A Novel Municipal-Level Approach to Uncover the Hidden Burden of Hepatitis C: A Replicable Model for National Elimination Strategies
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design, Inclusion Criteria, and Setting
2.2. Data Sources
2.2.1. Clinical Data
2.2.2. Population Data
2.3. Age Stratification
- 0–29 years: younger individuals (recent transmission routes, e.g., injection drug use).
- 30–45 years: younger adults with intermediate risk profiles.
- 46–56 years: middle-aged adults.
- >56 years: older adults, more likely exposed to historical iatrogenic sources (e.g., unscreened blood transfusions and non-sterile procedures).
2.4. Urban/Rural Classification
- Urban areas (≥5000 inhabitants and ≥150 inhabitants/km2): characterized by higher population density, greater availability of healthcare services (e.g., hospitals, diagnostic centers), and more diversified economic activities. These included 38 municipalities, totaling 791,298 inhabitants.
- Rural areas (<5000 inhabitants and/or <150 inhabitants/km2): characterized by lower population density, economies based primarily on agriculture or tourism, and reduced access to specialist healthcare services. These comprised 101 municipalities, totaling 247,430 inhabitants.
2.5. Prevalence of Infection and Hidden Burden Estimation
2.5.1. Observed Prevalence
2.5.2. Expected Prevalence of Active HCV Infection
2.5.3. Hidden Burden of HCV
2.5.4. Bayesian Prevalence
2.6. Municipality Classification Criteria
- Underdiagnosed areas: Observed cases significantly below expected (p < 0.05), suggesting gaps in detection or healthcare access.
- Hotspot areas: Observed cases significantly exceeded expected values (p < 0.05), indicating possible local transmission clusters or enhanced screening programs.
- Other area: No significant difference (p ≥ 0.05) between observed and expected cases.
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Study Population and Demographics
3.2. Municipal-Level Analysis and Geographic Classification
- Underdiagnosed (UD): Municipalities with significantly fewer observed cases than expected, p < 0.05 (observed < expected), indicating high hidden burden (e.g., Acerno, Agropoli, and others).
- Hotspots (HS): Municipalities with significantly more observed cases than expected, p < 0.05 (observed > expected), suggesting effective screening or localized outbreaks (e.g., Salento).
- Other (OTH): Municipalities with no significant difference between observed and expected cases, p ≥ 0.05. (e.g., Albanella, Atrani, and others).
3.3. Age-Specific Prevalence and Diagnostic Gaps
3.4. Multivariate Predictors of Advanced Liver Disease (F3–F4 Fibrosis)
- Patients aged ≥56 years had more than fourfold increased odds compared to those aged 0–29 years, OR = 4.50; 95% CI: 2.14–10.6; p <0.001.
- Those aged 46–56 years had an OR of 2.93 (95% CI: 1.38–6.95; p = 0.008).
- Those aged 30–45 years had an OR of 1.28 (95% CI: 0.590–3.07; p = 0.56).
3.5. Temporal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCV | Hepatitis C Virus |
| WHO | World Health Organization |
| PWID | People who inject drugs |
| MSM | Men who have sex with men |
| GPs | General practitioners |
| DAAs | Direct-acting antivirals |
| ISTAT | Istituto nazionale di statistica |
| SVR | Sustained virologic response |
| VCTE | Vibration-controlled transient elastography |
| OECD | Organisation for Economic Co-operation and Development |
| Eurostat | European Statistical Office |
| SEIEVA | Sistema epidemiologico integrato delle epatiti virali acute |
| SD | Standard deviation |
| MUN | Municipality |
| POP | Population |
| OBS_C | Observed Cases |
| OBS_P | Observed Prevalence |
| BAY_P | Bayesian Prevalence |
| EXP_Min-Max | Expected cases, minimum–maximum |
| HB_Min-Max | Hidden Burden, minimum–maximum |
| CLS | Classification |
| UD | Underdiagnosed |
| HS | Hotspots |
| OTH | Other |
| QGIS | Quantum Geographic Information System |
| ORs | Odds ratios |
| CIs | Confidence intervals |
| IDU | Injecting drug use |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| ECHO | Extension for Community Healthcare Outcomes |
References
- WHO. Hepatitis C-Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 29 August 2024).
- Torre, P.; Festa, M.; Sarcina, T.; Masarone, M.; Persico, M. Elimination of HCV Infection: Recent Epidemiological Findings, Barriers, and Strategies for the Coming Years. Viruses 2024, 16, 1792. [Google Scholar] [CrossRef]
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Heath, K.; Hill, A. WHO hepatitis C elimination targets: The global equity challenge. Lancet Gastroenterol. Hepatol. 2024, 9, 286–288. [Google Scholar] [CrossRef]
- WHO. Global health sector strategy on viral hepatitis 2016–2021. In Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Thomadakis, C.; Gountas, I.; Duffell, E.; Gountas, K.; Bluemel, B.; Seyler, T.; Pericoli, F.M.; Kászoni-Rückerl, I.; El-Khatib, Z.; Busch, M.; et al. Prevalence of chronic HCV infection in EU/EEA countries in 2019 using multiparameter evidence synthesis. Lancet Reg. Health–Eur. 2024, 36, 100792. [Google Scholar] [CrossRef] [PubMed]
- The CDA Foundation. Hepatitis C. Available online: https://cdafound.org/polaris/elimination-maps/ (accessed on 19 September 2025).
- Ridefinizione dei Criteri di Trattamento Per la Terapia Dell’Epatite C Cronica. Available online: https://www.aifa.gov.it/sites/default/files/Determina_n._500-2017_Epatite-C.pdf (accessed on 12 May 2023).
- Razavi, H.; Sanchez Gonzalez, Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Aghemo, A.; Andreoni, M.; Galli, M.; Rossi, A.; Babudieri, S.; Nava, F.; Leonardi, C.; Mennini, F.S.; Gardini, I.; et al. Milestones to reach Hepatitis C Virus (HCV) elimination in Italy: From free-of-charge screening to regional roadmaps for an HCV-free nation. Dig. Liver Dis. 2022, 54, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Torre, P.; Coppola, R.; Masarone, M.; Persico, M. Country-Wide HCV Elimination Strategies Need to Reach Older Patients in the General Population: The Italian Experience. Viruses 2023, 15, 2199. [Google Scholar] [CrossRef]
- Kondili, L.A.; Andreoni, M.; Alberti, A.; Lobello, S.; Babudieri, S.; Roscini, A.S.; Merolla, R.; Marrocco, W.; Craxì, A. Estimated prevalence of undiagnosed HCV infected individuals in Italy: A mathematical model by route of transmission and fibrosis progression. Epidemics 2021, 34, 100442. [Google Scholar] [CrossRef]
- Torre, P.; Aglitti, A.; Masarone, M.; Persico, M. Viral hepatitis: Milestones, unresolved issues, and future goals. World J. Gastroenterol. 2021, 27, 4603–4638. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Safreed-Harmon, K.; Thursz, M.R.; Dillon, J.F.; El-Sayed, M.H.; Elsharkawy, A.M.; Hatzakis, A.; Jadoul, M.; Prestileo, T.; Razavi, H.; et al. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin. Liver Dis. 2018, 38, 181–192. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wiktor, S.; Colombo, M.; Thursz, M. Micro-elimination–A path to global elimination of hepatitis C. J. Hepatol. 2017, 67, 665–666. [Google Scholar] [CrossRef]
- Arora, S.; Thornton, K.; Murata, G.; Deming, P.; Kalishman, S.; Dion, D.; Parish, B.; Burke, T.; Pak, W.; Dunkelberg, J.; et al. Outcomes of Treatment for Hepatitis C Virus Infection by Primary Care Providers. N. Engl. J. Med. 2011, 364, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Astell-Burt, T.; Flowerdew, R.; Boyle, P.; Dillon, J. Is travel-time to a specialist centre a risk factor for non-referral, non-attendance and loss to follow-up among patients with hepatitis C (HCV) infection? Soc. Sci. Med. 2012, 75, 240–247. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.E.; Fried, M.W. Barriers to hepatitis C treatment. Liver Int. 2012, 32 (Suppl. 1), 151–156. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Willing, A.R.; Kairouz, A.; Cunningham, E.B.; Wheeler, A.; O’Brien, N.; Perera, V.; Ward, J.W.; Hiebert, L.; Degenhardt, L.; et al. Direct-acting antiviral therapies for hepatitis C infection: Global registration, reimbursement, and restrictions. Lancet Gastroenterol. Hepatol. 2024, 9, 366–382. [Google Scholar] [CrossRef]
- Morisco, F.; Loperto, I.; Stroffolini, T.; Lombardo, F.L.; Cossiga, V.; Guarino, M.; De Feo, A.; Caporaso, N. Prevalence and risk factors of HCV infection in a metropolitan area in southern Italy: Tail of a cohort infected in past decades. J. Med. Virol. 2017, 89, 291–297. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL recommendations on treatment of hepatitis C: Final update of the series (☆). J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- ISTAT. Popolazione e Famiglie. Available online: https://esploradati.istat.it/databrowser/ (accessed on 1 April 2025).
- ISTAT. Available online: https://www.istat.it/it/files/2023/03/Dinamica-demografica2022.pdf (accessed on 1 April 2025).
- Holtzman, D.; Asher, A.K.; Schillie, S. The Changing Epidemiology of Hepatitis C Virus Infection in the United States During the Years 2010 to 2018. Am. J. Public Health 2021, 111, 949–955. [Google Scholar] [CrossRef]
- Kondili, L.A.; Andreoni, M.; Aghemo, A.; Mastroianni, C.M.; Merolla, R.; Gallinaro, V.; Craxì, A. Prevalence of hepatitis C virus estimates of undiagnosed individuals in different Italian regions: A mathematical modelling approach by route of transmission and fibrosis progression with results up to January 2021. New Microbiol. 2022, 45, 249–259. [Google Scholar]
- EUROSTAT. Territorial Typologies Manual–Degree of Urbanization. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Territorial_typologies_manual_-_degree_of_urbanisation (accessed on 1 April 2025).
- ISTAT. Territorio. Available online: https://www.istat.it/storage/ASI/2024/capitoli/C01.pdf (accessed on 1 April 2025).
- Nevola, R.; Messina, V.; Marrone, A.; Coppola, N.; Rescigno, C.; Esposito, V.; Sangiovanni, V.; Claar, E.; Pisaturo, M.; Fusco, F.M.; et al. Epidemiology of HCV and HBV in a High Endemic Area of Southern Italy: Opportunities from the COVID-19 Pandemic-Standardized National Screening or One Tailored to Local Epidemiology? Biology 2022, 11, 609. [Google Scholar] [CrossRef]
- Torre, P.; Annunziata, M.; Sciorio, R.; Coppola, C.; Masarone, M.; Persico, M. Hepatitis C screening during SARS-CoV-2 testing or vaccination. Experience in an area of southern Italy in the province of Salerno. Liver Int. 2022, 42, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Coppola, C.; Masarone, M.; Bartoli, M.; Staiano, L.; Coppola, R.; Torre, P.; Conforti, M.; Amoruso, D.; Gardini, I.; Persico, M. Associated screening for HCV and SARS-Cov2 infection in an urban area of Southern Italy: A cohort study. J. Viral Hepat. 2022, 29, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Timmerman, K. Awareness and knowledge of hepatitis C among health care providers and the public: A scoping review. Can. Commun. Dis. Rep. 2018, 44, 157–165. [Google Scholar] [CrossRef]
- Raffaele, A.; Valenti, M.; Iovenitti, M.; Matani, A.; Bruno, M.L.; Altobelli, E.; D’Alessandro, A.; Barnabei, R.; Leonardis, B.; Taglieri, G. High prevalence of HCV infection among the general population in a rural area of central Italy. Eur. J. Epidemiol. 2001, 17, 41–46. [Google Scholar] [CrossRef]
- Tillakeratne, S.; Valerio, H.; Alavi, M.; Hajarizadeh, B.; Martinello, M.; Petoumenos, K.; George, J.; Amin, J.; Matthews, G.V.; Grebely, J.; et al. Missed opportunities in HCV care: Trends in late diagnosis and treatment. JHEP Rep. 2025, 7, 101474. [Google Scholar] [CrossRef]
- Andriulli, A.; Stroffolini, T.; Mariano, A.; Valvano, M.R.; Grattagliano, I.; Ippolito, A.M.; Grossi, A.; Brancaccio, G.; Coco, C.; Russello, M.; et al. Declining prevalence and increasing awareness of HCV infection in Italy: A population-based survey in five metropolitan areas. Eur. J. Intern. Med. 2018, 53, 79–84. [Google Scholar] [CrossRef]
- Gardini, I.; Bartoli, M.; Conforti, M.; Mennini, F.S.; Marcellusi, A. Estimation of the number of HCV-positive patients in Italy. PLoS ONE 2019, 14, e0223668. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Capunzo, M. The Power of Environment: A Comprehensive Review of the Exposome’s Role in Healthy Aging, Longevity, and Preventive Medicine-Lessons from Blue Zones and Cilento. Nutrients 2025, 17, 722. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Donato, A.; Funk, R.H.W.; Capunzo, M. A Narrative Review Exploring the Similarities between Cilento and the Already Defined “Blue Zones” in Terms of Environment, Nutrition, and Lifestyle: Can Cilento Be Considered an Undefined “Blue Zone”? Nutrients 2024, 16, 729. [Google Scholar] [CrossRef]
- Aliberti, S.M.; De Caro, F.; Funk, R.H.W.; Schiavo, L.; Gonnella, J.; Boccia, G.; Capunzo, M. Extreme Longevity: Analysis of the Direct or Indirect Influence of Environmental Factors on Old, Nonagenarians, and Centenarians in Cilento, Italy. Int. J. Environ. Res. Public Health 2022, 19, 1589. [Google Scholar] [CrossRef]
- Barouki, R.; Samson, M.; Blanc, E.B.; Colombo, M.; Zucman-Rossi, J.; Lazaridis, K.N.; Miller, G.W.; Coumoul, X. The exposome and liver disease-how environmental factors affect liver health. J. Hepatol. 2023, 79, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Harrison, S.A. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol. Hepatol. 2012, 8, 305–312. [Google Scholar]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Pisanti, S. The mystery of longevity in Cilento: A mix of a good dose of genetic predisposition and a balanced diet based on the Mediterranean model. Eur. J. Clin. Nutr. 2017, 71, 1020–1021. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Piccinelli, S.; Beccalli, B.; Spinetti, A.; Puoti, M.; Fagiuoli, S.; Magni, C.F.; Vavassori, A.; Sacchi, P.; Castaldi, S.; et al. A territory-wide opportunistic, hospital-based HCV screening in the general population from northern Italy: The 1969–1989 birth-cohort. Liver Int. 2023, 43, 2645–2656. [Google Scholar] [CrossRef]
- Torre, P.; Coppola, C.; Masarone, M.; Persico, M. Screening for hepatitis C at the time of the pandemic: Need to adjust the shot. Liver Int. 2022, 42, 1918–1919. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Rizzardini, G.; Puoti, M.; Fagiuoli, S.; Anolli, M.P.; Gabiati, C.; D’Amico, F.; Pasulo, L.; Restelli, U.; Colombo, M.; et al. Implementation of HCV screening in the 1969-1989 birth-cohort undergoing COVID-19 vaccination. Liver Int. 2022, 42, 1012–1016. [Google Scholar] [CrossRef]
- Kondili, L.A.; Craxì, L.; Nava, F.; Babudieri, S.; D’Ambrosio, R.; Marcellusi, A.; Mennini, F.S.; Valle, S.; Russo, P.; Olimpieri, P.P.; et al. From Prioritization to Universal Treatment: Successes and Challenges of Hepatitis C Virus Elimination in Italy. J. Infect. Dis. 2023, 228, S211–S220. [Google Scholar] [CrossRef]
- HCV: Eradicazione del Virus Sul Territorio Nazionale. Available online: https://www.epac.it/cm-files/2019/10/03/position-paper-hcv-def.pdf (accessed on 4 July 2023).
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Megid, S.A.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef]
- Arora, S.; Kalishman, S.; Thornton, K.; Dion, D.; Murata, G.; Deming, P.; Parish, B.; Brown, J.; Komaromy, M.; Colleran, K.; et al. Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology 2010, 52, 1124–1133. [Google Scholar] [CrossRef]
| Characteristic | Value | p-Value |
|---|---|---|
| Total Provincial Population | 1,054,766 | - |
| Population Covered (139 Municipalities) | 1,038,728 (98.5% of the province) | - |
| Population Missing (19 Municipalities) | 16,038 (1.5% of the province) | - |
| Total Confirmed HCV Cases | 3528 | - |
| Urban Municipalities | 38 (791,298 inhabitants, 3067 cases, 86.9%) | |
| Rural Municipalities | 101 (247,430 inhabitants, 461 cases, 13.1%) | <0.001 * |
| Age Distribution | ||
| 0–29 years | 294,967 (38 cases, 1.1%) | |
| 30–45 years | 203,548 (414 cases, 11.7%) | |
| 46–56 years | 171,274 (766 cases, 21.7%) | |
| >56 years | 368,939 (2310 cases, 65.5%) | <0.001 ** |
| Mean Age (years) ± SD | 62.7 (14.1) | - |
| HCV Genotype | 1b (38.6%) | - |
| 2 (31.3%) | ||
| 3 (13.3%) | ||
| 1a (12.0%) | ||
| 4 (4.6%) | ||
| Others (0.3%) | ||
| Sex | - | |
| Female | 1598 (45.3%) | |
| Male | 1930 (54.7%) | |
| Disease Stage | 1757 (49.8%) F0–F2 | - |
| 1771 (50.2%) F3–F4 | ||
| Study Period | 2015–2022 | - |
| Parameter | Urban | Rural |
|---|---|---|
| Population | 791,298 | 247,430 |
| Observed cases | 3067 | 461 |
| Observed prevalence per 1000 | 3.88 | 1.86 |
| Expected prevalence range | 0.6–0.8% | 0.8–1.0% |
| Expected cases | 4748–6330 | 1979–2474 |
| Hidden burden (undiagnosed cases) | 1681–3263 | 1518–2013 |
| Prevalence of hidden burden per 1000 | 3.12 | 7.14 |
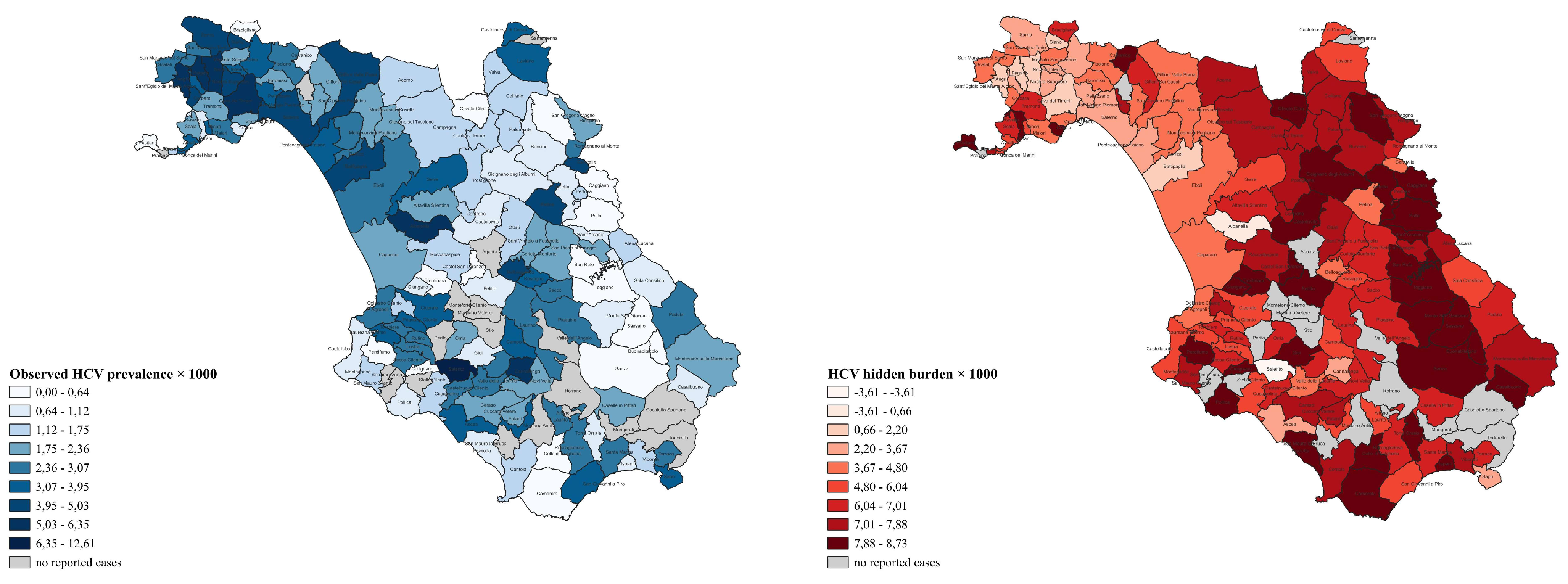
| MUN | Type | POP | OBS_C | OBS_P | BAY_P | EXP_Min–Max | HB_Min–Max | p | CLS |
|---|---|---|---|---|---|---|---|---|---|
| Agropoli | Urban | 21,283 | 62 | 2.91 | 2.79 | 127.7–170.3 | 65.7–108.3 | <0.001 | UD |
| Albanella | Urban | 6311 | 40 | 6.34 | 5.48 | 37.9–50.5 | −2.1–10.5 | 0.051 | OTH |
| Angri | Urban | 34,136 | 205 | 6.01 | 5.84 | 204.8–273.1 | −0.2–68.1 | 0.002 | UD |
| Ascea | Urban | 5846 | 21 | 3.59 | 3.07 | 35.1–46.8 | 14.1–25.8 | <0.001 | UD |
| Baronissi | Urban | 16,859 | 39 | 2.31 | 2.19 | 101.2–134.9 | 62.2–95.9 | <0.001 | UD |
| Battipaglia | Urban | 49,395 | 237 | 4.80 | 4.71 | 296.4–395.2 | 59.4–158.2 | <0.001 | UD |
| Bellizzi | Urban | 13,299 | 36 | 2.71 | 2.52 | 79.8–106.4 | 43.8–70.4 | <0.001 | UD |
| Bracigliano | Urban | 5291 | 2 | 0.38 | 0.32 | 31.7–42.3 | 29.7–40.3 | <0.001 | UD |
| Capaccio Paestum | Urban | 22,412 | 53 | 2.36 | 2.27 | 134.5–179.3 | 81.5–126.3 | <0.001 | UD |
| Casal Velino | Urban | 5360 | 8 | 1.49 | 1.26 | 32.2–42.9 | 24.2–34.9 | <0.001 | UD |
| Castel San Giorgio | Urban | 13,705 | 31 | 2.26 | 2.11 | 82.2–109.6 | 51.2–78.6 | <0.001 | UD |
| Castellabate | Urban | 8679 | 9 | 1.04 | 0.94 | 52.1–69.4 | 43.1–60.4 | <0.001 | UD |
| Cava de’ Tirreni | Urban | 49,754 | 273 | 5.49 | 5.39 | 298.5–398.0 | 25.5–125.0 | <0.001 | UD |
| Eboli | Urban | 37,578 | 91 | 2.42 | 2.37 | 225.5–300.6 | 134.5–209.6 | <0.001 | UD |
| Fisciano | Urban | 14,110 | 36 | 2.55 | 2.39 | 84.7–112.9 | 48.7–76.9 | <0.001 | UD |
| Maiori | Urban | 5217 | 16 | 3.07 | 2.58 | 31.3–41.7 | 15.3–25.7 | <0.001 | UD |
| Mercato San Severino | Urban | 21,451 | 75 | 3.50 | 3.35 | 128.7–171.6 | 53.7–96.6 | <0.001 | UD |
| Montecorvino Pugliano | Urban | 11,119 | 29 | 2.61 | 2.40 | 66.7–89.0 | 37.7–60.0 | <0.001 | UD |
| Montecorvino Rovella | Urban | 12,286 | 27 | 2.20 | 2.04 | 73.7–98.3 | 46.7–71.3 | <0.001 | UD |
| Nocera Inferiore | Urban | 43,424 | 245 | 5.64 | 5.52 | 260.5–347.4 | 15.5–102.4 | <0.001 | UD |
| Nocera Superiore | Urban | 23,495 | 102 | 4.34 | 4.17 | 141.0–188.0 | 39.0–86.0 | <0.001 | UD |
| Olevano sul Tusciano | Urban | 6608 | 15 | 2.27 | 1.98 | 39.6–52.9 | 24.6–37.9 | <0.001 | UD |
| Pagani | Urban | 35,086 | 196 | 5.59 | 5.44 | 210.5–280.7 | 14.5–84.7 | <0.001 | UD |
| Pellezzano | Urban | 10,884 | 39 | 3.58 | 3.29 | 65.3–87.1 | 26.3–48.1 | <0.001 | UD |
| Pontecagnano Faiano | Urban | 26,581 | 105 | 3.95 | 3.81 | 159.5–212.6 | 54.5–107.6 | <0.001 | UD |
| Roccapiemonte | Urban | 8705 | 29 | 3.33 | 2.99 | 52.2–69.6 | 23.2–40.6 | <0.001 | UD |
| Sala Consilina | Urban | 12,142 | 13 | 1.07 | 1.00 | 72.9–97.1 | 59.9–84.1 | <0.001 | UD |
| Salerno | Urban | 125,958 | 559 | 4.44 | 4.41 | 755.7–1007.7 | 196.7–448.7 | <0.001 | UD |
| San Cipriano Picentino | Urban | 6674 | 15 | 2.25 | 1.96 | 40.0–53.4 | 25.0–38.4 | <0.001 | UD |
| San Marzano sul Sarno | Urban | 10,192 | 27 | 2.65 | 2.42 | 61.2–81.5 | 34.2–54.5 | <0.001 | UD |
| San Valentino Torio | Urban | 10,922 | 31 | 2.84 | 2.61 | 65.5–87.4 | 34.5–56.4 | <0.001 | UD |
| Sant’Egidio del Monte Albino | Urban | 7676 | 26 | 3.39 | 3.00 | 46.1–61.4 | 20.1–35.4 | <0.001 | UD |
| Sapri | Urban | 6345 | 23 | 3.62 | 3.14 | 38.1–50.8 | 15.1–27.8 | <0.001 | UD |
| Sarno | Urban | 30,751 | 133 | 4.33 | 4.20 | 184.5–246.0 | 51.5–113.0 | <0.001 | UD |
| Scafati | Urban | 47,706 | 136 | 2.85 | 2.80 | 286.2–381.6 | 150.2–245.6 | <0.001 | UD |
| Siano | Urban | 9278 | 45 | 4.85 | 4.38 | 55.7–74.2 | 10.7–29.2 | 0.002 | UD |
| Vallo della Lucania | Urban | 7835 | 22 | 2.81 | 2.50 | 47.0–62.7 | 25.0–40.7 | <0.001 | UD |
| Vietri sul Mare | Urban | 6945 | 16 | 2.30 | 2.02 | 41.7–55.6 | 25.7–39.6 | <0.001 | UD |
| Acerno | Rural | 2464 | 3 | 1.22 | 0.87 | 19.7–24.6 | 16.7–21.6 | <0.001 | UD |
| Altavilla Silentina | Rural | 7032 | 15 | 2.13 | 1.88 | 56.3–70.3 | 41.3–55.3 | <0.001 | UD |
| Amalfi | Rural | 4611 | 18 | 3.90 | 3.22 | 36.9–46.1 | 18.9–28.1 | <0.001 | UD |
| Atena Lucana | Rural | 2380 | 3 | 1.26 | 0.89 | 19.0–23.8 | 16.0–20.8 | <0.001 | UD |
| Atrani | Rural | 764 | 3 | 3.93 | 1.70 | 6.1–7.6 | 3.1–4.6 | 0.056 | OTH |
| Auletta | Rural | 2143 | 2 | 0.93 | 0.64 | 17.1–21.4 | 15.1–19.4 | <0.001 | UD |
| Bellosguardo | Rural | 679 | 3 | 4.42 | 1.79 | 5.4–6.8 | 2.4–3.8 | 0.084 | OTH |
| Buccino | Rural | 4473 | 5 | 1.12 | 0.92 | 35.8–44.7 | 30.8–39.7 | <0.001 | UD |
| Buonabitacolo | Rural | 2431 | 1 | 0.41 | 0.30 | 19.4–24.3 | 18.4–23.3 | <0.001 | UD |
| Caggiano | Rural | 2479 | 1 | 0.40 | 0.29 | 19.8–24.8 | 18.8–23.8 | <0.001 | UD |
| Calvanico | Rural | 1399 | 1 | 0.71 | 0.42 | 11.2–14.0 | 10.2–13.0 | <0.001 | UD |
| Camerota | Rural | 6774 | 2 | 0.30 | 0.27 | 54.2–67.7 | 52.2–65.7 | <0.001 | UD |
| Campagna | Rural | 17,060 | 25 | 1.47 | 1.39 | 136.5–170.6 | 111.5–145.6 | <0.001 | UD |
| Campora | Rural | 311 | 1 | 3.22 | 0.76 | 2.5–3.1 | 1.5–2.1 | 0.170 | OTH |
| Cannalonga | Rural | 945 | 6 | 6.35 | 3.09 | 7.6–9.5 | 1.6–3.5 | 0.106 | OTH |
| Casalbuono | Rural | 1018 | 1 | 0.98 | 0.50 | 8.1–10.2 | 7.1–9.2 | <0.001 | UD |
| Caselle in Pittari | Rural | 1865 | 4 | 2.14 | 1.40 | 14.9–18.7 | 10.9–14.7 | <0.001 | UD |
| Castelcivita | Rural | 1383 | 1 | 0.72 | 0.42 | 11.1–13.8 | 10.1–12.8 | <0.001 | UD |
| Castelnuovo Cilento | Rural | 2831 | 7 | 2.47 | 1.83 | 22.6–28.3 | 15.6–21.3 | <0.001 | UD |
| Castelnuovo di Conza | Rural | 561 | 2 | 3.57 | 1.28 | 4.5–5.6 | 2.5–3.6 | 0.082 | OTH |
| Castel San Lorenzo | Rural | 2174 | 1 | 0.46 | 0.32 | 17.4–21.7 | 16.4–20.7 | <0.001 | UD |
| Celle di Bulgheria | Rural | 1685 | 1 | 0.59 | 0.38 | 13.5–16.9 | 12.5–15.9 | <0.001 | UD |
| Centola | Rural | 4954 | 7 | 1.41 | 1.18 | 39.6–49.5 | 32.6–42.5 | <0.001 | UD |
| Ceraso | Rural | 2205 | 4 | 1.81 | 1.25 | 17.6–22.1 | 13.6–18.1 | <0.001 | UD |
| Cetara | Rural | 1919 | 2 | 1.04 | 0.69 | 15.4–19.2 | 13.4–17.2 | <0.001 | UD |
| Cicerale | Rural | 1165 | 4 | 3.43 | 1.85 | 9.3–11.7 | 5.3–7.7 | 0.014 | UD |
| Colliano | Rural | 3397 | 5 | 1.47 | 1.14 | 27.2–34.0 | 22.2–29.0 | <0.001 | UD |
| Conca dei Marini | Rural | 636 | 2 | 3.14 | 1.23 | 5.1–6.4 | 3.1–4.4 | 0.054 | OTH |
| Contursi Terme | Rural | 3158 | 4 | 1.27 | 0.97 | 25.3–31.6 | 21.3–27.6 | <0.001 | UD |
| Controne | Rural | 771 | 1 | 1.30 | 0.57 | 6.2–7.7 | 5.2–6.7 | 0.007 | UD |
| Corbara | Rural | 2484 | 11 | 4.43 | 3.16 | 19.9–24.8 | 8.9–13.8 | 0.003 | UD |
| Corleto Monteforte | Rural | 479 | 1 | 2.09 | 0.68 | 3.8–4.8 | 2.8–3.8 | 0.058 | OTH |
| Cuccaro Vetere | Rural | 511 | 1 | 1.96 | 0.66 | 4.1–5.1 | 3.1–4.1 | 0.046 | UD |
| Felitto | Rural | 1175 | 1 | 0.85 | 0.46 | 9.4–11.8 | 8.4–10.8 | <0.001 | UD |
| Furore | Rural | 680 | 1 | 1.47 | 0.60 | 5.4–6.8 | 4.4–5.8 | 0.013 | UD |
| Futani | Rural | 1052 | 4 | 3.80 | 1.95 | 8.4–10.5 | 4.4–6.5 | 0.026 | UD |
| Giffoni Sei Casali | Rural | 4950 | 10 | 2.02 | 1.69 | 39.6–49.5 | 29.6–39.5 | <0.001 | UD |
| Giffoni Valle Piana | Rural | 11,456 | 53 | 4.63 | 4.26 | 91.6–114.6 | 38.6–61.6 | <0.001 | UD |
| Gioi | Rural | 1072 | 1 | 0.93 | 0.49 | 8.6–10.7 | 7.6–9.7 | <0.001 | UD |
| Giungano | Rural | 1284 | 1 | 0.78 | 0.44 | 10.3–12.8 | 9.3–11.8 | <0.001 | UD |
| Ispani | Rural | 959 | 1 | 1.04 | 0.51 | 7.7–9.6 | 6.7–8.6 | 0.002 | UD |
| Laureana Cilento | Rural | 1270 | 4 | 3.15 | 1.77 | 10.2–12.7 | 6.2–8.7 | 0.008 | UD |
| Laurino | Rural | 1214 | 3 | 2.47 | 1.36 | 9.7–12.1 | 6.7–9.1 | 0.004 | UD |
| Laurito | Rural | 663 | 2 | 3.02 | 1.21 | 5.3–6.6 | 3.3–4.6 | 0.046 | UD |
| Laviano | Rural | 1278 | 4 | 3.13 | 1.76 | 10.2–12.8 | 6.2–8.8 | 0.007 | UD |
| Lustra | Rural | 1014 | 3 | 2.96 | 1.49 | 8.1–10.1 | 5.1–7.1 | 0.014 | UD |
| Minori | Rural | 2559 | 9 | 3.52 | 2.54 | 20.5–25.6 | 11.5–16.6 | <0.001 | UD |
| Moio della Civitella | Rural | 1812 | 5 | 2.76 | 1.78 | 14.5–18.1 | 9.5–13.1 | <0.001 | UD |
| Montecorice | Rural | 2579 | 4 | 1.55 | 1.12 | 20.6–25.8 | 16.6–21.8 | <0.001 | UD |
| Monte San Giacomo | Rural | 1372 | 1 | 0.73 | 0.43 | 11.0–13.7 | 10.0–12.7 | <0.001 | UD |
| Montesano sulla Marcellana | Rural | 6231 | 12 | 1.93 | 1.67 | 49.8–62.3 | 37.8–50.3 | <0.001 | UD |
| Novi Velia | Rural | 2349 | 5 | 2.13 | 1.50 | 18.8–23.5 | 13.8–18.5 | <0.001 | UD |
| Ogliastro Cilento | Rural | 2291 | 4 | 1.75 | 1.22 | 18.3–22.9 | 14.3–18.9 | <0.001 | UD |
| Oliveto Citra | Rural | 3602 | 2 | 0.56 | 0.44 | 28.8–36.0 | 26.8–34.0 | <0.001 | UD |
| Omignano | Rural | 1641 | 1 | 0.61 | 0.38 | 13.1–16.4 | 12.1–15.4 | <0.001 | UD |
| Orria | Rural | 912 | 2 | 2.19 | 1.05 | 7.3–9.1 | 5.3–7.1 | 0.009 | UD |
| Ottati | Rural | 579 | 1 | 1.73 | 0.64 | 4.6–5.8 | 3.6–4.8 | 0.028 | UD |
| Padula | Rural | 4794 | 12 | 2.50 | 2.08 | 38.4–47.9 | 26.4–35.9 | <0.001 | UD |
| Palomonte | Rural | 3768 | 5 | 1.33 | 1.06 | 30.1–37.7 | 25.1–32.7 | <0.001 | UD |
| Perdifumo | Rural | 1803 | 1 | 0.55 | 0.36 | 14.4–18.0 | 13.4–17.0 | <0.001 | UD |
| Pertosa | Rural | 649 | 1 | 1.54 | 0.61 | 5.2–6.5 | 4.2–5.5 | 0.017 | UD |
| Petina | Rural | 994 | 5 | 5.03 | 2.51 | 8.0–9.9 | 3.0–4.9 | 0.062 | OTH |
| Piaggine | Rural | 1077 | 3 | 2.79 | 1.45 | 8.6–10.8 | 5.6–7.8 | 0.009 | UD |
| Pisciotta | Rural | 2404 | 2 | 0.83 | 0.59 | 19.2–24.0 | 17.2–22.0 | <0.001 | UD |
| Polla | Rural | 5078 | 2 | 0.39 | 0.34 | 40.6–50.8 | 38.6–48.8 | <0.001 | UD |
| Pollica | Rural | 2102 | 2 | 0.95 | 0.65 | 16.8–21.0 | 14.8–19.0 | <0.001 | UD |
| Positano | Rural | 3678 | 1 | 0.27 | 0.22 | 29.4–36.8 | 28.4–35.8 | <0.001 | UD |
| Postiglione | Rural | 1998 | 3 | 1.50 | 1.01 | 16.0–20.0 | 13.0–17.0 | <0.001 | UD |
| Prignano Cilento | Rural | 1081 | 4 | 3.70 | 1.93 | 8.6–10.8 | 4.6–6.8 | 0.022 | UD |
| Ravello | Rural | 2332 | 2 | 0.86 | 0.61 | 18.7–23.3 | 16.7–21.3 | <0.001 | UD |
| Ricigliano | Rural | 1064 | 2 | 1.88 | 0.97 | 8.5–10.6 | 6.5–8.6 | 0.003 | UD |
| Roccadaspide | Rural | 6880 | 11 | 1.60 | 1.40 | 55.0–68.8 | 44.0–57.8 | <0.001 | UD |
| Roccagloriosa | Rural | 1551 | 4 | 2.58 | 1.57 | 12.4–15.5 | 8.4–11.5 | 0.001 | UD |
| Romagnano al Monte | Rural | 378 | 1 | 2.65 | 0.73 | 3.0–3.8 | 2.0–2.8 | 0.113 | OTH |
| Roscigno | Rural | 579 | 2 | 3.45 | 1.27 | 4.6–5.8 | 2.6–3.8 | 0.074 | OTH |
| Rutino | Rural | 750 | 2 | 2.67 | 1.15 | 6.0–7.5 | 4.0–5.5 | 0.027 | UD |
| Sacco | Rural | 409 | 1 | 2.44 | 0.71 | 3.3–4.1 | 2.3–3.1 | 0.093 | OTH |
| Salento | Rural | 1744 | 22 | 12.61 | 8.02 | 14.0–17.4 | −8.0–4.6 | 0.028 | HS |
| Salvitelle | Rural | 467 | 2 | 4.28 | 1.37 | 3.7–4.7 | 1.7–2.7 | 0.132 | OTH |
| San Giovanni a Piro | Rural | 3571 | 12 | 3.36 | 2.63 | 28.6–35.7 | 16.6–23.7 | <0.001 | UD |
| San Gregorio Magno | Rural | 3866 | 2 | 0.52 | 0.42 | 30.9–38.7 | 28.9–36.7 | <0.001 | UD |
| San Mango Piemonte | Rural | 2660 | 5 | 1.88 | 1.37 | 21.3–26.6 | 16.3–21.6 | <0.001 | UD |
| San Pietro al Tanagro | Rural | 1669 | 3 | 1.80 | 1.13 | 13.4–16.7 | 10.4–13.7 | <0.001 | UD |
| San Rufo | Rural | 1591 | 1 | 0.63 | 0.39 | 12.7–15.9 | 11.7–14.9 | <0.001 | UD |
| Sant’Angelo a Fasanella | Rural | 500 | 1 | 2.00 | 0.67 | 4.0–5.0 | 3.0–4.0 | 0.050 | OTH |
| Sant’Arsenio | Rural | 2645 | 2 | 0.76 | 0.56 | 21.2–26.5 | 19.2–24.5 | <0.001 | UD |
| Santa Marina | Rural | 3219 | 8 | 2.49 | 1.90 | 25.8–32.2 | 17.8–24.2 | <0.001 | UD |
| Sanza | Rural | 2330 | 1 | 0.43 | 0.31 | 18.6–23.3 | 17.6–22.3 | <0.001 | UD |
| Sassano | Rural | 4666 | 2 | 0.43 | 0.36 | 37.3–46.7 | 35.3–44.7 | <0.001 | UD |
| Scala | Rural | 1504 | 3 | 1.99 | 1.20 | 12.0–15.0 | 9.0–12.0 | <0.001 | UD |
| Serre | Rural | 3646 | 12 | 3.29 | 2.59 | 29.2–36.5 | 17.2–24.5 | <0.001 | UD |
| Sessa Cilento | Rural | 1129 | 3 | 2.66 | 1.41 | 9.0–11.3 | 6.0–8.3 | 0.007 | UD |
| Sicignano degli Alburni | Rural | 3172 | 3 | 0.95 | 0.73 | 25.4–31.7 | 22.4–28.7 | <0.001 | UD |
| Teggiano | Rural | 6993 | 4 | 0.57 | 0.51 | 55.9–69.9 | 51.9–65.9 | <0.001 | UD |
| Torchiara | Rural | 1903 | 5 | 2.63 | 1.73 | 15.2–19.0 | 10.2–14.0 | <0.001 | UD |
| Torraca | Rural | 1229 | 3 | 2.44 | 1.35 | 9.8–12.3 | 6.8–9.3 | 0.004 | UD |
| Torre Orsaia | Rural | 1938 | 2 | 1.03 | 0.69 | 15.5–19.4 | 13.5–17.4 | <0.001 | UD |
| Tramonti | Rural | 4184 | 9 | 2.15 | 1.74 | 33.5–41.8 | 24.5–32.8 | <0.001 | UD |
| Trentinara | Rural | 1561 | 1 | 0.64 | 0.40 | 12.5–15.6 | 11.5–14.6 | <0.001 | UD |
| Valva | Rural | 1534 | 2 | 1.30 | 0.79 | 12.3–15.3 | 10.3–13.3 | <0.001 | UD |
| Vibonati | Rural | 3205 | 5 | 1.56 | 1.20 | 25.6–32.1 | 20.6–27.1 | <0.001 | UD |
| Age Group | Population | Observed Cases | Observed Prevalence Per 1000 | Expected Prevalence % | Expected Cases | Hidden Burden | p-Value |
|---|---|---|---|---|---|---|---|
| 0–29 years | 294,967 | 38 | 0.13 | 0.12 | 354 | 316 | <0.001 |
| 30–45 years | 203,548 | 414 | 2.03 | 0.6 | 1221 | 807 | <0.001 |
| 46–56 years | 171,274 | 766 | 4.47 | 1.2 | 2055 | 1289 | <0.001 |
| >56 years | 368,939 | 2310 | 6.26 | 2.5 | 9223 | 6913 | <0.001 |
| Total | 1,038,728 | 3528 | 3.40 | 0.72 | 7479 | 3951 | <0.001 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Age group | |||
| 0–29 years | 1 a | ||
| 30–45 years | 1.28 | 0.590–3.07 | 0.56 |
| 46–56 years | 2.93 | 1.38–6.95 | 0.008 |
| >56 years | 4.50 | 2.14–10.6 | <0.001 |
| Sex | |||
| Female | 1 a | ||
| Male | 1.63 | 1.42–1.89 | <0.001 |
| Urban/rural | |||
| Rural | 1 a | ||
| Urban | 1.29 | 1.05–1.58 | 0.01 |
| Genotype | |||
| Others | 1 a | ||
| 1b | 1.56 | 1.35–1.80 | <0.001 |
| Parameter | 2015–2018 | 2019–2022 | p-Value |
|---|---|---|---|
| Expected HCV cases (period start) | 7479 | 5237 | <0.001 * |
| Observed/linked-to-care cases | 2242 | 1286 | |
| Hidden burden (period end) | 5237 | 3951 | |
| Mean Age (years) ± SD | 63.2 (13.4) | 61.9 (15.0) | 0.02 |
| F3–F4 fibrosis (%) | 1363 (60.8%) | 408 (31.7%) | <0.001 |
| Urban residence (%) | 1955 (87.2%) | 1112 (86.5%) | 0.5362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, P.; Aliberti, S.M.; Sarcina, T.; Festa, M.; D’Amore, C.; D’Adamo, G.; Gambardella, M.; Santonicola, A.; Manzi, G.; Masarone, M.; et al. A Novel Municipal-Level Approach to Uncover the Hidden Burden of Hepatitis C: A Replicable Model for National Elimination Strategies. Viruses 2025, 17, 1392. https://doi.org/10.3390/v17101392
Torre P, Aliberti SM, Sarcina T, Festa M, D’Amore C, D’Adamo G, Gambardella M, Santonicola A, Manzi G, Masarone M, et al. A Novel Municipal-Level Approach to Uncover the Hidden Burden of Hepatitis C: A Replicable Model for National Elimination Strategies. Viruses. 2025; 17(10):1392. https://doi.org/10.3390/v17101392
Chicago/Turabian StyleTorre, Pietro, Silvana Mirella Aliberti, Tommaso Sarcina, Mariano Festa, Chiara D’Amore, Giuseppe D’Adamo, Michele Gambardella, Antonella Santonicola, Gaetano Manzi, Mario Masarone, and et al. 2025. "A Novel Municipal-Level Approach to Uncover the Hidden Burden of Hepatitis C: A Replicable Model for National Elimination Strategies" Viruses 17, no. 10: 1392. https://doi.org/10.3390/v17101392
APA StyleTorre, P., Aliberti, S. M., Sarcina, T., Festa, M., D’Amore, C., D’Adamo, G., Gambardella, M., Santonicola, A., Manzi, G., Masarone, M., Capunzo, M., & Persico, M. (2025). A Novel Municipal-Level Approach to Uncover the Hidden Burden of Hepatitis C: A Replicable Model for National Elimination Strategies. Viruses, 17(10), 1392. https://doi.org/10.3390/v17101392












