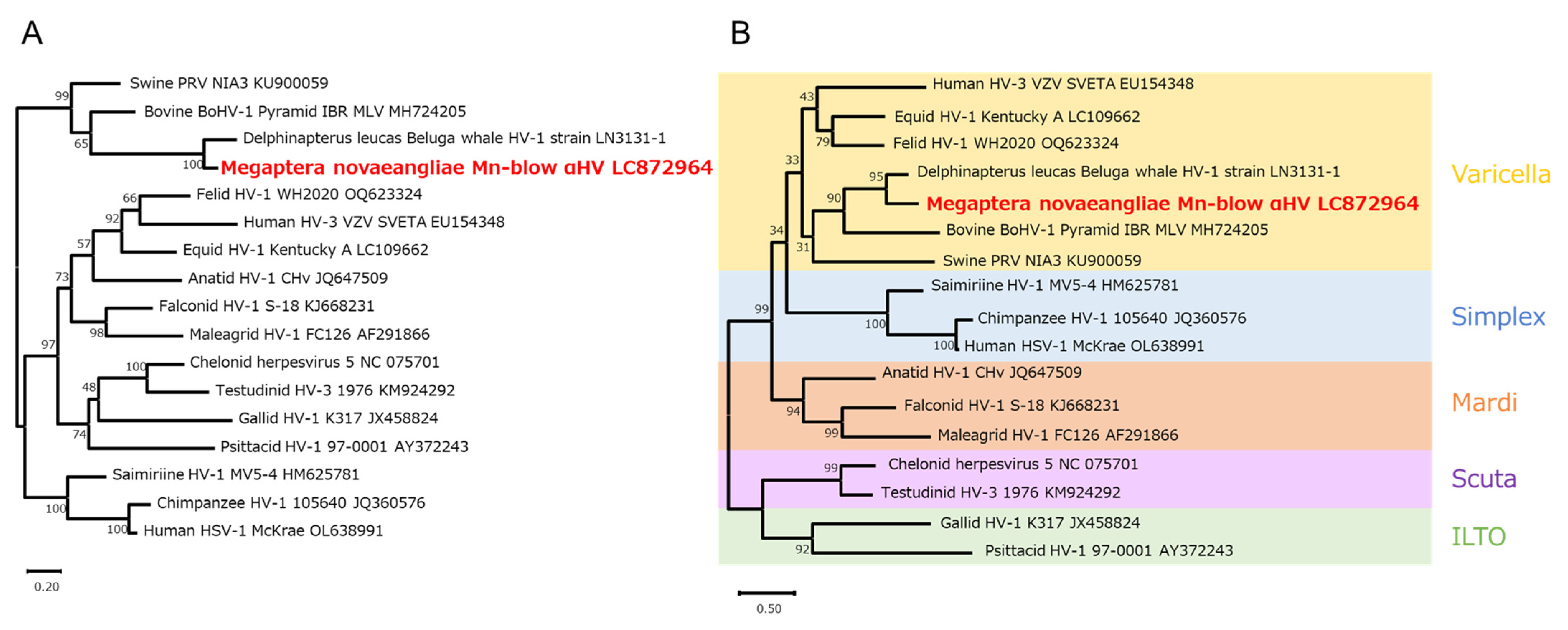
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chami, R.; Cosimano, T.; Fullenkamp, C.; Oztosun, S. Nature’s solution to climate change. Financ. Dev. 2019, 56, 34–38. [Google Scholar]
- West, K.L.; Silva-Krott, I.; Landrau-Giovannetti, N.; Rotstein, D.; Saliki, J.; Raverty, S.; Nielsen, O.; Popov, V.L.; Davis, N.; Walker, W.A.; et al. Novel cetacean morbillivirus in a rare Fraser’s dolphin (Lagenodelphis hosei) stranding from Maui, Hawai’i. Sci. Rep. 2021, 11, 1598. [Google Scholar]
- Domingo, M. Evidence for chronic morbillivirus infection in the mediterranean striped dolphin (Stenella coeruleoalba). Vet. Microbiol. 1995, 44, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.; Fernández, A.; Zucca, D.; Câmara, N.; Felipe-Jiménez, I.; Suárez-Santana, C.; De Quirós, Y.; Díaz-Delgado, J.; Arbelo, M. Morbillivirus infection in Risso’s dolphin grampus griseus: A phylogenetic and pathological study of cases from the Canary Islands. Dis. Aquat. Org. 2018, 129, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, S.; Sacristán, C.; Duarte-Benvenuto, A.; Ewbank, A.C.; Soares, R.M.; Carvalho, V.L.; Castilho, P.V.; Cremer, M.J.; Vieira, J.V.; Lemos, G.G.; et al. Morbillivirus and coronavirus survey in stranded cetaceans, Brazil. PLoS ONE 2025, 20, e0316050. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Peletto, S.; Mattioda, V.; Goria, M.; Serracca, L.; Varello, K.; Sánchez-Vizcaíno, J.M.; Puleio, R.; Nocera, F.D.; Lucifora, G.; et al. Epidemiological and genetic analysis of cetacean morbillivirus circulating on the Italian coast between 2018 and 2021. Front. Vet. Sci. 2023, 10, 1216838. [Google Scholar] [CrossRef]
- Mihindukulasuriya, K.A.; Wu, G.; St. Leger, J.; Nordhausen, R.W.; Wang, D. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef]
- Wang, L.; Maddox, C.; Terio, K.; Lanka, S.; Fredrickson, R.; Novick, B.; Parry, C.; McClain, A.; Ross, K. Detection and charac-terization of new coronavirus in bottlenose dolphin, United States, 2019. Emerg. Infect. Dis. 2020, 26, 1610–1612. [Google Scholar] [CrossRef]
- Mandler, J.; Gorman, O.T.; Ludwig, S.; Schroeder, E.; Fitch, W.M.; Webster, R.G.; Scholtissek, C. Derivation of the nucleoproteins (NP) of influenza A viruses isolated from marine mammals. Virology 1990, 176, 255–261. [Google Scholar] [CrossRef]
- Hinshaw, V.S.; Bean, W.J.; Geraci, J.; Fiorelli, P.; Early, G.; Webster, R.G. Characterization of two influenza A viruses from a pilot whale. J. Virol. 1986, 58, 655–656. [Google Scholar] [CrossRef]
- Lvov, D.K.; Zdanov, V.M.; Sazonov, A.A.; Braude, N.A.; Vladimirtceva, E.A.; Agafonova, L.V.; Oserovic, A.M.; Berzin, A.A.; Mjasnikova, I.A.; Podcernjaeva, R.Y.; et al. Comparison of influenza viruses isolated from man and from whales. Bull. World Health Organ. 1978, 56, 923–930. [Google Scholar] [PubMed]
- St. Leger, J. West Nile virus infection in killer whale, Texas, USA, 2007. Emerg. Infect. Dis. 2011, 17, 1531–1533. [Google Scholar]
- Miyoshi, K.; Nishida, S.; Sone, E.; Tajima, Y.; Makara, M.; Yoshioka, M.; Nakamura, M.; Yamada, T.K.; Koike, H. Molecular identification of novel alpha- and gammaherpesviruses from cetaceans stranded on Japanese coasts. Zool. Sci. 2011, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Shimoda, H.; Terada, Y.; Shimojima, M.; Kohyama, K.; Inoshima, Y.; Maeda, K. Isolation of a novel herpesvirus from a pacific white-sided dolphin. Arch. Virol. 2013, 158, 695–699. [Google Scholar] [CrossRef]
- Davison, A.J.; Nielsen, O.; Subramaniam, K.; Jacob, J.M.; Romero, C.H.; Burek-Huntington, K.A.; Waltzek, T.B. Genome sequence of an alphaherpesvirus from a beluga whale (Delphinapterus leucas). Genome Announc. 2017, 5, e01100-17. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, K.L.; Kim, S.W.; Jung, W.J.; Park, D.S.; Lee, S.; Giri, S.S.; Kim, S.G.; Jo, S.J.; Park, J.H.; et al. Novel gammaherpesvirus infections in narrow-ridged finless porpoise (Neophocaena Asiaeorientalis) and false killer whales (Pseudorca Crassidens) in the Republic of Korea. Viruses 2024, 16, 1234. [Google Scholar] [CrossRef]
- Pietroluongo, G.; Tucciarone, C.M.; Cecchinato, M.; Si, H.; Spadotto, L.; Danyer, I.A.; Isuru, H.; Wijesundera, K.; Ekanayake, L.; Centelleghe, C.; et al. Coinfection with dolphin morbillivirus (DMV) and gammaherpesvirus in a spinner dolphin (Stenella longirostris) stranded in Sri Lanka. Viruses 2024, 16, 1662. [Google Scholar] [CrossRef]
- Saliki, J.T.; Cooper, E.J.; Rotstein, D.S.; Caseltine, S.L.; Pabst, D.A.; McLellan, W.A.; Govett, P.; Harms, C.; Smolarek, K.A.; Romero, C.H. A novel gammaherpesvirus associated with genital lesions in a Blainville’s beaked whale (Mesoplodon densirostris). J. Wildl. Dis. 2006, 42, 142–148. [Google Scholar] [CrossRef]
- Sacristán, C.; Ewbank, A.C.; Duarte-Benvenuto, A.; Sacristán, I.; Zamana-Ramblas, R.; Costa-Silva, S.; Lanes Ribeiro, V.; Bertozzi, C.P.; Del Rio Do Valle, R.; Castilho, P.V.; et al. Survey of selected viral agents (herpesvirus, adenovirus and hepatitis E virus) in liver and lung samples of cetaceans, Brazil. Sci. Rep. 2024, 14, 2689. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Crespo-Picazo, J.L.; Jiménez Martínez, M.Á.; Muñoz-Baquero, M.; Marco-Cabedo, V.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Molecular detection of herpesvirus in a skin lesion of a humpback whale (Megaptera novaeangliae) from the western mediterranean sea. Eur. J. Wildl. Res. 2024, 70, 31. [Google Scholar] [CrossRef]
- Arbelo, M.; Bellière, E.N.; Sierra, E.; Sacchinni, S.; Esperón, F.; Andrada, M.; Rivero, M.; Diaz-Delgado, J.; Fernández, A. Herpes virus infection associated with interstitial nephritis in a beaked whale (Mesoplodon densirostris). BMC Vet. Res. 2012, 8, 243. [Google Scholar] [CrossRef]
- Apprill, A.; Miller, C.A.; Moore, M.J.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G. Extensive core microbiome in drone-captured whale blow supports a framework for health monitoring. mSystems 2017, 2, e00119-17. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, V.; Smith, A.; Ostrowski, M.; Russell, D.; Jonsen, I.D.; Grech, A.; Harcourt, R. An economical custom-built drone for assessing whale health. Front. Mar. Sci. 2017, 4, 42. [Google Scholar] [CrossRef]
- Costa, H.; Rogan, A.; Zadra, C.; Larsen, O.; Rikardsen, A.; Waugh, C. Blowing in the wind: Using a consumer drone for the collection of humpback whale (Megaptera novaeangliae) blow samples during the Arctic Polar nights. Drones 2022, 7, 15. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kondo, S.; Tsujii, K.; Oki, K.; Hida, M.; Okabe, H.; Yoshikawa, T.; Ogawa, R.; Lee, C.; Higashi, N.; et al. Interchanges and movements of humpback whales in Japanese waters: Okinawa, Ogasawara, Amami, and Hokkaido, using an automated matching system. PLoS ONE 2022, 17, e027776. [Google Scholar] [CrossRef]
- Ahrens, A.K.; Selinka, H.-C.; Wylezich, C.; Wonnemann, H.; Sindt, O.; Hellmer, H.H.; Pfaff, F.; Höper, D.; Mettenleiter, T.C.; Beer, M.; et al. Investigating environmental matrices for use in avian influenza virus surveillance—Surface water, sediments, and avian fecal samples. Microbiol. Spectr. 2023, 11, e02664-2. [Google Scholar] [CrossRef]
- Deboosere, N.; Horm, S.V.; Pinon, A.; Gachet, J.; Coldefy, C.; Buchy, P.; Vialette, M. Development and validation of a concentration method for the detection of influenza A viruses from large volumes of surface water. Appl. Environ. Microbiol. 2011, 77, 3802–3808. [Google Scholar] [CrossRef]
- Rönnqvist, M.; Ziegler, T.; Von Bonsdorff, C.-H.; Maunula, L. Detection method for avian influenza viruses in water. Food Environ. Virol. 2012, 4, 26–33. [Google Scholar] [CrossRef]
- Cuevas-Ferrando, E.; Pérez-Cataluña, A.; Allende, A.; Guix, S.; Randazzo, W.; Sánchez, G. Recovering coronavirus from large volumes of water. Sci. Total Environ. 2021, 762, 143101. [Google Scholar] [CrossRef]
- Igarashi, K.; Tanabe, A.; Sahara, H.; Nozaki, R.; Kondo, H.; Katsumata, T.; Tamura, S.; Yamakoshi, T.; Mori, M.; Miyagi, M.; et al. Application of DNA methylation–based age estimation to construct an age structure of humpback whales in a newly emerged wintering ground around Hachijojima Island, Tokyo metropolis, Japan. Ecol. Evol. 2025, 15, e7085. [Google Scholar] [CrossRef]
- Katsumata, T.; Hirose, A.; Nakajo, K.; Shibata, C.; Murata, H.; Yamakoshi, T.; Nakamura, G.; Kato, H. Evidence of winter migration of humpback whales to the Hachijo Island, Izu archipelago off the southern coast of Tokyo, Japan. Cetacean Popul. Stud. 2021, 3, 164–174. [Google Scholar]
- Urayama, S.; Takaki, Y.; Nishi, S.; Yoshida-Takashima, Y.; Deguchi, S.; Takai, K.; Nunoura, T. Unveiling the RNA virosphere associated with marine microorganisms. Mol. Ecol. Resour. 2018, 18, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Hamza, I.A.; Jurzik, L.; Überla, K.; Wilhelm, M. Evaluation of pepper mild mottle virus, human picobirnavirus and torque teno virus as indicators of fecal contamination in river water. Water Res. 2011, 45, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Suntsova, M.; Garazha, A.; Ivanova, A.; Kaminsky, D.; Zhavoronkov, A.; Buzdin, A. Molecular functions of human endogenous retroviruses in health and disease. Cell. Mol. Life Sci. 2015, 72, 3653–3675. [Google Scholar] [CrossRef]
- Ruprecht, K.; Mayer, J.; Sauter, M.; Roemer, K.; Mueller-Lantzsch, N. Endogenous retroviruses: Endogenous retroviruses and cancer. Cell. Mol. Life Sci. 2008, 65, 3366–3382. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Pirotta, V.; Harvey, E.; Smith, A.; Buchmann, J.P.; Ostrowski, M.; Eden, J.-S.; Harcourt, R.; Holmes, E.C. Virological sampling of inaccessible wildlife with drones. Viruses 2018, 10, 300. [Google Scholar] [CrossRef]
- Lodder, W.; De Roda Husman, A.M. SARS-CoV-2 in wastewater: Potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020, 5, 533–534. [Google Scholar] [CrossRef]
- La Rosa, G.; Bonadonna, L.; Lucentini, L.; Kenmoe, S.; Suffredini, E. Coronavirus in water environments: Occurrence, persistence and concentration methods—A scoping review. Water Res. 2020, 179, 115899. [Google Scholar] [CrossRef]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering Earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef]
- Kim, Y.; Van Bonn, W.; Aw, T.G.; Rose, J.B. Aquarium viromes: Viromes of human-managed aquatic systems. Front. Microbiol. 2017, 8, 123. [Google Scholar] [CrossRef]
- Simmonds, P.; Adams, M.J.; Benkő, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Holmes, E.C. Predicting virus emergence amid evolutionary noise. Open Biol. 2017, 7, 170189. [Google Scholar] [CrossRef]
- Obbard, D.J. Expansion of the metazoan virosphere: Progress, pitfalls, and prospects. Curr. Opin. Virol. 2018, 31, 17–23. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Friedlaender, A.S.; Calambokidis, J.; McKenna, M.F.; Simon, M.; Nowacek, D.P. Integrative approaches to the study of baleen whale diving behavior, feeding performance, and foraging ecology. BioScience 2013, 63, 90–100. [Google Scholar] [CrossRef]
- Álvarez-González, M.; Suarez-Bregua, P.; Pierce, G.J.; Saavedra, C. Unmanned aerial vehicles (UAVs) in marine mammal research: A review of current applications and challenges. Drones 2023, 7, 667. [Google Scholar] [CrossRef]
- De Oliveira, L.L.; Andriolo, A.; Cremer, M.J.; Zerbini, A.N. Aerial photogrammetry techniques using drones to estimate morphometric measurements and body condition in South American small cetaceans. Mar. Mammal Sci. 2023, 39, 811–829. [Google Scholar] [CrossRef]
- Irschick, D.J.; Martin, J.; Siebert, U.; Kristensen, J.H.; Madsen, P.T.; Christiansen, F. Creation of accurate 3D models of harbor porpoises (Phocoena phocoena) using 3D photogrammetry. Mar. Mammal Sci. 2021, 37, 482–491. [Google Scholar] [CrossRef]
- Afridi, S.; Laporte-Devylder, L.; Kline, J.M.; Penny, S.G.; Hlebowicz, K.; Cawthorne, D.; Lundquist, U.P.S. Impact of drone disturbances on wildlife: A review. Drones 2025, 9, 311. [Google Scholar] [CrossRef]
- Schilling, A.-K.; Mazzamuto, M.V.; Romeo, C. A review of non-invasive sampling in wildlife disease and health research: What’s new? Animals 2022, 12, 1719. [Google Scholar] [CrossRef]
- Melero, M.; Crespo-Picazo, J.L.; Rubio-Guerri, C.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. First molecular determination of herpesvirus from two mysticete species stranded in the Mediterranean sea. BMC Vet. Res. 2015, 11, 283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, W.; Kawasaki, J.; Ohira, K.; Li, K.; Katayama, M.; Ichikawa, A.; Wakabayashi, Y.; Takenaka-Uema, A.; Murakami, S.; Horimoto, T. First Detection of an Alphaherpesvirus Gene in Humpback Whale Blow Samples Collected Noninvasively Using Unmanned Aerial Vehicles. Viruses 2025, 17, 1411. https://doi.org/10.3390/v17111411
Sekine W, Kawasaki J, Ohira K, Li K, Katayama M, Ichikawa A, Wakabayashi Y, Takenaka-Uema A, Murakami S, Horimoto T. First Detection of an Alphaherpesvirus Gene in Humpback Whale Blow Samples Collected Noninvasively Using Unmanned Aerial Vehicles. Viruses. 2025; 17(11):1411. https://doi.org/10.3390/v17111411
Chicago/Turabian StyleSekine, Wataru, Junna Kawasaki, Kosuke Ohira, Kaixin Li, Misa Katayama, Ayano Ichikawa, Yuta Wakabayashi, Akiko Takenaka-Uema, Shin Murakami, and Taisuke Horimoto. 2025. "First Detection of an Alphaherpesvirus Gene in Humpback Whale Blow Samples Collected Noninvasively Using Unmanned Aerial Vehicles" Viruses 17, no. 11: 1411. https://doi.org/10.3390/v17111411
APA StyleSekine, W., Kawasaki, J., Ohira, K., Li, K., Katayama, M., Ichikawa, A., Wakabayashi, Y., Takenaka-Uema, A., Murakami, S., & Horimoto, T. (2025). First Detection of an Alphaherpesvirus Gene in Humpback Whale Blow Samples Collected Noninvasively Using Unmanned Aerial Vehicles. Viruses, 17(11), 1411. https://doi.org/10.3390/v17111411





