Metabolic Hostile Takeover: How Influenza Virus Reprograms Cellular Metabolism for Replication
Abstract
1. Introduction
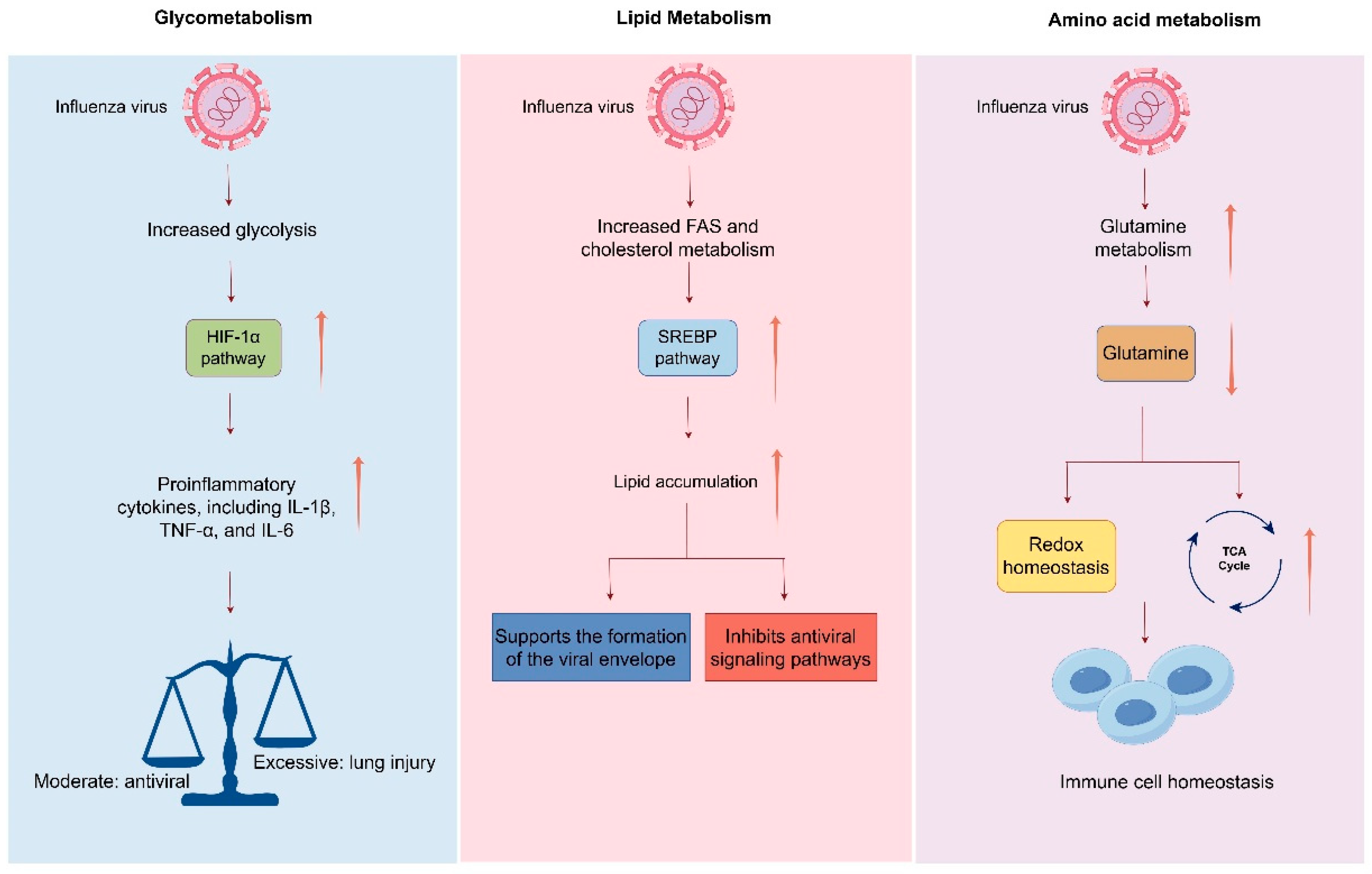
2. Glycolysis and Glucose Metabolic Reprogramming Following Influenza Virus Infection
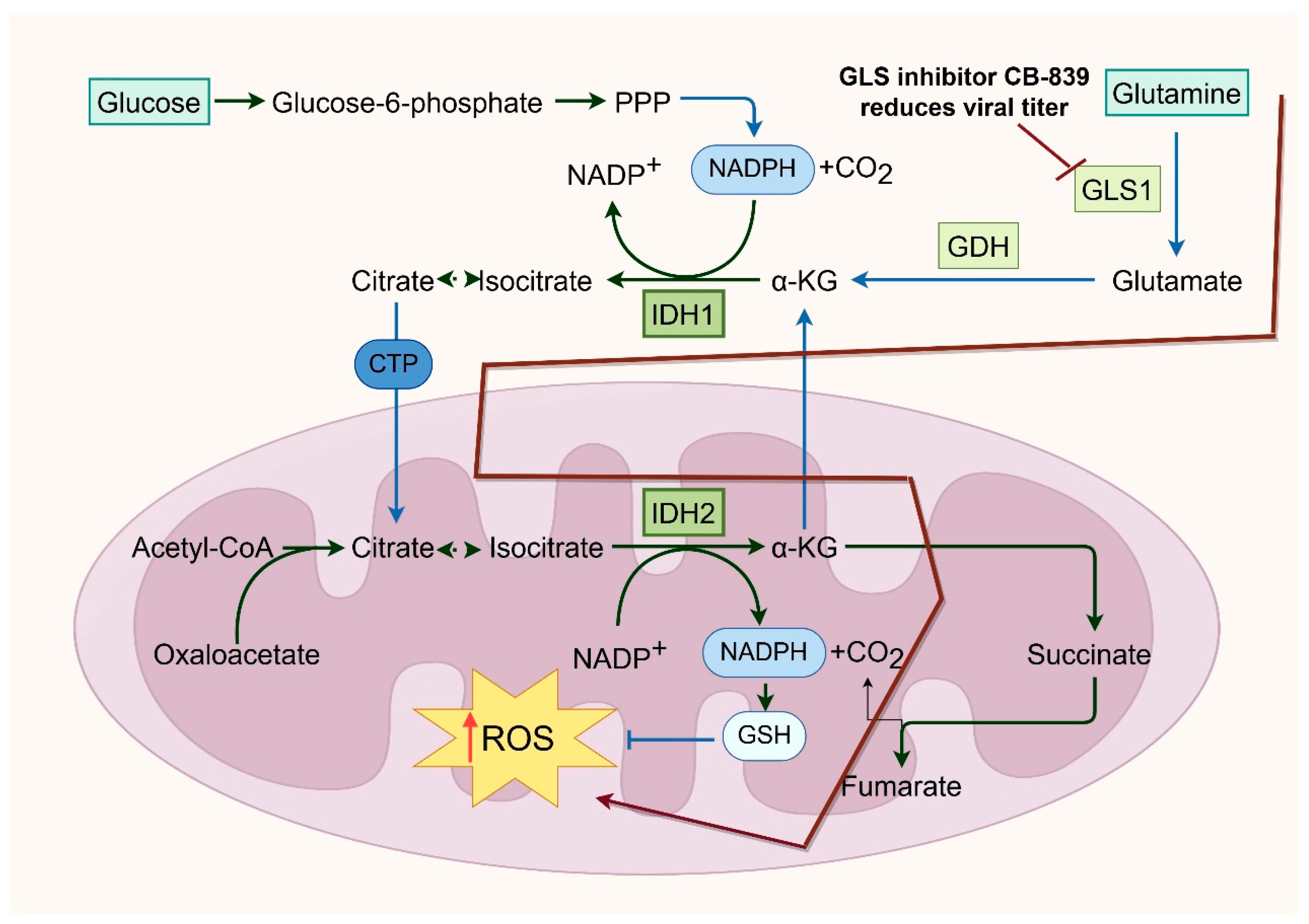
3. Influenza Virus Infection and Its Crosstalk with the TCA Cycle and Mitochondrial Function
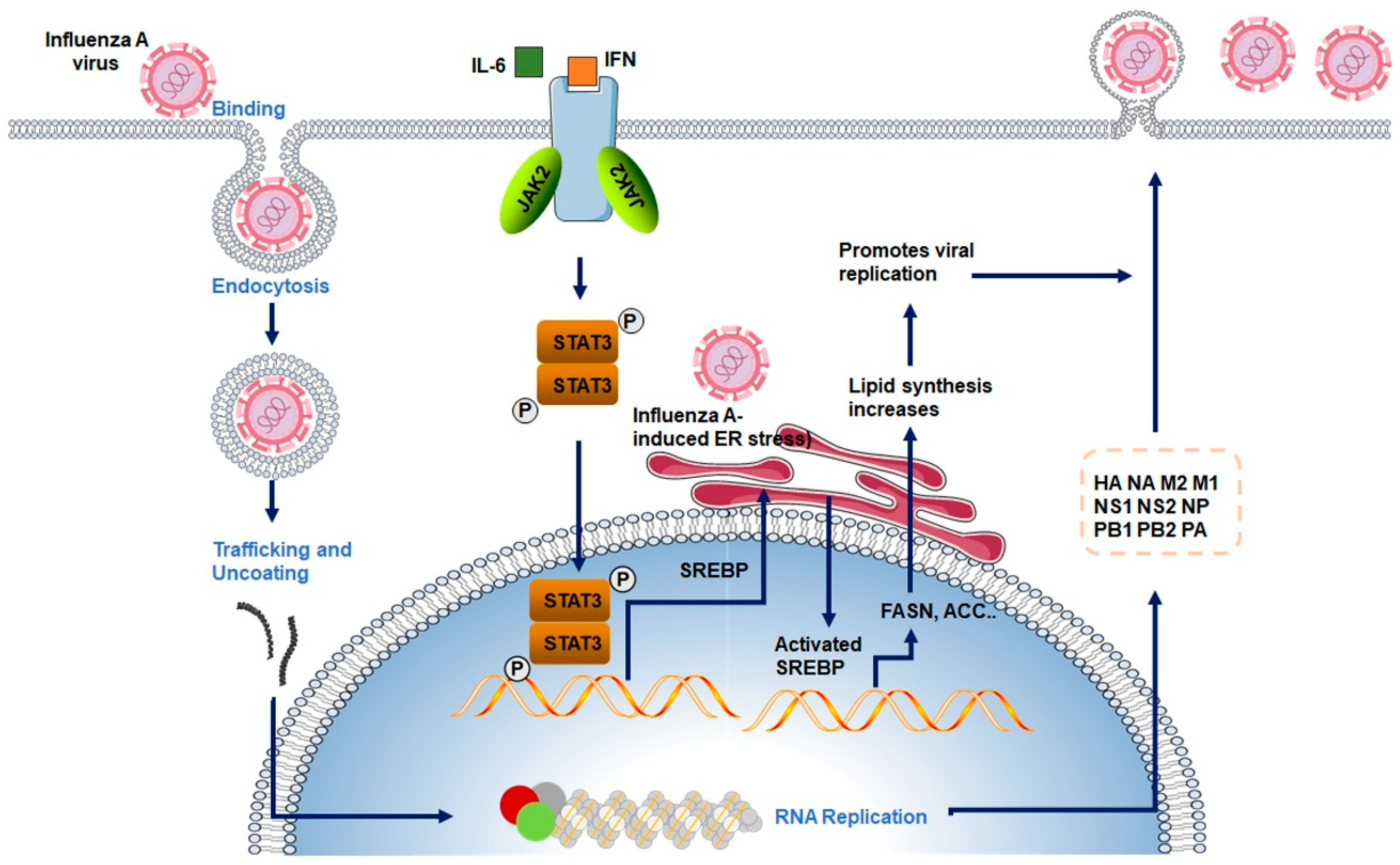
4. The Interplay Between Influenza Virus Infection and Lipid Metabolism
5. Interplay Between Influenza Virus Infection and Amino Acid Metabolism and One-Carbon Metabolism
6. Interplay Between Metabolic Reprogramming and Host Immune Responses During Influenza Virus Infection
7. Therapeutic Implications: Targeting Host Metabolism as an Antiviral Strategy Against Influenza
8. Conclusions and Future Perspectives
- (1)
- Systematic Mapping of Virus–Host Metabolic Interactions
- (2)
- Functional Validation and Mechanistic Elucidation
- (3)
- Development of Novel Metabolism-Targeting Antivirals
- (4)
- Integration of Metabolic Interventions with Existing Therapies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, T.H.; Zhu, X.; Wang, S.; Zhang, D.; McBride, R.; Yu, W.; Babarinde, S.; Paulson, J.C.; Wilson, I.A. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science 2024, 386, 1128–1134. [Google Scholar] [CrossRef]
- Hui, X.; Cao, L.; Xu, T.; Zhao, L.; Huang, K.; Zou, Z.; Ren, P.; Mao, H.; Yang, Y.; Gao, S.; et al. PSMD12-Mediated M1 Ubiquitination of Influenza A Virus at K102 Regulates Viral Replication. J. Virol. 2022, 96, e0078622. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kam, Y.W. Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness. Viruses 2024, 16, 458. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Xu, J.; Luo, Q.; Huang, Y.; Li, J.; Ye, W.; Yan, R.; Zhou, X.; He, Z.; Liu, G.; Zhu, Q. Influenza neuraminidase mutations and resistance to neuraminidase inhibitors. Emerg. Microbes Infect. 2024, 13, 2429627. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Jiang, F.; Zhang, Z.; Yang, J.; Li, L.; Zhang, Q. Metabolism-associated protein network constructing and host-directed anti-influenza drug repurposing. Brief. Bioinform. 2025, 26, bbaf163. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, W.; Zhang, J.; Zhang, J.; Zhang, H.; Zhu, Y.; Meng, X.; Yi, Z.; Wang, R. Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication. Virol. Sin. 2021, 36, 1532–1542. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, Y.; Yang, W.; Zhang, J.; Jin, W.; Tian, R.; Yang, Z.; Wang, R. HIF-1alpha promotes virus replication and cytokine storm in H1N1 virus-induced severe pneumonia through cellular metabolic reprogramming. Virol. Sin. 2024, 39, 81–96. [Google Scholar] [CrossRef]
- Mayer, K.A.; Stockl, J.; Zlabinger, G.J.; Gualdoni, G.A. Hijacking the Supplies: Metabolism as a Novel Facet of Virus-Host Interaction. Front. Immunol. 2019, 10, 1533. [Google Scholar] [CrossRef]
- Al-Shalan, H.A.M.; Zhou, L.; Dong, Z.; Wang, P.; Nicholls, P.K.; Boughton, B.; Stumbles, P.A.; Greene, W.K.; Ma, B. Systemic perturbations in amino acids/amino acid derivatives and tryptophan pathway metabolites associated with murine influenza A virus infection. Virol. J. 2023, 20, 270. [Google Scholar] [CrossRef]
- Ohno, M.; Sekiya, T.; Nomura, N.; Daito, T.J.; Shingai, M.; Kida, H. Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci. Rep. 2020, 10, 10879. [Google Scholar] [CrossRef]
- Pila-Castellanos, I.; Molino, D.; McKellar, J.; Lines, L.; Da Graca, J.; Tauziet, M.; Chanteloup, L.; Mikaelian, I.; Meyniel-Schicklin, L.; Codogno, P.; et al. Mitochondrial morphodynamics alteration induced by influenza virus infection as a new antiviral strategy. PLoS Pathog. 2021, 17, e1009340. [Google Scholar] [CrossRef]
- Awad, K.; Abdelhadi, M.; Awad, A.M. High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells. Int. J. Mol. Sci. 2025, 26, 2975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Wang, Y.; Liu, P.; Liu, K.; Sun, J.; Zhang, P.; Wang, X.; Liu, X.; Xu, X. Influenza A virus infection activates STAT3 to enhance SREBP2 expression, cholesterol biosynthesis, and virus replication. iScience 2024, 27, 110424. [Google Scholar] [CrossRef]
- Bezgovsek, J.; Gulbins, E.; Friedrich, S.K.; Lang, K.S.; Duhan, V. Sphingolipids in early viral replication and innate immune activation. Biol. Chem. 2018, 399, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Rezinciuc, S.; Bezavada, L.; Bahadoran, A.; Duan, S.; Wang, R.; Lopez-Ferrer, D.; Finkelstein, D.; McGargill, M.A.; Green, D.R.; Pasa-Tolic, L.; et al. Dynamic metabolic reprogramming in dendritic cells: An early response to influenza infection that is essential for effector function. PLoS Pathog. 2020, 16, e1008957. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Gowda, S.G.B.; Sekiya, T.; Nomura, N.; Shingai, M.; Hui, S.P.; Kida, H. The elucidation of plasma lipidome profiles during severe influenza in a mouse model. Sci. Rep. 2023, 13, 14210. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, A.; Wang, H.; Xu, X.; Chen, L.; Luo, L. Emerging role of the TCA cycle and its metabolites in lung disease. Front. Physiol. 2025, 16, 1621013. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Nguyen, N.T.; Bhutia, Y.D.; Sivaprakasam, S.; Ganapathy, V. Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth. Cancers 2024, 16, 504. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Arcos, S.; Rothamel, K.; Jian, J.; Rose, K.L.; McDonald, W.H.; Bian, Y.; Reasoner, S.; Barrows, N.J.; Bradrick, S.; et al. Discovery of Widespread Host Protein Interactions with the Pre-replicated Genome of CHIKV Using VIR-CLASP. Mol. Cell 2020, 78, 624–640.E7. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, L.; Xin, X.; Qiao, Y.; Qiao, W.; Ping, J.; Xia, J.; Su, J. Influenza A virus-induced glycolysis facilitates virus replication by activating ROS/HIF-1alpha pathway. Free Radic. Biol. Med. 2024, 225, 910–924. [Google Scholar] [CrossRef]
- Darweesh, M.; Mohammadi, S.; Rahmati, M.; Al-Hamadani, M.; Al-Harrasi, A. Metabolic reprogramming in viral infections: The interplay of glucose metabolism and immune responses. Front. Immunol. 2025, 16, 1578202. [Google Scholar] [CrossRef] [PubMed]
- Thyrsted, J.; Storgaard, J.; Blay-Cadanet, J.; Heinz, A.; Thielke, A.L.; Crotta, S.; de Paoli, F.; Olagnier, D.; Wack, A.; Hiller, K.; et al. Influenza A induces lactate formation to inhibit type I IFN in primary human airway epithelium. iScience 2021, 24, 103300. [Google Scholar] [CrossRef]
- O’Carroll, S.M.; Henkel, F.D.R.; O’Neill, L.A.J. Metabolic regulation of type I interferon production. Immunol. Rev. 2024, 323, 276–287. [Google Scholar] [CrossRef]
- Agani, F.; Jiang, B.H. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr. Cancer Drug Targets 2013, 13, 245–251. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Csizmar, S.H.; Samec, M.; Brockmueller, A.; Sudomova, M.; Biringer, K.; Kudela, E.; Pec, M.; Samuel, S.M.; et al. Significance of flavonoids targeting PI3K/Akt/HIF-1alpha signaling pathway in therapy-resistant cancer cells-A potential contribution to the predictive, preventive, and personalized medicine. J. Adv. Res. 2024, 55, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Corrales, N.; Galvez, N.M.S.; Bueno, S.M.; Kalergis, A.M.; Gonzalez, P.A. Contribution of hypoxia inducible factor-1 during viral infections. Virulence 2020, 11, 1482–1500. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.X.; Wang, X.Q.; Liu, X.F. NS1: A Key Protein in the “Game” Between Influenza A Virus and Host in Innate Immunity. Front. Cell Infect. Microbiol. 2021, 11, 670177. [Google Scholar] [CrossRef]
- Dubois, J.; Traversier, A.; Julien, T.; Padey, B.; Lina, B.; Bourdon, J.C.; Marcel, V.; Boivin, G.; Rosa-Calatrava, M.; Terrier, O. The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4. J. Virol. 2019, 93, 1–19. [Google Scholar] [CrossRef]
- Aslam, S.; Sanchez-Aparicio, M.T.; Siempelkamp, B.D.; Dornan, G.L.; Tsolakos, N.; Burke, J.E.; Hale, B.G.; Garcia-Sastre, A.; Ayllon, J. Influenza A virus NS1 protein mimics oncogenic PI3K resulting in isoform specific cellular redistribution and activation. Proc. Natl. Acad. Sci. USA 2025, 122, e2423066122. [Google Scholar] [CrossRef]
- Larcombe, D.E.; Bohovych, I.M.; Pradhan, A.; Ma, Q.; Hickey, E.; Leaves, I.; Cameron, G.; Avelar, G.M.; de Assis, L.J.; Childers, D.S.; et al. Glucose-enhanced oxidative stress resistance-A protective anticipatory response that enhances the fitness of Candida albicans during systemic infection. PLoS Pathog. 2023, 19, e1011505. [Google Scholar] [CrossRef]
- Zevini, A.; Palermo, E.; Di Carlo, D.; Alexandridi, M.; Rinaldo, S.; Paone, A.; Cutruzzola, F.; Etna, M.P.; Coccia, E.M.; Olagnier, D.; et al. Inhibition of Glycolysis Impairs Retinoic Acid-Inducible Gene I-Mediated Antiviral Responses in Primary Human Dendritic Cells. Front. Cell Infect. Microbiol. 2022, 12, 910864. [Google Scholar] [CrossRef]
- Huckestein, B.R.; Alcorn, J.F. Improving Mitochondrial Function in Viral Infection: Targeting Cellular Metabolism. Am. J. Respir. Cell Mol. Biol. 2022, 66, 598–600. [Google Scholar] [CrossRef]
- Sanchez-Garcia, F.J.; Perez-Hernandez, C.A.; Rodriguez-Murillo, M.; Moreno-Altamirano, M.M.B. The Role of Tricarboxylic Acid Cycle Metabolites in Viral Infections. Front. Cell Infect. Microbiol. 2021, 11, 725043. [Google Scholar] [CrossRef]
- Changaei, M.; Azimzadeh Tabrizi, Z.; Karimi, M.; Kashfi, S.A.; Koochaki Chahardeh, T.; Hashemi, S.M.; Soudi, S. From powerhouse to modulator: Regulating immune system responses through intracellular mitochondrial transfer. Cell Commun. Signal 2025, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, Y.; Jin, X.; Li, S.; Qiu, H.J. Crosstalk between Dysfunctional Mitochondria and Proinflammatory Responses during Viral Infections. Int. J. Mol. Sci. 2024, 25, 9206. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, S.J.; Yun, J.; Shin, O.S. Nonstructural Protein NS1 of Influenza Virus Disrupts Mitochondrial Dynamics and Enhances Mitophagy via ULK1 and BNIP3. Viruses 2021, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Garcia-Sastre, A.; Xiao, X.; Wang, R.; Palese, P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog. 2005, 1, e4. [Google Scholar] [CrossRef]
- Varga, Z.T.; Grant, A.; Manicassamy, B.; Palese, P. Influenza virus protein PB1-F2 inhibits the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J. Virol. 2012, 86, 8359–8366. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Ichinohe, T.; Sasaki, O.; Otera, H.; Kawabata, S.; Mihara, K.; Koshiba, T. Influenza A virus protein PB1-F2 translocates into mitochondria via Tom40 channels and impairs innate immunity. Nat. Commun. 2014, 5, 4713. [Google Scholar] [CrossRef]
- Palmer, C.S. Innate metabolic responses against viral infections. Nat. Metab. 2022, 4, 1245–1259. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Deng, H.; Li, S.; Qiu, H.J. Cellular metabolism hijacked by viruses for immunoevasion: Potential antiviral targets. Front. Immunol. 2023, 14, 1228811. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef]
- Pei, Y.; Robertson, E.S. The Crosstalk of Epigenetics and Metabolism in Herpesvirus Infection. Viruses 2020, 12, 1377. [Google Scholar] [CrossRef]
- Girdhar, K.; Powis, A.; Raisingani, A.; Chrudinova, M.; Huang, R.; Tran, T.; Sevgi, K.; Dogus Dogru, Y.; Altindis, E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu. Rev. Virol. 2021, 8, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyas, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Effects of oxidative stress on viral infections: An overview. npj Viruses 2025, 3, 27. [Google Scholar] [CrossRef]
- Kirkpatrick, C.T.; Wang, Y.; Leiva Juarez, M.M.; Shivshankar, P.; Pantaleon Garcia, J.; Plumer, A.K.; Kulkarni, V.V.; Ware, H.H.; Gulraiz, F.; Chavez Cavasos, M.A.; et al. Inducible Lung Epithelial Resistance Requires Multisource Reactive Oxygen Species Generation To Protect against Viral Infections. mBio 2018, 9, e00696-18. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospisil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Fernandez-Sesma, A. The influenza virus NS1 protein: Inhibitor of innate and adaptive immunity. Infect. Disord. Drug Targets 2007, 7, 336–343. [Google Scholar] [CrossRef]
- Koshiba, T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta 2013, 1833, 225–232. [Google Scholar] [CrossRef]
- Duan, X.; Liu, R.; Lan, W.; Liu, S. The Essential Role of Mitochondrial Dynamics in Viral Infections. Int. J. Mol. Sci. 2025, 26, 1955. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Konakova, T.E.; Wolff, T.; Klenk, H.D. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 2002, 76, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.E.; Datan, E.; Matassov, D.; Zakeri, Z.F. Lack of Bax prevents influenza A virus-induced apoptosis and causes diminished viral replication. J. Virol. 2009, 83, 8233–8246. [Google Scholar] [CrossRef]
- Wurzer, W.J.; Planz, O.; Ehrhardt, C.; Giner, M.; Silberzahn, T.; Pleschka, S.; Ludwig, S. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003, 22, 2717–2728. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Q.; Florence, J.M.; Jung, B.G.; Kurdowska, A.K.; Samten, B.; Idell, S.; Tang, H. Influenza Virus Infection Induces ZBP1 Expression and Necroptosis in Mouse Lungs. Front. Cell Infect. Microbiol. 2019, 9, 286. [Google Scholar] [CrossRef]
- Goellner, S.; Enkavi, G.; Prasad, V.; Denolly, S.; Eu, S.; Mizzon, G.; Witte, L.; Kulig, W.; Uckeley, Z.M.; Lavacca, T.M.; et al. Zika virus prM protein contains cholesterol binding motifs required for virus entry and assembly. Nat. Commun. 2023, 14, 7344. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Chan, J.F.; Ye, Z.W.; Wen, L.; Yan, B.; Lai, P.M.; Tee, K.M.; Huang, J.; Chen, D.; et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar] [CrossRef]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007, 9, 1089–1097. [Google Scholar] [CrossRef]
- Madsen, J.J.; Rossman, J.S. Cholesterol and M2 Rendezvous in Budding and Scission of Influenza A Virus. Subcell. Biochem. 2023, 106, 441–459. [Google Scholar] [CrossRef]
- Bao, D.; Lu, C.; Ma, T.; Xu, G.; Mao, Y.; Xin, L.; Niu, S.; Wu, Z.; Li, X.; Teng, Q.; et al. Hydrophobic Residues at the Intracellular Domain of the M2 Protein Play an Important Role in Budding and Membrane Integrity of Influenza Virus. J. Virol. 2022, 96, e0037322. [Google Scholar] [CrossRef]
- Kolokouris, D.; Kalenderoglou, I.E.; Duncan, A.L.; Corey, R.A.; Sansom, M.S.P.; Kolocouris, A. The Role of Cholesterol in M2 Clustering and Viral Budding Explained. J. Chem. Theory Comput. 2025, 21, 912–932. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Tian, J.; Su, R.; Sun, J.; Li, Y.; Wang, L.; Zhou, H.; Sha, S.; Xiao, J.; et al. SREBP2-dependent lipid droplet formation enhances viral replication and deteriorates lung injury in mice following IAV infection. Emerg. Microbes Infect. 2025, 14, 2470371. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef]
- Hendrix, S.; Kingma, J.; Ottenhoff, R.; Valiloo, M.; Svecla, M.; Zijlstra, L.F.; Sachdev, V.; Kovac, K.; Levels, J.H.M.; Jongejan, A.; et al. Hepatic SREBP signaling requires SPRING to govern systemic lipid metabolism in mice and humans. Nat. Commun. 2023, 14, 5181. [Google Scholar] [CrossRef]
- Hale, B.G.; Jackson, D.; Chen, Y.H.; Lamb, R.A.; Randall, R.E. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 14194–14199. [Google Scholar] [CrossRef]
- Kang, K.; Reilly, S.M.; Karabacak, V.; Gangl, M.R.; Fitzgerald, K.; Hatano, B.; Lee, C.H. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008, 7, 485–495. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Mazzon, M.; Mercer, J. Lipid interactions during virus entry and infection. Cell Microbiol. 2014, 16, 1493–1502. [Google Scholar] [CrossRef]
- Barman, S.; Nayak, D.P. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J. Virol. 2007, 81, 12169–12178. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Hirohama, M.; Harada, Y.; Osari, S.; Nagata, K. Influenza Virus Induces Cholesterol-Enriched Endocytic Recycling Compartments for Budozone Formation via Cell Cycle-Independent Centrosome Maturation. PLoS Pathog. 2015, 11, e1005284. [Google Scholar] [CrossRef]
- Vandermeer, M.L.; Thomas, A.R.; Kamimoto, L.; Reingold, A.; Gershman, K.; Meek, J.; Farley, M.M.; Ryan, P.; Lynfield, R.; Baumbach, J.; et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: A multistate study. J. Infect. Dis. 2012, 205, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Bartenschlager, R. Flaviviridae Replication Organelles: Oh, What a Tangled Web We Weave. Annu. Rev. Virol. 2015, 2, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Sweet, M.J.; Parton, R.G.; Pol, A. Lipid droplets and the host-pathogen dynamic: FATal attraction? J. Cell Biol. 2021, 220, e202104005. [Google Scholar] [CrossRef]
- Bosch, M.; Sanchez-Alvarez, M.; Fajardo, A.; Kapetanovic, R.; Steiner, B.; Dutra, F.; Moreira, L.; Lopez, J.A.; Campo, R.; Mari, M.; et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 2020, 370, eaay8085. [Google Scholar] [CrossRef]
- Zhou, Y.; Pu, J.; Wu, Y. The Role of Lipid Metabolism in Influenza A Virus Infection. Pathogens 2021, 10, 303. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Bartenschlager, R. Membranous replication factories induced by plus-strand RNA viruses. Viruses 2014, 6, 2826–2857. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Petakh, P.; Lushchak, O.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin in Antiviral Therapy: Evidence and Perspectives. Viruses 2024, 16, 1938. [Google Scholar] [CrossRef]
- Lee, H.S.; Noh, J.Y.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Metformin reduces the risk of developing influenza A virus related cardiovascular disease. Heliyon 2023, 9, e20284. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19. Clin. Infect. Dis. 2024, 79, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Hong, K.S.; Pagan, K.; Whalen, W.; Harris, R.; Yang, J.; Stout-Delgado, H.; Cho, S.J. The Role of Glutathione Reductase in Influenza Infection. Am. J. Respir. Cell Mol. Biol. 2022, 67, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Meiser, J.; Vazquez, A. Give it or take it: The flux of one-carbon in cancer cells. FEBS J. 2016, 283, 3695–3704. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Pham, V.N.; Bruemmer, K.J.; Toh, J.D.W.; Ge, E.J.; Tenney, L.; Ward, C.C.; Dingler, F.A.; Millington, C.L.; Garcia-Prieto, C.A.; Pulos-Holmes, M.C.; et al. Formaldehyde regulates S-adenosylmethionine biosynthesis and one-carbon metabolism. Science 2023, 382, eabp9201. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, J.; Taryma-Lesniak, O.; Luczkowska, K.; Niedzwiedz, A.; Lechowicz, K.; Strapagiel, D.; Jarczak, J.; Davalos, V.; Pujol, A.; Esteller, M.; et al. Epigenetic activation of antiviral sensors and effectors of interferon response pathways during SARS-CoV-2 infection. Biomed. Pharmacother. 2022, 153, 113396. [Google Scholar] [CrossRef]
- Green, R.; Ireton, R.C.; Gale, M., Jr. Interferon-stimulated genes: New platforms and computational approaches. Mamm. Genome 2018, 29, 593–602. [Google Scholar] [CrossRef]
- Marazzi, I.; Ho, J.S.; Kim, J.; Manicassamy, B.; Dewell, S.; Albrecht, R.A.; Seibert, C.W.; Schaefer, U.; Jeffrey, K.L.; Prinjha, R.K.; et al. Suppression of the antiviral response by an influenza histone mimic. Nature 2012, 483, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Xiao, Y.; Zhou, X.; Chang, J.H.; Chang, M.; Cheng, X.; Blonska, M.; Lin, X.; Sun, S.C. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef]
- Luan, H. Cell-Autonomous and Non-Cell-Autonomous Antiviral Immunity via siRNA-Directed RNAi in Drosophila melanogaster. Immune Discov. 2025, 1, 10001. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- York, A.G.; Williams, K.J.; Argus, J.P.; Zhou, Q.D.; Brar, G.; Vergnes, L.; Gray, E.E.; Zhen, A.; Wu, N.C.; Yamada, D.H.; et al. Limiting Cholesterol Biosynthetic Flux Spontaneously Engages Type I IFN Signaling. Cell 2015, 163, 1716–1729. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Llibre, A.; Kucuk, S.; Gope, A.; Certo, M.; Mauro, C. Lactate: A key regulator of the immune response. Immunity 2025, 58, 535–554. [Google Scholar] [CrossRef]
- Hayden, F.G.; Shindo, N. Influenza virus polymerase inhibitors in clinical development. Curr. Opin. Infect. Dis. 2019, 32, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza. Ann. Pharmacother. 2019, 53, 754–759. [Google Scholar] [CrossRef]
- Fukao, K.; Ando, Y.; Noshi, T.; Kitano, M.; Noda, T.; Kawai, M.; Yoshida, R.; Sato, A.; Shishido, T.; Naito, A. Baloxavir marboxil, a novel cap-dependent endonuclease inhibitor potently suppresses influenza virus replication and represents therapeutic effects in both immunocompetent and immunocompromised mouse models. PLoS ONE 2019, 14, e0217307. [Google Scholar] [CrossRef]
- Andreev, K.; Jones, J.C.; Seiler, P.; Kandeil, A.; Turner, J.C.M.; Barman, S.; Rubrum, A.M.; Webby, R.J.; Govorkova, E.A. Antiviral Susceptibility of Highly Pathogenic Avian Influenza A(H5N1) Viruses Circulating Globally in 2022–2023. J. Infect. Dis. 2024, 229, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Chien, K.Y.; Lai, C.H.; Li, G.J.; Lin, J.F.; Ho, H.Y. Metabolic Reprogramming of Host Cells in Response to Enteroviral Infection. Cells 2020, 9, 473. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, M.; He, Y.; Jiang, B.; He, M.L. Metabolic alterations upon SARS-CoV-2 infection and potential therapeutic targets against coronavirus infection. Signal Transduct. Target. Ther. 2023, 8, 237. [Google Scholar] [CrossRef]
- Flores-Torres, A.S.; Rezinciuc, S.; Bezavada, L.; Shulkin, B.L.; Cormier, S.A.; Smallwood, H.S. Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways. Viruses 2025, 17, 703. [Google Scholar] [CrossRef] [PubMed]
- Chermahini, F.A.; Arvejeh, P.M.; Marincola, F.M.; Ahmad, S.; Naderian, R.; Pajand, O.; Eslami, M.; Hasannia, M.; Sanami, S. Investigating how dengue virus-induced metabolic changes affect the host immune response and how to develop Immunomodulatory strategies. Virol. J. 2025, 22, 117. [Google Scholar] [CrossRef] [PubMed]


| Target Pathway | Agent | Mechanism of Action | Antiviral Effect | Stage |
|---|---|---|---|---|
| Fatty acid synthesis | Orlistat | Inhibits FASN, blocks fatty acid biosynthesis | Suppresses viral RNA replication and protein synthesis | Preclinical |
| Cholesterol synthesis | Statins | Inhibit HMG-CoA reductase, reduce cholesterol synthesis | Impairs viral budding and infectivity | Preclinical/Early clinical |
| SREBP activation | Betulin | Inhibits SREBP cleavage and nuclear translocation | Decreases lipid synthesis and viral replication | Preclinical |
| AMPK/mTOR | Metformin | Activates AMPK, inhibits mTOR, suppresses SREBP activity indirectly | Restores metabolic balance, limits viral replication [91] | Early clinical (repurposing) [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, X.; Tian, X.; Ding, S.; Gao, G.; Zhao, X.; Cui, J.; Hou, Y.; Zhao, T.; Wang, H. Metabolic Hostile Takeover: How Influenza Virus Reprograms Cellular Metabolism for Replication. Viruses 2025, 17, 1386. https://doi.org/10.3390/v17101386
Hui X, Tian X, Ding S, Gao G, Zhao X, Cui J, Hou Y, Zhao T, Wang H. Metabolic Hostile Takeover: How Influenza Virus Reprograms Cellular Metabolism for Replication. Viruses. 2025; 17(10):1386. https://doi.org/10.3390/v17101386
Chicago/Turabian StyleHui, Xianfeng, Xiaowei Tian, Shihuan Ding, Ge Gao, Xin Zhao, Jiyan Cui, Yiru Hou, Tiesuo Zhao, and Hui Wang. 2025. "Metabolic Hostile Takeover: How Influenza Virus Reprograms Cellular Metabolism for Replication" Viruses 17, no. 10: 1386. https://doi.org/10.3390/v17101386
APA StyleHui, X., Tian, X., Ding, S., Gao, G., Zhao, X., Cui, J., Hou, Y., Zhao, T., & Wang, H. (2025). Metabolic Hostile Takeover: How Influenza Virus Reprograms Cellular Metabolism for Replication. Viruses, 17(10), 1386. https://doi.org/10.3390/v17101386






