Novel Bat Adenovirus Closely Related to Canine Adenoviruses Identified via Fecal Virome Surveillance of Bats in New Mexico, USA, 2020–2021
Abstract
1. Introduction
2. Materials and Methods
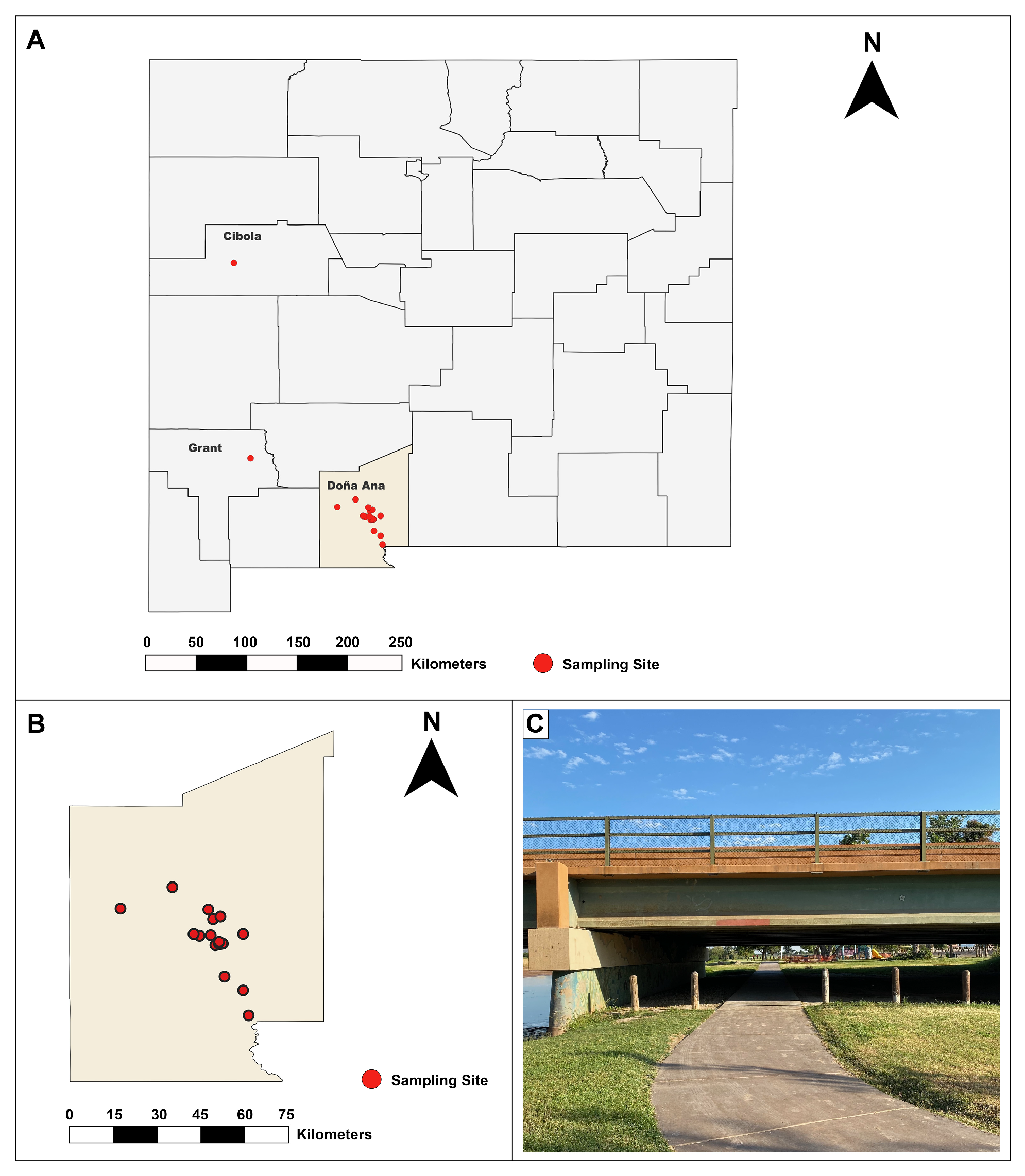
2.1. Study Site and Sample Collection
| Species 1 | Age Classes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (By Sex) | Migrates | Peridomestic | Group Size | Sexes Cohabit Roost | Synchronized Births | Unknown | Adult | Subadult | Juvenile | Total |
| Antrozous pallidus [22,23] | Yes | Moderate | Few to >100 | Yes | Yes | 1 | 31 | 3 | 35 | |
| Female | 25 | 3 | 28 | |||||||
| Male | 6 | 6 | ||||||||
| Not recorded | 1 | 1 | ||||||||
| Corynorhinus townsendii [24,25,26] | Yes | Low | 40-100 | Yes | Yes | 9 | 9 | |||
| Female | 8 | 8 | ||||||||
| Male | 1 | 1 | ||||||||
| Eptesicus fuscus [27,28,29,30,31] | Yes | High | >100 | Yes | Yes | 11 | 2 | 13 | ||
| Female | 6 | 2 | 8 | |||||||
| Male | 5 | 5 | ||||||||
| Lasiurus cinereus [32,33] | Yes | Low | 1 | No | No | 3 | 3 | |||
| Female | 0 | |||||||||
| Male | 3 | 3 | ||||||||
| Myotis species 2 [29,34,35,36] | Yes | Moderate | >100 | Varies | Yes | 73 | 35 | 11 | 119 | |
| Female | 48 | 16 | 4 | 68 | ||||||
| Male | 25 | 19 | 7 | 51 | ||||||
| Tadarida brasiliensis [37,38,39,40] | Mix | Moderate | >1000 | Yes | Yes | 2 | 60 | 13 | 75 | |
| Female | 36 | 4 | 40 | |||||||
| Male | 24 | 9 | 33 | |||||||
| Not recorded | 2 | 2 | ||||||||
| Total | 3 | 187 | 53 | 11 | 254 |
2.2. RNA Extraction
2.3. Pan-Coronavirus RT-PCR
2.4. Metagenomic Sequencing
2.5. Bioinformatics
2.6. Phylogenetic Analysis
3. Results
3.1. Coronavirus Surveillance Yielded No Detections
3.2. Novel Virus Identification and Genomic Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olival, K.J.; Cryan, P.M.; Amman, B.R.; Baric, R.S.; Blehert, D.S.; Brook, C.E.; Calisher, C.H.; Castle, K.T.; Coleman, J.T.H.; Daszak, P.; et al. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: A case study of bats. PLoS Pathog. 2020, 16, e1008758. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsugo, H.; Maruyama, J.; Kamiki, H.; Takada, A.; Maeda, K.; Takenaka-Uema, A.; Tohya, Y.; Murakami, S.; Horimoto, T.; et al. Characterization of a novel species of adenovirus from Japanese microbat and role of CXADR as its entry factor. Sci. Rep. 2019, 9, 573. [Google Scholar] [CrossRef]
- Valitutto, M.T.; Aung, O.; Tun, K.Y.N.; Vodzak, M.E.; Zimmerman, D.; Yu, J.H.; Win, Y.T.; Maw, M.T.; Thein, W.Z.; Win, H.H.; et al. Detection of novel coronaviruses in bats in Myanmar. PLoS ONE 2020, 15, e0230802. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, E.F.; Haskew, A.N.; Gates, J.E.; Huynh, J.; Moore, C.J.; Frieman, M.B. Metagenomic Analysis of the Viromes of Three North American Bat Species: Viral Diversity among Different Bat Species That Share a Common Habitat. J. Virol. 2010, 84, 13004–13018. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Ojeda-Flores, R.; Rico-Chávez, O.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Rostal, M.K.; Epstein, J.H.; Tipps, T.; Liang, E.; Sanchez-Leon, M.; et al. Coronaviruses in bats from Mexico. J. Gen. Virol. 2013, 94, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, S.R.; O’Shea, T.J.; Oko, L.M.; Holmes, K.V. Detection of group 1 coronaviruses in bats in North America. Emerg. Infect. Dis. 2007, 13, 1295–1300. [Google Scholar] [CrossRef]
- Li, L.; Victoria, J.G.; Wang, C.; Jones, M.; Fellers, G.M.; Kunz, T.H.; Delwart, E. Bat guano virome: Predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 2010, 84, 6955–6965. [Google Scholar] [CrossRef]
- Li, Y.; Altan, E.; Reyes, G.; Halstead, B.; Deng, X.; Delwart, E. Virome of Bat Guano from Nine Northern California Roosts. J. Virol. 2021, 95, e01713–e01720. [Google Scholar] [CrossRef]
- Osborne, C.; Cryan, P.M.; O’Shea, T.J.; Oko, L.M.; Ndaluka, C.; Calisher, C.H.; Berglund, A.D.; Klavetter, M.L.; Bowen, R.A.; Holmes, K.V.; et al. Alphacoronaviruses in new World bats: Prevalence, persistence, phylogeny, and potential for interaction with humans. PLoS ONE 2011, 6, 19156. [Google Scholar] [CrossRef]
- Jolliffe, T.; Kepel, A.; Kingston, T.; Leopardi, S.; Mclean, J.; Mendehall, I.H.; Parsons, S.; Russo, D.; Shapiro, J.T.; Viquez-R, L.; et al. IUCN SSC Bat Specialist Group (BSG) Recommendations to Reduce the Risk of Transmission of SARS-CoV-2 From Humans to Bats in Bat Rescue and Rehabilitation Centers; IUCN SSC Bat Specialist Group: Gland, Switzerland, 2021. [Google Scholar]
- Shapiro, J.T.; Phelps, K.; Racey, P.; Vicente-Santos, A.; Viquez-R, L.; Walsh, A.; Weinberg, M.; Kingston, T.; Leopardi, S.; Mclean, J.; et al. IUCN SSC BSG Guidelines for Field Hygiene 2024; IUCN SSC Bat Specialist Group: Gland, Switzerland, 2024. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Fieldhouse, J.K.; Seto, D.; Gray, G.C. Are adenoviruses zoonotic? A systematic review of the evidence. Emerg. Microbes Infect. 2019, 8, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhu, C.; Zhang, W.; He, T.; Ke, Y.; Wu, J.; Yin, W.; Zou, X.; Ding, C.; Luo, Y.; et al. Genomic characteristics and pathogenicity of a new bat adenoviruses strains that was isolated in at sites along the southeastern coasts of the P.R. of China from 2015 to 2019. Virus Res. 2022, 308, 198653. [Google Scholar] [CrossRef] [PubMed]
- Diakoudi, G.; Lanave, G.; Moreno, A.; Chiapponi, C.; Sozzi, E.; Prosperi, A.; Larocca, V.; Losurdo, M.; Decaro, N.; Martella, V.; et al. Surveillance for Adenoviruses in Bats in Italy. Viruses 2019, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.V.; Lanzarini, N.M.; de Moraes, M.T.B.; Nordgren, J.; Moura, P.E.B.; Moratelli, R.; Novaes, R.L.M.; Costa-Neto, S.F.; Veríssimo, I.; Miagostovich, M.P.; et al. First molecular detection of adenoviruses in bats from an urban Atlantic Forest in Rio de Janeiro, Brazil. Infect. Genet. Evol. 2024, 126, 105687. [Google Scholar] [CrossRef]
- Jansen Van Vuren, P.; Allam, M.; Wiley, M.R.; Ismail, A.; Storm, N.; Birkhead, M.; Markotter, W.; Palacios, G.; Paweska, J.T. A novel adenovirus isolated from the Egyptian fruit bat in South Africa is closely related to recent isolates from China. Sci. Rep. 2018, 8, 9584. [Google Scholar] [CrossRef]
- Karamendin, K.; Kydyrmanov, A.; Sabyrzhan, T.; Nuralibekov, S.; Kasymbekov, Y.; Khan, Y. Detection and Phylogenetic Characterization of a Novel Adenovirus Found in Lesser Mouse-Eared Bat (Myotis blythii) in South Kazakhstan. Viruses 2023, 15, 1139. [Google Scholar] [CrossRef]
- Lee, D.N.; Angiel, M. Two novel adenoviruses found in Cave Myotis bats (Myotis velifer) in Oklahoma. Virus Genes 2019, 56, 99. [Google Scholar] [CrossRef]
- Sikes, R.S. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Brunet-Rossinni, A.K. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S.E., Eds.; Johns Hopkins University Press: Baltimore, MA, USA, 2009. [Google Scholar]
- Lewis, S.E. Effect of climatic variation on reproduction by pallid bats (Antrozous pallidus). Can. J. Zool. 1993, 71, 1429–1433. [Google Scholar] [CrossRef]
- Hermanson, J.W.; O’Shea, T.J. Antrozous pallidus. Mamm. Species 1983, 3, 1–8. [Google Scholar] [CrossRef]
- Sherwin, R.E.; Stricklan, D.; Rogers, D.S. Roosting Affinities of Townsend’s Big-Eared Bat (Corynorhinus townsendii) in Northern Utah. J. Mammal. 2000, 81, 939–947. [Google Scholar] [CrossRef]
- Lucas, J.S.; Loeb, S.C.; Jodice, P.G.R. Roost selection by rafinesque’s big-eared bats (Corynorhinus rafinesquii) in a pristine habitat at three spatial scales. Acta Chiropterologica 2015, 17, 131–141. [Google Scholar] [CrossRef]
- Pearson, O.P.; Koford, M.R.; Pearson, A.K. Reproduction of the Lump-Nosed Bat (Corynorhinus rafinesquei) in California. J. Mammal. 1952, 33, 273. [Google Scholar] [CrossRef]
- McGuire, L.P.; Boyle, W.A. Altitudinal migration in bats: Evidence, patterns, and drivers. Biol. Rev. 2013, 88, 767–786. [Google Scholar] [CrossRef]
- Agosta, S.J. Habitat use, diet and roost selection by the Big Brown Bat (Eptesicus fuscus) in North America: A case for conserving an abundant species. Mamm. Rev. 2002, 32, 179–198. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; South, J.M. Life history, ecology and longevity in bats. Aging Cell 2002, 1, 124–131. [Google Scholar] [CrossRef]
- Wilkinson, L.C.; Barclay, R.M.R. Differences in the foraging behaviour of male and female big brown bats (Eptesicus fuscus) during the reproductive period. Écoscience 1997, 4, 279–285. [Google Scholar] [CrossRef]
- Mayrberger, S. Exit/entry Sequences, Roost Fidelity and Transport of Young by Big Brown Bats (Eptesicus fuscus). Master’s Thesis, University of Michigan-Flint, Flint, MI, USA, 2003. [Google Scholar]
- Shump, K.A.; Shump, A.U. Lasiurus cinereus. Mamm. Species 1982, 3, 1–5. [Google Scholar] [CrossRef]
- Rolseth, S.L.; Koehler, C.E.; Barclay, R.M.R. Differences in the Diets of Juvenile and Adult Hoary Bats, Lasiurus cinereus. J. Mammal. 1994, 75, 394–398. [Google Scholar] [CrossRef]
- Krutzsch, P.H. Notes on the habits of the bat, Myotis californicus. J. Mammal. 1954, 35, 539–545. [Google Scholar] [CrossRef]
- Oxberry, B.A. Female reproductive patterns in hibernating bats. J. Reprod. Fertil. 1979, 56, 359–367. [Google Scholar] [CrossRef]
- Wimsatt, W.A. An analysis of parturition in Chiroptera, including new observations on Myotis lucifugus. J. Mammal. 1960, 41, 183–200. [Google Scholar] [CrossRef]
- Russell, A.L.; Medellín, R.A.; Mccracken, G.F. Genetic variation and migration in the Mexican free-tailed bat (Tadarida brasiliensis mexicana). Mol. Ecol. 2005, 14, 2207–2222. [Google Scholar] [CrossRef]
- Wilkins, K.T. Tadarida brasiliensis. Mamm. Species 1989, 3, 1–10. [Google Scholar] [CrossRef]
- Altenbach, J.S.; Geluso, K.N.; Wilson, D.E. Population size of Tadarida. Biological Investigations in the Guadalupe Mountains National Park, Texas; National Park Service: Lubbock, TX, USA, 1975; p. 341.
- Krutzsch, P.H.; Fleming, T.H.; Crichton, E.G. Reproductive biology of male Mexican free-tailed bats (Tadarida brasiliensis mexicana). J. Mammal. 2002, 83, 489–500. [Google Scholar] [CrossRef]
- Bennett, A.J.; Bushmaker, T.; Cameron, K.; Ondzie, A.; Niama, F.R.; Parra, H.J.; Mombouli, J.V.; Olson, S.H.; Munster, V.J.; Goldberg, T.L. Diverse RNA viruses of arthropod origin in the blood of fruit bats suggest a link between bat and arthropod viromes. Virology 2019, 528, 64. [Google Scholar] [CrossRef]
- Bennett, A.J.; Paskey, A.C.; Kuhn, J.H.; Bishop-Lilly, K.A.; Goldberg, T.L. Diversity, Transmission, and Cophylogeny of Ledanteviruses (Rhabdoviridae: Ledantevirus) and Nycteribiid Bat Flies Parasitizing Angolan Soft-Furred Fruit Bats in Bundibugyo District, Uganda. Microorganisms 2020, 8, 750. [Google Scholar] [CrossRef]
- Goldberg, T.L.; Bennett, A.J.; Kityo, R.; Kuhn, J.H.; Chapman, C.A. Kanyawara Virus: A Novel Rhabdovirus Infecting Newly Discovered Nycteribiid Bat Flies Infesting Previously Unknown Pteropodid Bats in Uganda. Sci. Rep. 2017, 7, 5287. [Google Scholar] [CrossRef]
- Allander, T.; Emerson, S.U.; Engle, R.E.; Purcell, R.H.; Bukh, J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. USA 2001, 98, 11609–11614. [Google Scholar] [CrossRef]
- Xiu, L.; Binder, R.A.; Alarja, N.A.; Kochek, K.; Coleman, K.K.; Than, S.T.; Bailey, E.S.; Bui, V.N.; Toh, T.-H.; Erdmann, D.D.; et al. A RT-PCR assay for the detection of coronaviruses from four genera. J. Clin. Virol. 2020, 128, 104391. [Google Scholar] [CrossRef]
- Tan, C.C.S.; Trew, J.; Peacock, T.P.; Mok, K.Y.; Hart, C.; Lau, K.; Ni, D.; Orme, C.D.L.; Ransome, E.; Pearse, W.D.; et al. Genomic screening of 16 UK native bat species through conservationist networks uncovers coronaviruses with zoonotic potential. Nat. Commun. 2023, 14, 3322. [Google Scholar] [CrossRef]
- Brnić, D.; Lojkić, I.; Krešić, N.; Zrnčić, V.; Ružanović, L.; Mikuletič, T.; Bosilj, M.; Steyer, A.; Keros, T.; Habrun, B.; et al. Circulation of SARS-CoV-Related Coronaviruses and Alphacoronaviruses in Bats from Croatia. Microorganisms 2023, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.L.; Sibley, S.; Pinkerton, M.; Dunn, C.; Long, L.; White, L. Multidecade mortality and a homolog of hepatitis C virus in bald eagles (Haliaeetus leucocephalus), the national bird of the USA. Sci. Rep. 2019, 9, 14953. [Google Scholar] [CrossRef] [PubMed]
- Toohey-Kurth, K.; Sibley, S.D.; Goldberg, T.L. Metagenomic assessment of adventitious viruses in commercial bovine sera. Biologicals 2017, 47, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Negrey, J.D.; Mitani, J.C.; Wrangham, R.W.; Otali, E.; Reddy, R.B.; Pappas, T.E.; Grindle, K.A.; Gern, J.E.; Machanda, Z.P.; Muller, M.N.; et al. Viruses associated with ill health in wild chimpanzees. Am. J. Primatol. 2022, 84, e23358. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gish, W.; States, D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993, 3, 266–272. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018.
- Maeda, K.; Hondo, E.; Terakawa, J.; Kiso, Y.; Nakaichi, N.; Endoh, D.; Sakai, K.; Morikawa, S.; Mizutani, T. Isolation of novel adenovirus from fruit bat (Pteropus dasymallus yayeyamae). Emerg. Infect. Dis. 2008, 14, 347–349. [Google Scholar] [CrossRef]
- Kohl, C.; Vidovszky, M.Z.; Mühldorfer, K.; Dabrowski, P.W.; Radonić, A.; Nitsche, A.; Wibbelt, G.; Kurth, A.; Harrach, B. Genome Analysis of Bat Adenovirus 2: Indications of Interspecies Transmission. J. Virol. 2012, 86, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, M.; Mühldorfer, K.; Speck, S.; Wibbelt, G.; Kurth, A. New Adenovirus in Bats, Germany. Emerg. Infect. Dis. 2009, 15, 2052–2055. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Martella, V.; Buonavoglia, C. Canine Adenoviruses and Herpesvirus. Vet. Clin. North. Am. Small Anim. Pract. 2008, 38, 799. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Marziani, E.; Aziz, C.; Brown, C.M.; Cohn, L.A.; Lea, C.; Moore, G.E.; Taneja, N. 2022 AAHA Canine Vaccination Guidelines (2024 Update). J. Am. Anim. Hosp. Assoc. 2024, 60, 1–19. [Google Scholar] [CrossRef]
- Mietzsch, M.; Li, Y.; Kurian, J.; Smith, J.K.; Chipman, P.; McKenna, R.; Yang, L.; Agbandje-McKenna, M. Structural characterization of a bat Adeno-associated virus capsid. J. Struct. Biol. 2020, 211, 107547. [Google Scholar] [CrossRef]
- Orłowska, A.; Smreczak, M.; Potyrało, P.; Bomba, A.; Trębas, P.; Rola, J. First Detection of Bat Astroviruses (BtAstVs) among Bats in Poland: The Genetic BtAstVs Diversity Reveals Multiple Co-Infection of Bats with Different Strains. Viruses 2021, 13, 158. [Google Scholar] [CrossRef]
- Harding, C.; Larsen, B.B.; Otto, H.W.; Potticary, A.L.; Kraberger, S.; Custer, J.M.; Suazo, C.; Upham, N.S.; Worobey, M.; Van Doorslaer, K.; et al. Diverse DNA virus genomes identified in fecal samples of Mexican free-tailed bats (Tadarida brasiliensis) captured in Chiricahua Mountains of southeast Arizona (USA). Virology 2023, 580, 98–111. [Google Scholar] [CrossRef]
- Trejo-Chávez, A.; Castillo-Velázquez, U.; Méndez-Bernal, A.; Flores-Martínez, K.; Hernández-Vidal, G.; Rodríguez-Tovar, L.E.; Villarreal-Villarreal, J.P. Infection by Adenovirus Type 2 in a Short-Tailed Bat in Mexico. Case Rep. Vet. Med. 2025, 2025, 2431526. [Google Scholar] [CrossRef]
- Niu, Y.; McKee, C.D. Bat Viral Shedding: A Review of Seasonal Patterns and Risk Factors. Vector-Borne Zoonotic Dis. 2025, 25, 229–239. [Google Scholar] [CrossRef]
- Simon, A.Y.; Badmalia, M.D.; Paquette, S.J.; Manalaysay, J.; Czekay, D.; Kandel, B.S.; Sultana, A.; Lung, O.; Babuadze, G.G.; Shahhosseini, N. Evolutionary Relationships of Unclassified Coronaviruses in Canadian Bat Species. Viruses 2024, 16, 1878. [Google Scholar] [CrossRef]
- Jiménez-Rico, M.A.; Vigueras-Galván, A.L.; Hernández-Villegas, E.N.; Martínez-Duque, P.; Roiz, D.; Falcón, L.I.; Vázquez-Domínguez, E.; Gaona, O.; Arnal, A.; Roche, B.; et al. Bat coronavirus surveillance across different habitats in Yucatán, México. Virology 2025, 603, 110401. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, S.; Rapin, N.; Bollinger, T.K.; Hill, J.E.; Donaldson, M.E.; Davy, C.M.; Warnecke, L.; Turner, J.M.; Kyle, C.J.; Willis, C.K.R.; et al. A persistently infecting coronavirus in hibernating Myotis lucifugus, the North American little brown bat. J. Gen. Virol. 2017, 98, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Dumonceaux, T.; Dubois, J.; Willis, C.; Nadin-Davis, S.; Severini, A.; Wandeler, A.; Lindsay, R.; Artsob, H. Detection of polyoma and corona viruses in bats of Canada. J. Gen. Virol. 2009, 90, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Matson, M.J.; Yinda, C.K.; Seifert, S.N.; Bushmaker, T.; Fischer, R.J.; van Doremalen, N.; Lloyd-Smith, J.O.; Munster, V.J. Effect of Environmental Conditions on SARS-CoV-2 Stability in Human Nasal Mucus and Sputum. Emerg. Infect. Dis. 2020, 26, 2276. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl. Environ. Microbiol. 2010, 76, 2712. [Google Scholar] [CrossRef]
- Flanders, W.D.; Kleinbaum, D.G. Basic Models for Disease Occurrence in Epidemiology. Int. J. Epidemiol. 1995, 24, 1–7. [Google Scholar] [CrossRef]
- Eskew, E.A.; Olival, K.J.; Mazet, J.A.K.; Daszak, P. A global-scale dataset of bat viral detection suggests that pregnancy reduces viral shedding. Proc. R. Soc. B 2025, 292, 20242381. [Google Scholar] [CrossRef]
- Montecino-Latorre, D.; Goldstein, T.; Gilardi, K.; Wolking, D.; Van Wormer, E.; Kazwala, R.; Ssebide, B.; Nziza, J.; Sijali, Z.; Cranfield, M.; et al. Reproduction of East-African bats may guide risk mitigation for coronavirus spillover. One Health Outlook 2020, 2, 2. [Google Scholar] [CrossRef]
- Ntumvi, N.F.; Diffo, J.L.D.; Tamoufe, U.; Ndze, V.N.; Takuo, J.M.; Mouiche, M.M.M.; Nwobegahay, J.; Lebreton, M.; Gillis, A.; Rimoin, A.W.; et al. Evaluation of bat adenoviruses suggests co-evolution and host roosting behaviour as drivers for diversity. Microb. Genom. 2021, 7, 000561. [Google Scholar] [CrossRef]
- Cook, J.D.; Grant, E.H.C.; Coleman, J.T.H.; Sleeman, J.M.; Runge, M.C. Risks posed by SARS-CoV-2 to North American bats during winter fieldwork. Conserv. Sci. Pract. 2021, 3, e410. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.C.; Campbell Grant, E.H.; Coleman, J.T.H.; Reichard, J.D.; Gibbs, S.E.J.; Cryan, P.M.; Olival, K.J.; Walsh, D.P.; Blehert, D.S.; Hopkins, M.C.; et al. Assessing the Risks Posed by SARS-CoV-2 in and via North American Bats—Decision Framing and Rapid Risk Assessment; U.S. Geological Survey: Reston, VA, USA, 2020. [CrossRef]
- Hall, J.S.; Knowles, S.; Nashold, S.W.; Ip, H.S.; Leon, A.E.; Rocke, T.; Keller, S.; Carossino, M.; Balasuriya, U.; Hofmeister, E. Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS-CoV-2. Transbound. Emerg. Dis. 2021, 68, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.S.; Nashold, S.; Hofmeister, E.; Leon, A.E.; Falendysz, E.A.; Ip, H.S.; Malavé, C.M.; Rocke, T.E.; Carossino, M.; Balasuriya, U.; et al. Little Brown Bats (Myotis lucifugus) Are Resistant to SARS-CoV-2 Infection. J. Wildl. Dis. 2024, 60, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Hall, J.S.; Hofmeister, E.; Ip, H.S.; Nashold, S.W.; Leon, A.E.; Malavé, C.M.; Falendysz, E.A.; Rocke, T.E.; Carossino, M.; Balasuriya, U.; et al. Experimental Infection of Mexican Free-Tailed Bats (Tadarida brasiliensis) with SARS-CoV-2. mSphere 2023, 8, e00263-22. [Google Scholar] [CrossRef]
- Bálint, G.; Vörös-Horváth, B.; Széchenyi, A. Omicron: Increased transmissibility and decreased pathogenicity. Signal Transduct. Target. Ther. 2022, 7, 151. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Surendran-Nair, M.; Ruden, R.M.; Yon, M.; Nissly, R.H.; Vandegrift, K.J.; Nelli, R.K.; Li, L.; Jayarao, B.M.; Maranas, C.D.; et al. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc. Natl. Acad. Sci. USA 2022, 119, e2121644119. [Google Scholar] [CrossRef]
- Pickering, B.; Lung, O.; Maguire, F.; Kruczkiewicz, P.; Kotwa, J.D.; Buchanan, T.; Gagnier, M.; Guthrie, J.L.; Jardine, C.M.; Marchard-Austin, A.; et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat. Microbiol. 2022, 7, 2011–2024. [Google Scholar] [CrossRef]
- Munnink, B.B.O.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; Van Der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021, 371, 172. [Google Scholar] [CrossRef]
- Kabli, S.; Koschmann, J.R.; Robertstad, G.W.; Lawrence, J.; Ajello, L.; Redetzke, K. Endemic canine and feline histoplasmosis in El Paso, Texas. Med. Mycol. 1986, 24, 41–50. [Google Scholar] [CrossRef]

| Host Species | Age/Sex | Virus Name | Accession | Genome | Sequence Length (nt) 1 | Closest Match (Source, Location, Year, Accession) | % nt Similarity |
|---|---|---|---|---|---|---|---|
| Eptesicus fuscus | Geriatric female | Lacepfus virus (LCPV) | PV983329 | dsDNA | 31,279 | Canine mastadenovirus A (feces, Turkey, 2022, OQ596341) | 87.02 |
| Myotis sp. | Juvenile male | Bat adeno-associated virus 2259 | PV983328 | dsDNA | 4298 | Bat adeno-associated virus YNM (rectal swab, China, 2008, GU226971) | 77.09 |
| Myotis sp. | Juvenile male | Bat astrovirus 2259 | PV983331 | ssRNA(+) | 951 | Bat astrovirus BAstV/RB (guano, USA, 2020, MT734809) | 96.21 |
| Myotis sp. | Juvenile female | Bat genomovirus 2252 | PV983330 | ssDNA | 1303 | Chicken genomovirus mg4_1247 (tracheal swab, USA, 2017, MN379609) | 95.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weary, T.E.; Zhou, L.H.; MacDonald, L.; Ibañez IV, D.; Jaramillo, C.; Dunn, C.D.; Wright, T.F.; Hanley, K.A.; Goldberg, T.L.; Orr, T.J. Novel Bat Adenovirus Closely Related to Canine Adenoviruses Identified via Fecal Virome Surveillance of Bats in New Mexico, USA, 2020–2021. Viruses 2025, 17, 1349. https://doi.org/10.3390/v17101349
Weary TE, Zhou LH, MacDonald L, Ibañez IV D, Jaramillo C, Dunn CD, Wright TF, Hanley KA, Goldberg TL, Orr TJ. Novel Bat Adenovirus Closely Related to Canine Adenoviruses Identified via Fecal Virome Surveillance of Bats in New Mexico, USA, 2020–2021. Viruses. 2025; 17(10):1349. https://doi.org/10.3390/v17101349
Chicago/Turabian StyleWeary, Taylor E., Lawrence H. Zhou, Lauren MacDonald, Daniel Ibañez IV, Chance Jaramillo, Christopher D. Dunn, Timothy F. Wright, Kathryn A. Hanley, Tony L. Goldberg, and Teri J. Orr. 2025. "Novel Bat Adenovirus Closely Related to Canine Adenoviruses Identified via Fecal Virome Surveillance of Bats in New Mexico, USA, 2020–2021" Viruses 17, no. 10: 1349. https://doi.org/10.3390/v17101349
APA StyleWeary, T. E., Zhou, L. H., MacDonald, L., Ibañez IV, D., Jaramillo, C., Dunn, C. D., Wright, T. F., Hanley, K. A., Goldberg, T. L., & Orr, T. J. (2025). Novel Bat Adenovirus Closely Related to Canine Adenoviruses Identified via Fecal Virome Surveillance of Bats in New Mexico, USA, 2020–2021. Viruses, 17(10), 1349. https://doi.org/10.3390/v17101349






