Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spraying Assays
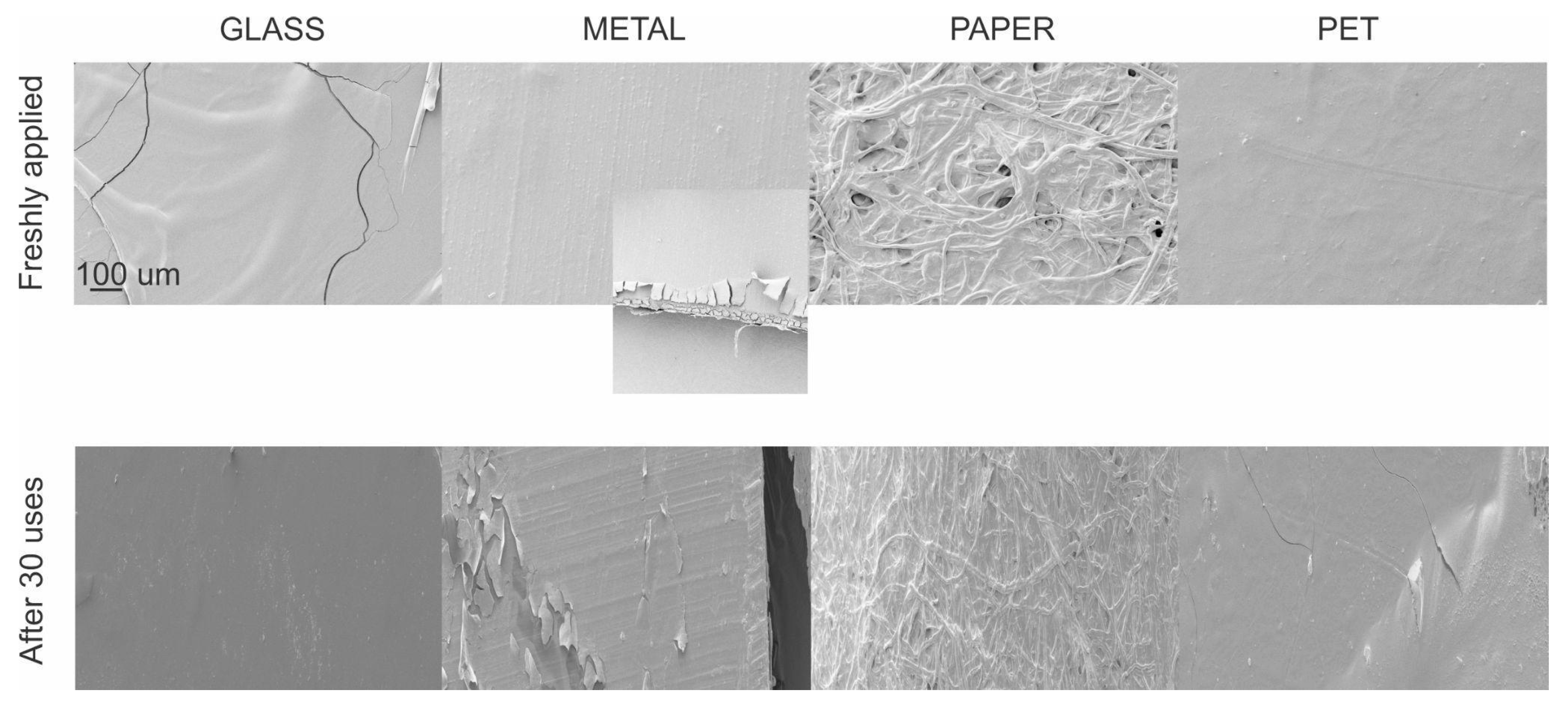
2.3. Scanning Electron Microscopy (SEM) Characterization
2.4. Cell Cultures
2.5. Virus Stocks
2.6. Virucidal Activity of Biopolymer
2.7. Virucidal Activity of Film-Coated Surfaces
2.7.1. Virucidal Activity of Several Film-Coated Surfaces
2.7.2. Kinetics of HSV-1 Inactivation in Films
2.7.3. Virucidal Activity of Film-Coated Surfaces After Repeated Inoculations
2.7.4. Virucidal Film Performance in Crowded Areas
2.8. Assessment of Antibacterial Activity of Formulation
2.9. Film Biosafety Analysis
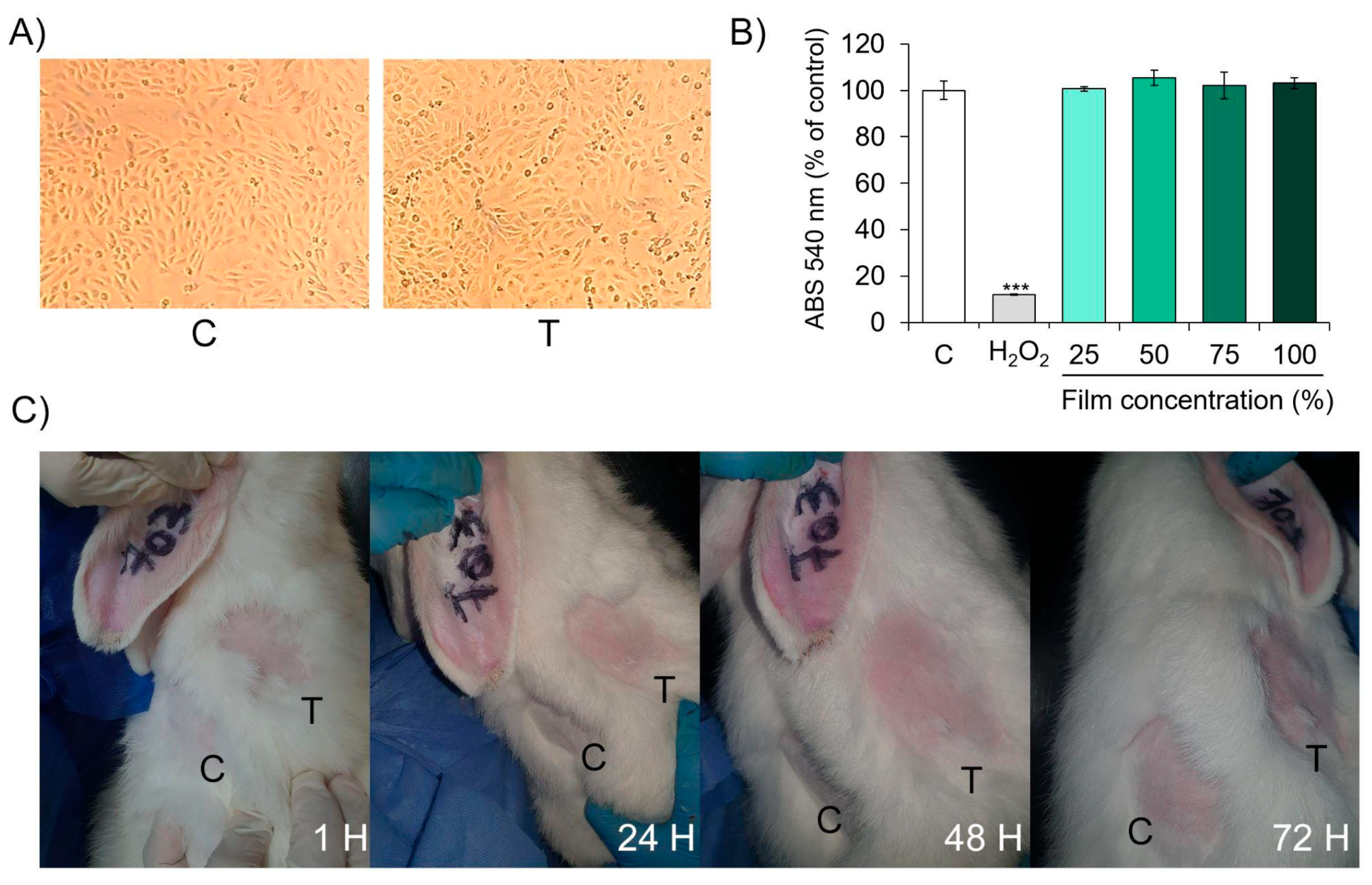
2.9.1. In Vitro Biocompatibility Evaluation
2.9.2. Estimation of Primary Dermal Irritation Index
2.10. Statistical Analysis
3. Results
3.1. Film CH.CA@Cu Coating Performance on Different Surfaces
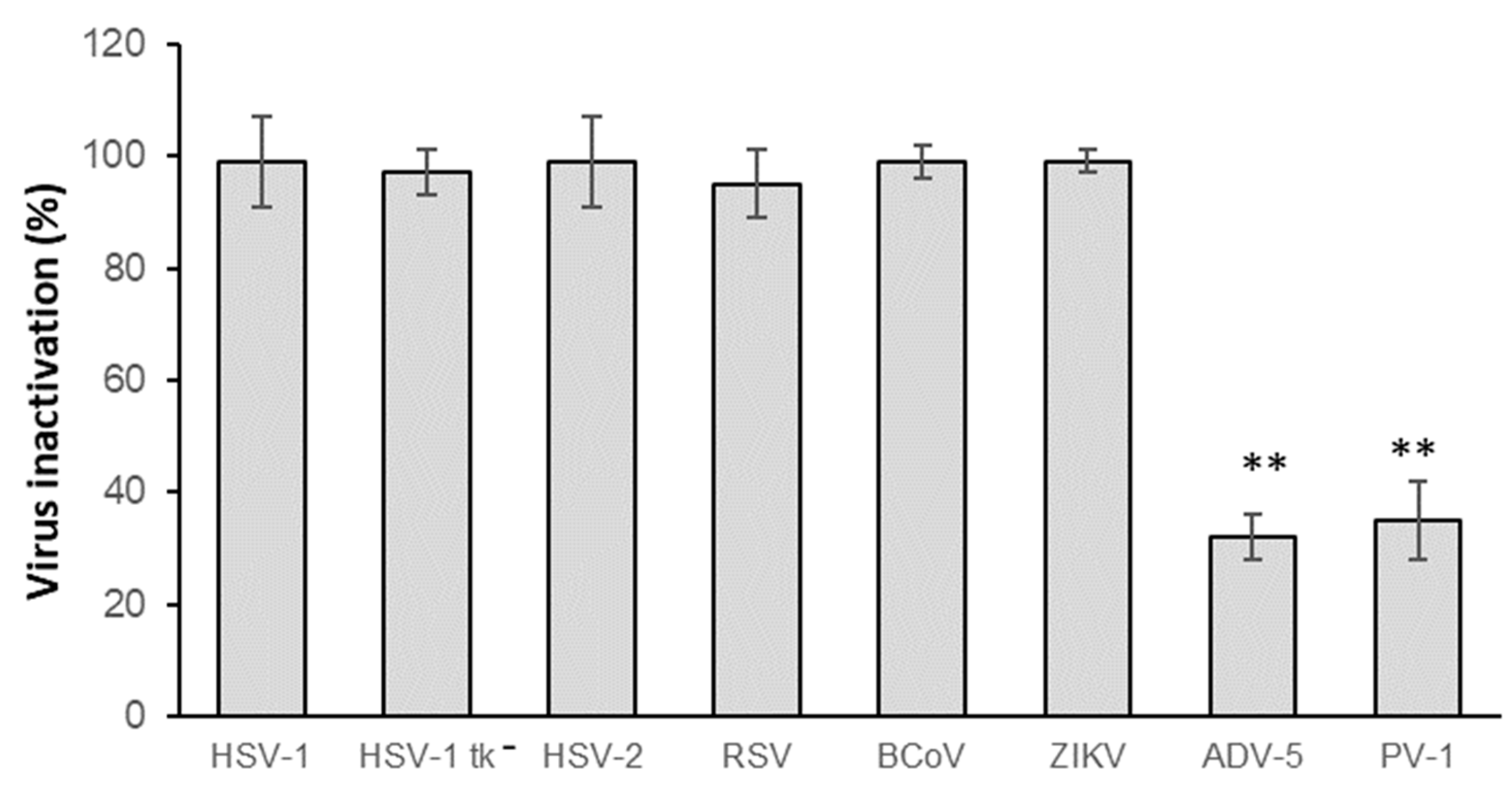
3.2. Virucidal Activity Spectrum of CH.CA@Cu Biopolymer Solution
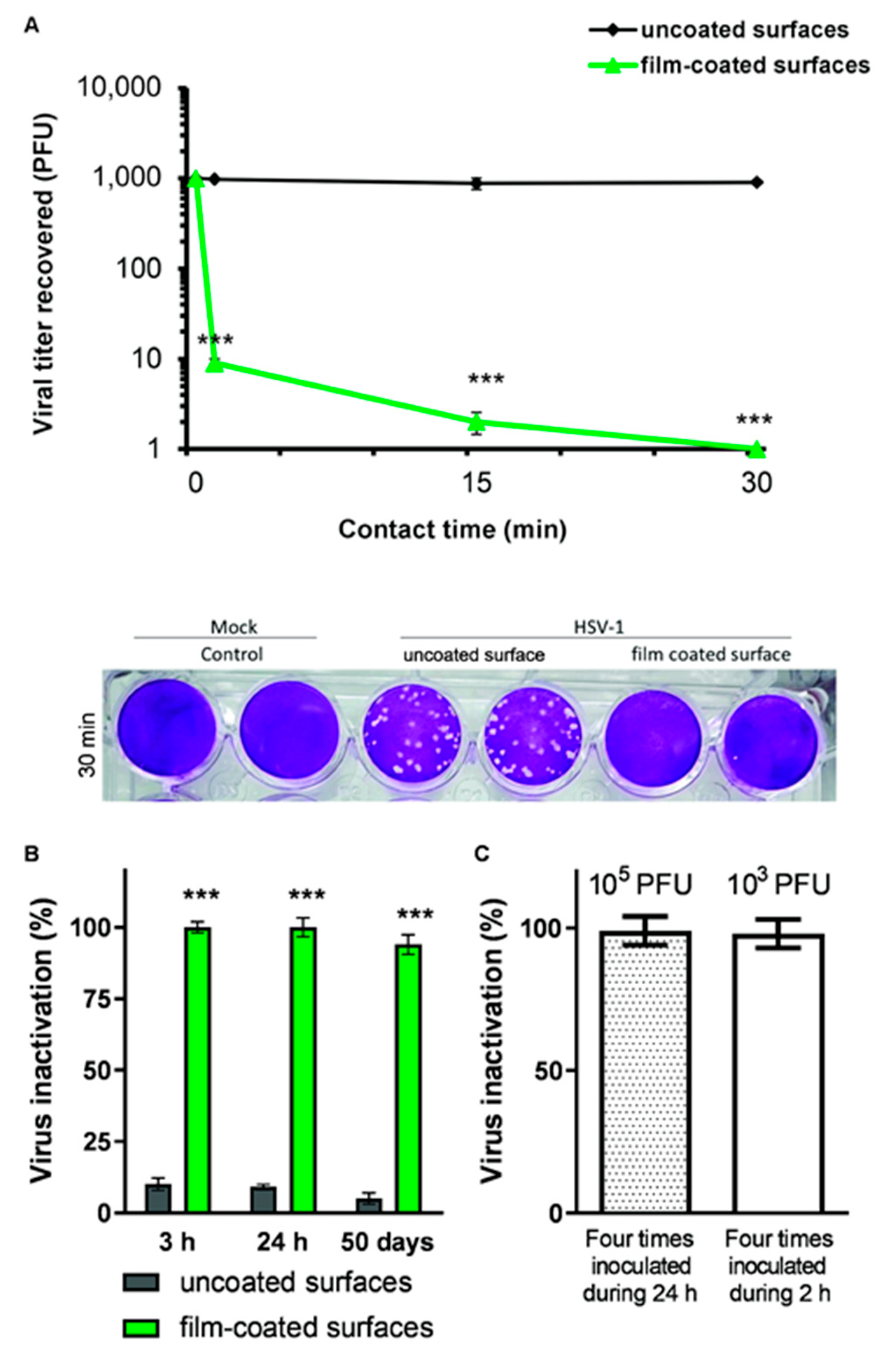
3.3. Persistence of Virucidal Activity on Spray-Coated Surfaces
3.4. Inactivating Performance After Repeated Inoculation
3.5. Virucidal Activity of Films Applied to Different Surfaces
3.6. Field Study of Virucidal Film Performance in Crowded Areas
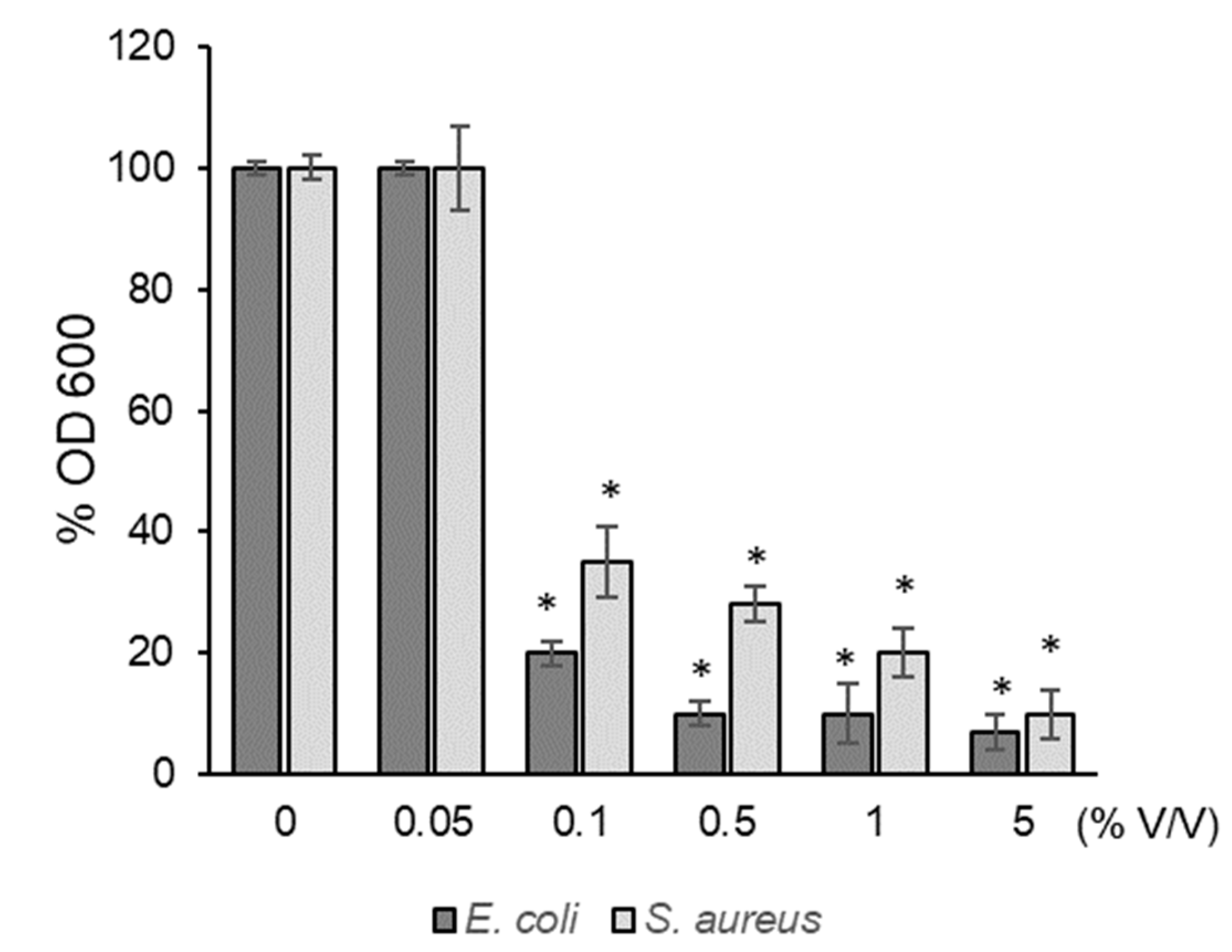
3.7. Antibacterial Activity
3.8. In Vitro Biocompatibility Evaluation
3.9. In Vivo Safety Analysis: Acute Dermal Irritation Index Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Citric acid |
| CH | Chitosan |
| Cu | Copper |
| PET | Polyethylene terephthalate |
| HSV-1 | Herpes simplex virus type 1 |
Appendix A
| Grade | Reactivity | Conditions of All Cultures |
|---|---|---|
| 0 | None | Discrete intracytoplasmic granules, no cell lysis, no reduction in cell growth. |
| 1 | Slight | Not more than 20 % of the cells are round, loosely attached and without intracytoplasmic granules, or show changes in morphology; occasional lysed cells are present; only slight growth inhibition observable. |
| 2 | Mild | Not more than 50% of the cells are round, devoid of intracytoplasmic granules, no extensive cell lysis; not more than 50% growth inhibition observable. |
| 3 | Moderate | Not more than 70% of the cell layers contain rounded cells or are lysed; cell layers not completely destroyed, but more than 50% growth inhibition observable. |
| 4 | Severe | Nearly complete or complete destruction of the cell layers. |
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- VanEpps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.; Sultan, O.; Mitchell, B.G.; Jenney, A.; Kiernan, M.; Brewster, D.J.; Russo, P.L. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Scoping Review. J. Hosp. Infect. 2024, 147, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-NCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Kwiecińska-Piróg, J.; Radtke, L.; Gospodarek-Komkowska, E.; Skowron, K. SARS-CoV-2 in the Environment—Non-Droplet Spreading Routes. Sci. Total Environ. 2021, 770, 145260. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Juthi, Z.T.; Jabeen, M.; Islam, M.R.; Biswas, P.; Ahmed, S. Biopolymer-Based Coating Materials for Antiviral and Antifungal Applications: Recent Advances in Formulations and Characterization. Chem. Eng. J. 2024, 498, 155000. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral Surfaces and Coatings and Their Mechanisms of Action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Chirkov, S.N. [The Antiviral Activity of Chitosan (Review)]. Prikl. Biokhim. Mikrobiol. 2002, 38, 5–13. [Google Scholar] [CrossRef]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the Immense Potential of Chitosan in Fighting a Wide Spectrum of Viruses: A Plausible Molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef]
- Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ku Ishak, K.M.; Shafiq, M.D.; Abdul Hamid, Z.A. Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers 2021, 13, 4234. [Google Scholar] [CrossRef]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the Antibacterial Activity of Silver, Zinc Oxide and Copper Oxide Nanoparticles against Poultry-Relevant Isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Favatela, M.F.; Otarola, J.; Ayala-Peña, V.B.; Dolcini, G.; Perez, S.; Torres Nicolini, A.; Alvarez, V.A.; Lassalle, V.L. Development and Characterization of Antimicrobial Textiles from Chitosan-Based Compounds: Possible Biomaterials Against SARS-CoV-2 Viruses. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1473–1486. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Jafari, R.; Momen, G. Design Strategies for Antiviral Coatings and Surfaces: A Review. Appl. Surf. Sci. Adv. 2022, 8, 100224. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Williams, M.D.; Hosseini, M.; Poon, L.L.M.; Chin, A.W.H.; Falkinham, J.O.; Ducker, W.A. Transparent and Sprayable Surface Coatings That Kill Drug-Resistant Bacteria Within Minutes and Inactivate SARS-CoV-2 Virus. ACS Appl. Mater. Interfaces 2021, 13, 54706–54714. [Google Scholar] [CrossRef] [PubMed]
- Das Jana, I.; Kumbhakar, P.; Banerjee, S.; Gowda, C.C.; Kedia, N.; Kuila, S.K.; Banerjee, S.; Das, N.C.; Das, A.K.; Manna, I.; et al. Copper Nanoparticle–Graphene Composite-Based Transparent Surface Coating with Antiviral Activity against Influenza Virus. ACS Appl. Nano. Mater. 2021, 4, 352–362. [Google Scholar] [CrossRef]
- Abe, K.; Sunada, K.; Mochizuki, Y.; Isobe, T.; Nagai, T.; Ishiguro, H.; Nakajima, A. Antibacterial and Antiviral Activities of Transparent PVA Coating Films Prepared by Using Solutions Containing Eluted Ions from Rare Earth Iodates. J. Coat. Technol. Res. 2025, 22, 471–480. [Google Scholar] [CrossRef]
- Ayala-Peña, V.B.; Martin, M.J.; Favatela, F.; Otarola, J.; Morán, P.; Ventura, M.; Gentili, C.; Salcedo, M.F.; Mansilla, A.; Pérez, S.; et al. Chitosan-Based Formulations Intended as Protective Spray for Mask Surfaces in Prevention of Coronavirus Dissemination. ChemistrySelect 2022, 7, e202202410. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Akbari, M.K.; Yadav, R.; Al-Tamimi, A.K.; Naebe, M. Fight against COVID-19: The Case of Antiviral Surfaces. APL Mater. 2021, 9, 031112. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 3 January 2022).
- Tardiff, R.G.; Hubner, R.P.; Gevecker Graves, C. Harmonization of Thresholds for Primary Skin Irritation from Results of Human Repeated Insult Patch Tests and Laboratory Animal Skin Irritation Tests. J. Appl. Toxicol. 2003, 23, 279–281. [Google Scholar] [CrossRef]
- Zanella, C.; Lekka, M.; Rossi, S.; Deflorian, F. Study of the Influence of Sonication during the Electrodeposition of Nickel Matrix Nanocomposite Coatings on the Protective Properties. Corros. Rev. 2011, 29, 253–260. [Google Scholar] [CrossRef]
- Arya, R.K.; Kaur, J.; Chandra, A.; Ahuja, S.; Rawat, M.; Sharma, J. Designing of Biodegradable Polycaprolactone: Binary and Ternary Coatings to Minimize the Defects and Cost of Solvent(s). J. Appl. Polym. Sci. 2021, 138, 50888. [Google Scholar] [CrossRef]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef]
- Nagarakanti, S.; Bishburg, E.; Bapat, A. Adenovirus, Herpes Simplex Virus and Cytomegalovirus Infection in a Lung Transplant Recipient. IDCases 2018, 11, 91–93. [Google Scholar] [CrossRef]
- Suissa, C.A.; Upadhyay, R.; Dabney, M.D.; Mack, R.J.; Masica, D.; Margulies, B.J. Investigating the Survival of Herpes Simplex Virus on Toothbrushes and Surrogate Phallic Devices. Int. J. STD AIDS 2023, 34, 152–158. [Google Scholar] [CrossRef]
- Castaño, N.; Cordts, S.C.; Kurosu Jalil, M.; Zhang, K.S.; Koppaka, S.; Bick, A.D.; Paul, R.; Tang, S.K.Y. Fomite Transmission, Physicochemical Origin of Virus–Surface Interactions, and Disinfection Strategies for Enveloped Viruses with Applications to SARS-CoV-2. ACS Omega 2021, 6, 6509–6527. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.A.; Gerba, C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl. Env. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef]
- Lopez, G.U.; Gerba, C.P.; Tamimi, A.H.; Kitajima, M.; Maxwell, S.L.; Rose, J.B. Transfer Efficiency of Bacteria and Viruses from Porous and Nonporous Fomites to Fingers under Different Relative Humidity Conditions. Appl. Env. Microbiol. 2013, 79, 5728–5734. [Google Scholar] [CrossRef]
- Müller, J.A.; Harms, M.; Schubert, A.; Jansen, S.; Michel, D.; Mertens, T.; Schmidt-Chanasit, J.; Münch, J. Inactivation and Environmental Stability of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1685–1687. [Google Scholar] [CrossRef]
- Brunner, G. Reactions of Synthetic Polymers with Water. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 511–523. [Google Scholar]
- Kramer, A.; Schwebke, I.; Kampf, G. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Milewska, A.; Kaminski, K.; Ciejka, J.; Kosowicz, K.; Zeglen, S.; Wojarski, J.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. HTCC: Broad Range Inhibitor of Coronavirus Entry. PLoS One 2016, 11, e0156552. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A Review of the Antiviral Activity of Chitosan, Including Patented Applications and Its Potential Use against COVID-19. J. Appl. Microbiol. 2022, 132, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.; Tezak, M. Variable Chain Length Carboxylic Acids as Modifiers to Enhance the Antiviral Efficacy of Sodium Dodecyl Sulfate; Sandia National Lab: Albuquerque, NM, USA, 2020. [Google Scholar]
- Cano-Vicent, A.; Tuñón-Molina, A.; Martí, M.; Serrano-Aroca, Á. Biocompatible Chitosan Films Containing Acetic Acid Manifested Potent Antiviral Activity against Enveloped and Non-Enveloped Viruses. Int. J. Mol. Sci. 2023, 24, 12028. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl Mater Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef]
- Goldman, E. Exaggerated Risk of Transmission of COVID-19 by Fomites. Lancet Infect. Dis. 2020, 20, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.U.; Colaneri, M.; Seminari, E.M.; Baldanti, F.; Bruno, R. Low Risk of SARS-CoV-2 Transmission by Fomites in Real-Life Conditions. Lancet Infect. Dis. 2021, 21, e112. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, A.; Dévora-Isiordia, G.; Sánchez-Duarte, R.; Villegas-Peralta, Y.; Orozco-Carmona, V.; Álvarez-Sánchez, J. Chitosan-Based Sustainable Coatings for Corrosion Inhibition of Aluminum in Seawater. Coatings 2023, 13, 1615. [Google Scholar] [CrossRef]
- Urmi, U.L.; Willcox, M.D.P.; Kuppusamy, R.; Attard, S.; Kumar, N.; Islam, S.; Chen, H.; Ren, X.; Vijay, A.K. Antiviral Activity of Peptide and Peptide Mimic Coated Surfaces. Appl. Surf. Sci. 2025, 702, 163242. [Google Scholar] [CrossRef]
- Prokhorov, E.; España-Sánchez, B.L.; Luna-Bárcenas, G.; Padilla-Vaca, F.; Cruz-Soto, M.-E.; Vázquez-Lepe, M.O.; Kovalenko, Y.; Elizalde-Peña, E.A. Chitosan/Copper Nanocomposites: Correlation between Electrical and Antibacterial Properties. Colloids Surf. B Biointerfaces 2019, 180, 186–192. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial Coatings Based on Chitosan to Prevent Implant-Associated Infections: A Systematic Review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Li, Y.; Eames, I.; Chan, P.K.S.; Ridgway, G.L. Factors Involved in the Aerosol Transmission of Infection and Control of Ventilation in Healthcare Premises. J. Hosp. Infect. 2006, 64, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.M.; Pavic, A. SALMONELLA | Introduction. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 322–331. [Google Scholar]
- Brüssow, H. ESCHERICHIA COLI | Detection of Enterotoxins of E. Coli. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 702–705. [Google Scholar]
- Schroeder, E.; Wuertz, S. Bacteria. In Handbook of Water and Wastewater Microbiology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 57–68. [Google Scholar]
- Ogungbesan, S.O.; Buxaderas, E.; Adedokun, R.A.; Moglie, Y.; Grijalvo, S.; García, M.T.; Bingbing, C.; Díaz Díaz, D.; Fu, G. Synthesis and Characterization of Chitosan–Silver Nanocomposite Film: Antibacterial and Cytotoxicity Study. ChemistrySelect 2024, 9, e202404909. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan Nanoparticle Toxicity: A Comprehensive Literature Review of in Vivo and in Vitro Assessments for Medical Applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef]
| HSV-1 Titer Reduction [%] | ||
|---|---|---|
| Treated Material | 1 min | 30 min |
| PET | 99.5 ± 0.7 | 99.9 ± 0.9 |
| Stainless steel | 95.1 ± 0.3 | 99.0 ± 0.1 |
| Nickel-plated steel | 99.9 ± 0.1 | 100.0 ± 0.2 |
| Aluminum | 56.0 ± 0.6 | 60.0 ± 0.1 |
| Wood | 99.6 ± 1.1 | 100.1 ± 0.1 |
| Leather | 99.8 ± 0.1 | 99.8 ± 1.1 |
| Cotton fabric | 90.1 ± 3.1 | 95.0 ± 0.2 |
| Glass | 98.1 ± 1.1 | 99.6 ± 2.6 |
| HSV-1 Titer Reduction (%) | ||
|---|---|---|
| Health Unit Primary Care | 16 h | 40 h |
| Eco-leather from chairs | 90.9 ± 0.2 | 95.0 ± 2.1 |
| Wood table reception | 97.8 ± 0.5 | 100.2 ± 1.2 |
| Glass window | 97.2 ± 0.7 | 100.0 ± 0.3 |
| Quartz countertops | 99.5 ± 1.2 | 98.1 ± 0.5 |
| Stretcher | 98.1 ± 0.5 | 96.8 ± 1.8 |
| Disposable friselina gown | 92.3 ± 1.2 | 99.5 ± 4.2 |
| Surgical mask | 98.3 ± 2.1 | 95.6 ± 0.5 |
| Buses (public transport) | 8 h | |
| Eco-leather from chairs | 99.5 ± 0.9 | |
| Glass windows | 99.8 ± 1.1 | |
| Stainless steel railings | 99.2 ± 1.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Peña, V.B.; Martin, M.J.; Otarola, J.; Favatela, F.; Gonzalez, J.S.; Conesa, A.L.; García, C.C.; Sepúlveda, C.S.; Alvarez, V.A.; Lassalle, V.L. Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces. Viruses 2025, 17, 1347. https://doi.org/10.3390/v17101347
Ayala-Peña VB, Martin MJ, Otarola J, Favatela F, Gonzalez JS, Conesa AL, García CC, Sepúlveda CS, Alvarez VA, Lassalle VL. Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces. Viruses. 2025; 17(10):1347. https://doi.org/10.3390/v17101347
Chicago/Turabian StyleAyala-Peña, Victoria Belen, María Julia Martin, Jessica Otarola, Florencia Favatela, Jimena Soledad Gonzalez, Ana Lucía Conesa, Cybele Carina García, Claudia Soledad Sepúlveda, Vera Alejandra Alvarez, and Verónica Leticia Lassalle. 2025. "Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces" Viruses 17, no. 10: 1347. https://doi.org/10.3390/v17101347
APA StyleAyala-Peña, V. B., Martin, M. J., Otarola, J., Favatela, F., Gonzalez, J. S., Conesa, A. L., García, C. C., Sepúlveda, C. S., Alvarez, V. A., & Lassalle, V. L. (2025). Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces. Viruses, 17(10), 1347. https://doi.org/10.3390/v17101347







