BCG Immunotherapy in Equine Sarcoid Treatment: Mechanisms, Clinical Efficacy, and Challenges in Veterinary Oncology
Abstract
1. Introduction
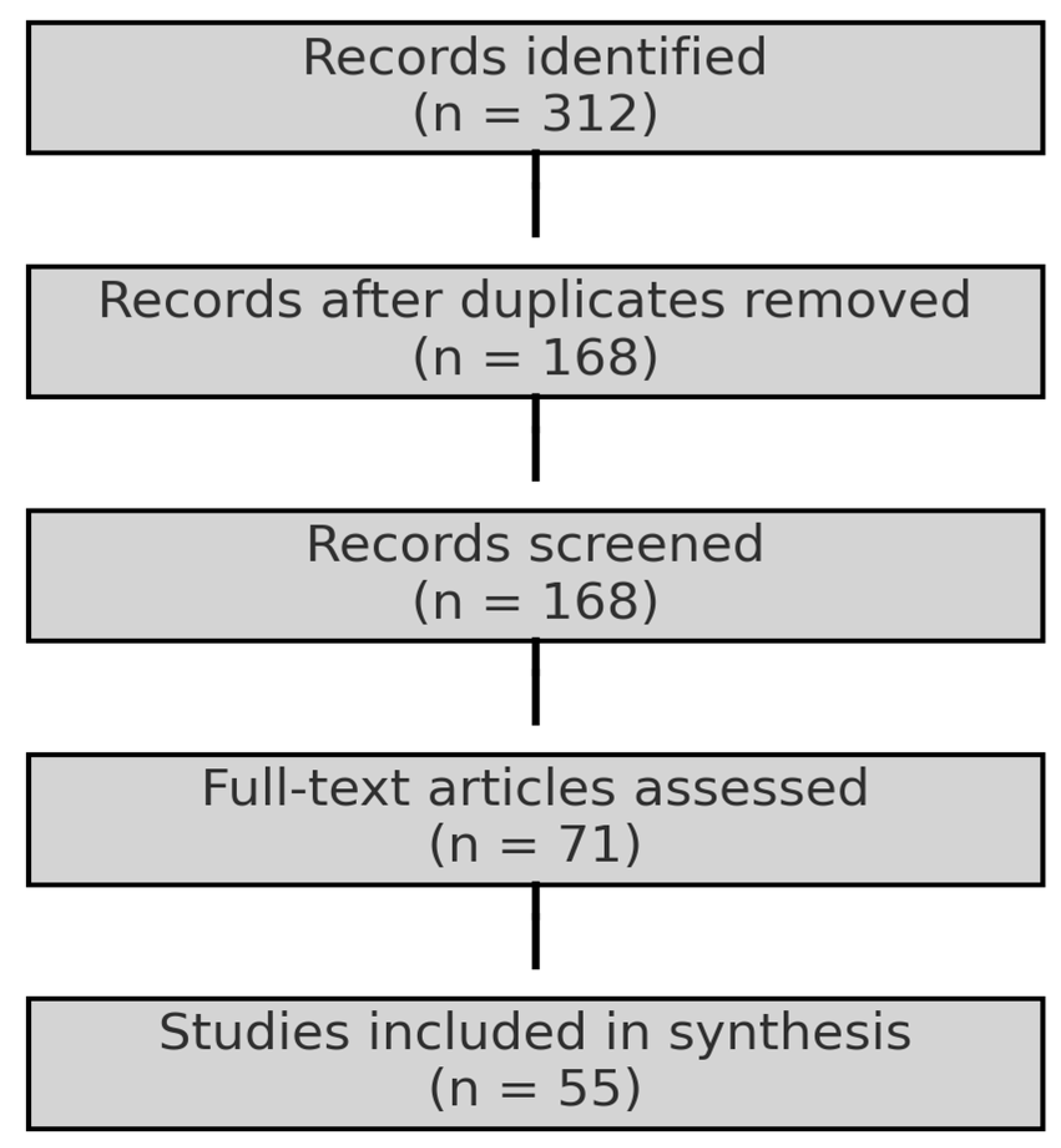
2. Materials and Methods
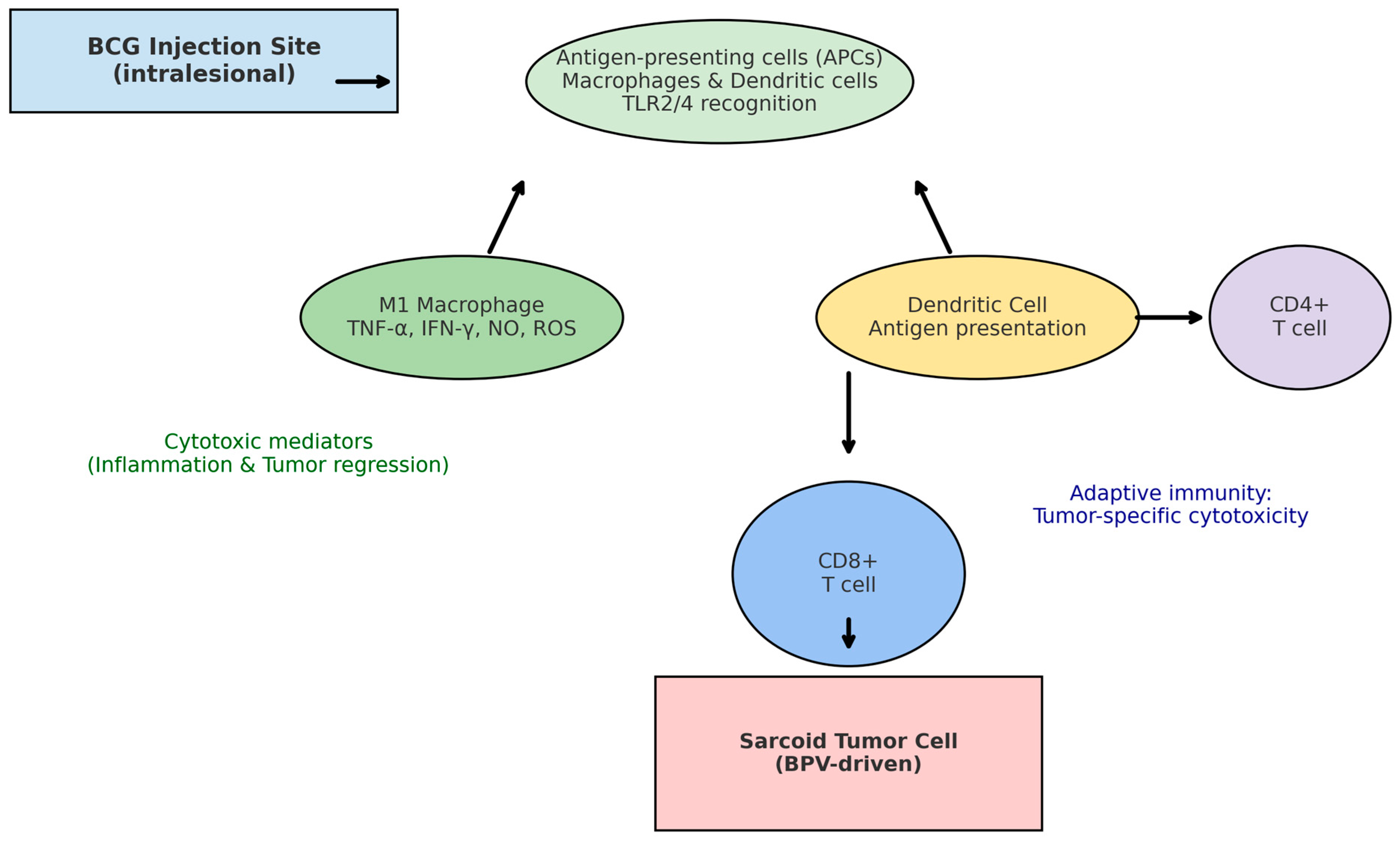
3. Immunological Mechanisms of BCG in Horses
4. Clinical Efficacy and Limitations of BCG
5. Opportunities in Comparative Oncology
6. Integration with the One Health Approach
7. Epidemiology and BCG Use in the Amazon Biome
8. Cost–Benefit Comparison of BCG vs. Conventional Therapies
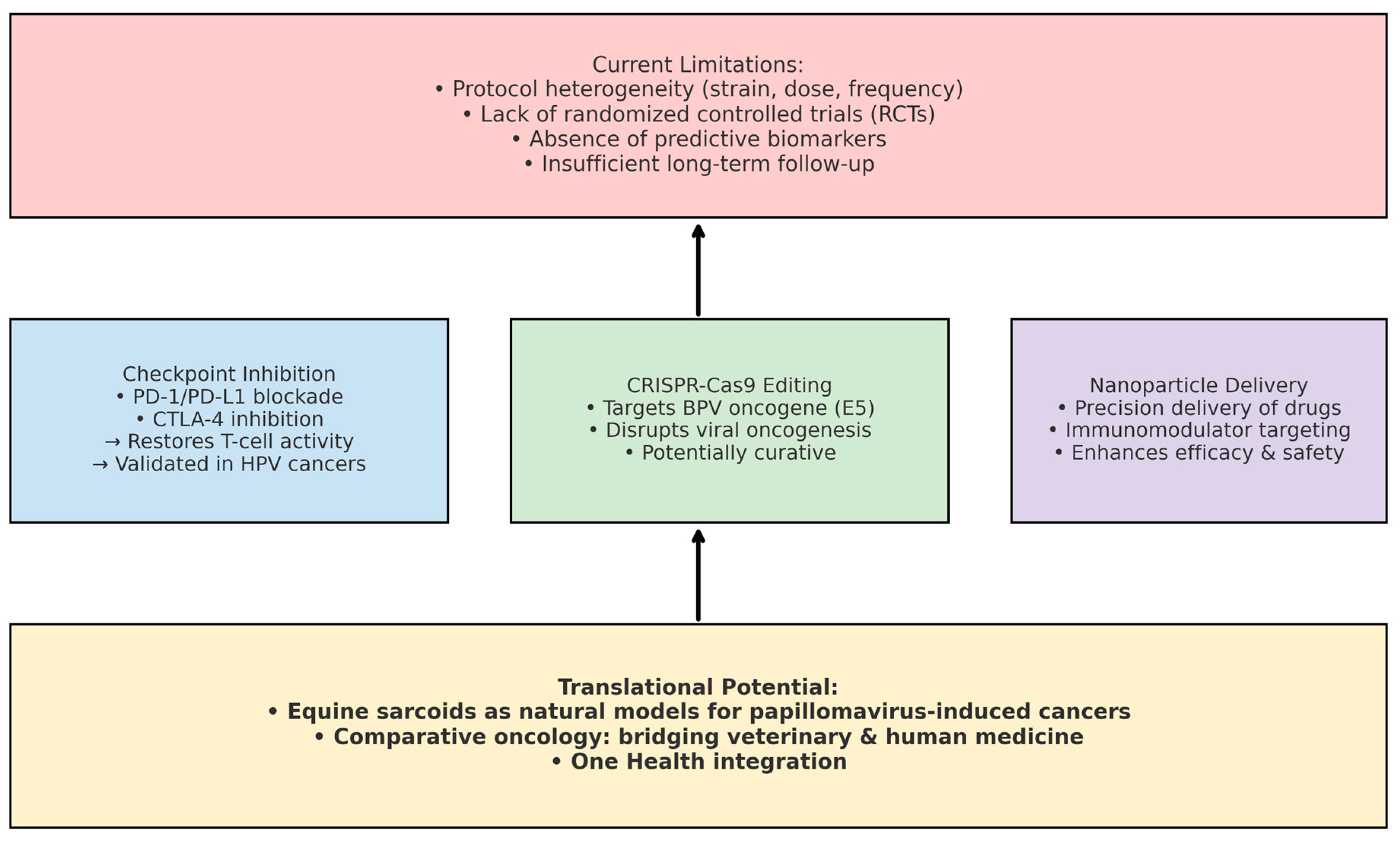
9. Future Directions, Gaps, and Novel Insights
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodrich, L.; Gerber, H.; Marti, E.; Antczak, D.F. Equine Sarcoids. Vet. Clin. N. Am. Equine Pract. 1998, 14, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Hollis, A.R. Management of Equine Sarcoids. Vet. J. 2023, 291, 105926. [Google Scholar] [CrossRef]
- Ogłuszka, M.; Starzyński, R.R.; Pierzchała, M.; Otrocka-Domagała, I.; Raś, A. Equine Sarcoids—Causes, Molecular Changes, and Clinicopathologic Features: A Review. Vet. Pathol. 2021, 58, 472–482. [Google Scholar] [CrossRef]
- Taylor, S.; Haldorson, G. A Review of Equine Sarcoid. Equine Vet. Educ. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Tamzali, Y.; Borde, L.; Rols, M.P.; Golzio, M.; Lyazrhi, F.; Teissie, J. Successful Treatment of Equine Sarcoids with Cisplatin Electrochemotherapy: A Retrospective Study of 48 Cases. Equine Vet. J. 2012, 44, 214–220. [Google Scholar] [CrossRef]
- Byam-Cook, K.L.; Henson, F.M.; Slater, J.D. Treatment of Periocular and Non-Ocular Sarcoids in 18 Horses by Interstitial Brachytherapy with Iridium-192. Vet. Rec. 2006, 159, 337–341. [Google Scholar] [CrossRef]
- Altamura, G.; Strazzullo, M.; Corteggio, A.; Francioso, R.; Roperto, F.; D’Esposito, M.; Roperto, S. O(6)-Methylguanine-DNA Methyltransferase in Equine Sarcoids: Molecular and Epigenetic Analysis. BMC Vet. Res. 2012, 8, 218. [Google Scholar] [CrossRef]
- Jindra, C.; Hainisch, E.K.; Brandt, S. Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines. Vaccines 2023, 11, 769. [Google Scholar] [CrossRef]
- Offer, K.S.; Dixon, C.E.; Sutton, D.G.M. Treatment of Equine Sarcoids: A Systematic Review. Equine Vet. J. 2024, 56, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, B.A.; Abetz, I.; Jackson, A.R. BCG Emulsion Immunotherapy of Equine Sarcoid. Equine Vet. J. 1988, 20, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; De Moor, A.; Vlaminck, L.; Pille, F.; Steenhaut, M. Evaluation of Excision, Cryosurgery and Local BCG Vaccination for the Treatment of Equine Sarcoids. Vet. Rec. 2001, 149, 665–669. [Google Scholar] [CrossRef]
- Compston, P.C.; Turner, T.; Wylie, C.E.; Payne, R.J. Laser Surgery as a Treatment for Histologically Confirmed Sarcoids in the Horse. Equine Vet. J. 2016, 48, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.R.; Bras, G.E.; Misdorp, W.; Steerenberg, P.A.; de Jong, W.H.; Tiesjema, R.H.; Kersjes, A.W.; Ruitenberg, E.J. Equine Sarcoid: BCG Immunotherapy Compared to Cryosurgery in a Prospective Randomised Clinical Trial. Cancer Immunol. Immunother. 1986, 21, 133–140. [Google Scholar] [CrossRef]
- Carvalho, M.C.P.; Almeida, G.B.; Vieira, R.R.; Oliveira, A.F.; Silva, F.L. Imunomodulação com vacina BCG para tratamento de sarcoide em um equino. In Proceedings of the XI Simpósio Internacional do Cavalo Atleta, Belo Horizonte, MG, Brazil, 11–13 May 2023; Available online: https://www.even3.com.br/anais/xi-simcav-304492/623917-IMUNOMODULACAO-COM-VACINA-BCG-PARA-TRATAMENTO-DE--SARCOIDE-EM-UM-EQUINO (accessed on 9 April 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lidagoster, S.; Ben-David, R.; De Leon, B.; Sfakianos, J.P. BCG and Alternative Therapies to BCG Therapy for Non-Muscle-Invasive Bladder Cancer. Curr. Oncol. 2024, 31, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Paixão, E.; Trajman, A.; de Souza, R.A.; da Natividade, M.S.; Pescarini, J.M.; Pereira, S.M.; Barreto, F.R.; Ximenes, R.; Dalcomo, M.; et al. The Need for Fast-Track, High-Quality and Low-Cost Studies about the Role of the BCG Vaccine in the Fight against COVID-19. Respir. Res. 2020, 21, 178. [Google Scholar] [CrossRef]
- Hannouneh, Z.A.; Hijazi, A.; Alsaleem, A.A.; Hami, S.; Kheyrbek, N.; Tanous, F.; Khaddour, K.; Abbas, A.; Alshehabi, Z. Novel immuno-therapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med. 2023, 12, 21944–21968. [Google Scholar] [CrossRef]
- Sultan, F.; Ganaie, B.A. Comparative Oncology: Integrating Human and Veterinary Medicine. Open Vet. J. 2018, 8, 25–34. [Google Scholar] [CrossRef]
- Youssef, E.; Palmer, D.; Fletcher, B.; Vaughn, R. Exosomes in Precision Oncology and Beyond: From Bench to Bedside in Diagnostics and Therapeutics. Cancers 2025, 17, 940. [Google Scholar] [CrossRef]
- Kader, M.; Smith, A.P.; Guiducci, C.; Wonderlich, E.R.; Normolle, D.; Watkins, S.C.; Barrat, F.J.; Barratt-Boyes, S.M. Blocking TLR7- and TLR9-Mediated IFN-α Production by Plasmacytoid Dendritic Cells Does Not Diminish Immune Activation in Early SIV Infection. PLoS Pathog. 2013, 9, e1003530. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The Future of Cancer Immunotherapy: DNA Vaccines Leading the Way. Med. Oncol. 2023, 40, 200. [Google Scholar] [CrossRef]
- Semik, E.; Gurgul, A.; Ząbek, T.; Ropka-Molik, K.; Koch, C.; Mählmann, K.; Bugno-Poniewierska, M. Transcriptome analysis of equine sarcoids. Vet. Comp. Oncol. 2017, 15, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Verkuijl, C.; Smit, J.; Green, J.M.H.; Nordquist, R.E.; Sebo, J.; Hayek, M.N.; Hötzel, M.J. Climate change, public health, and animal welfare: Towards a One Health approach to reducing animal agriculture’s climate footprint. Front. Anim. Sci. 2024, 5, 1281450. [Google Scholar] [CrossRef]
- Danasekaran, R. One Health: A Holistic Approach to Tackling Global Health Issues. Indian J. Community Med. 2024, 49, 260–263. [Google Scholar] [CrossRef]
- Feng, X.; Li, Z.; Liu, Y.; Chen, D.; Zhou, Z. CRISPR/Cas9 technology for advancements in cancer immunotherapy: From uncovering regulatory mechanisms to therapeutic applications. Exp. Hematol. Oncol. 2024, 13, 102. [Google Scholar] [CrossRef]
- Coman, M.A.; Marcu, A.; Chereches, R.M.; Leppälä, J.; Van Den Broucke, S. Educational Interventions to Improve Safety and Health Literacy Among Agricultural Workers: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1114. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.A.; Torres, S.M.; Malone, E.D.; Diaz, S.F.; Jessen, C.; Gilbert, S. Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: A pilot study. Vet. Dermatol. 2006, 17, 259–265. [Google Scholar] [CrossRef]
- Pagliusi, S.; Che, Y.; Dong, S. The art of partnerships for vaccines. Vaccine 2019, 37, 5909–5919. [Google Scholar] [CrossRef] [PubMed]
- Lorga, A.D.; Amaro, F.; Gomes, A.R.C.; Cocco, M.; Gularte, A.; Silva, Y.; Silva, J. Application of Euphorbia tirucalli Sap in Sarcoid Treatment in Horses—Case Report. Arq. Bras. Med. Vet. Zootec. 2022, 74, 509–513. [Google Scholar] [CrossRef]
- Knottenbelt, D.C.; Kelly, D.F. The Diagnosis and Treatment of Periorbital Sarcoid in the Horse: 445 Cases from 1974 to 1999. Vet. Ophthalmol. 2000, 3, 169–191. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. The Equine Sarcoid: Why Are There So Many Treatment Options? Vet. Clin. N. Am. Equine Pract. 2019, 35, 243–262. [Google Scholar] [CrossRef]
- Brum, J.S.; Souza, T.M.; Barros, C.S.L. Aspectos epidemiológicos e distribuição anatômica das diferentes formas clínicas do sarcoide equino no Rio Grande do Sul: 40 casos. Pesqui. Vet. Bras. 2010, 30, 839–843. [Google Scholar] [CrossRef]
- Anjos, B.L.; Silva, M.S.; Diefenbach, A.; Brito, M.F.; Seppa, G.S.; Brum, M.C.S. Sarcoide equino associado ao papilomavírus bovino BR-UEL-4. Ciênc. Rural 2010, 40, 1456–1459. [Google Scholar] [CrossRef]
- Alcântara, B.K.; Alfieri, A.A.; Headley, S.A.; Rodrigues, W.B.; Otonel, R.A.A.; Lunardi, M.; Alfieri, A.F. Caracterização molecular de DNA de Delta papillomavirus bovino (BPV1, 2–13) em sarcoides equinos. Pesqui. Vet. Bras. 2015, 35, 431–436. [Google Scholar] [CrossRef]
- Munday, J.S.; Orbell, G.; Fairley, R.A.; Hardcastle, M.; Vaatstra, B. Evidence from a Series of 104 Equine Sarcoids Suggests That Most Sarcoids in New Zealand are Caused by Bovine Papillomavirus Type 2, although Both BPV1 and BPV2 DNA are Detectable in around 10% of Sarcoids. Animals 2021, 11, 3093. [Google Scholar] [CrossRef]
- Karalus, W.; Subharat, S.; Orbell, G.; Vaatstra, B.; Munday, J.S. Equine Sarcoids: A Clinicopathologic Study of 49 Cases, with Mitotic Count and Clinical Type Predictive of Recurrence. Vet. Pathol. 2024, 61, 357–365. [Google Scholar] [CrossRef]
- Bogaert, L.; Martens, A.; Van Poucke, M.; Ducatelle, R.; De Cock, H.; Dewulf, J.; De Baere, C.; Peelman, L.; Gasthuys, F. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses. Vet. Microbiol. 2008, 129, 58–68. [Google Scholar] [CrossRef]
- McConaghy, F.F.; Davis, R.E.; Reppas, G.P.; Rawlinson, R.J.; McClintock, S.A.; Hutchins, D.R.; Hodgson, D.R. Management of equine sarcoids: 1975–1993. N. Z. Vet. J. 1994, 42, 180–184. [Google Scholar] [CrossRef]
- Horefti, E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens 2023, 12, 977. [Google Scholar] [CrossRef]
- Stewart, A.A.; Rush, B.; Davis, E. The efficacy of intratumoural 5-fluorouracil for the treatment of equine sarcoids. Aust. Vet. J. 2006, 84, 101–106. [Google Scholar] [CrossRef]
- Stadler, S.; Kainzbauer, C.; Haralambus, R.; Brehm, W.; Hainisch, E.; Brandt, S. Successful treatment of equine sarcoids by topical aciclovir application. Vet. Rec. 2011, 168, 187. [Google Scholar] [CrossRef]
- Walker, M.; Adams, W.; Hoskinson, J.; Held, J.P.; Blackford, J.; Geiser, D.; Henton, J. Iridium-192 brachytherapy for equine sarcoid, one and two year remission rates. Vet. Radiol. 1991, 32, 206–208. [Google Scholar] [CrossRef]
- Spoormakers, T.J.; Klein, W.R.; Jacobs, J.J.; Van Den Ingh, T.S.; Koten, J.W.; Den Otter, W. Comparison of the efficacy of local treatment of equine sarcoids with IL-2 or cisplatin/IL-2. Cancer Immunol. Immunother. 2003, 52, 179–184. [Google Scholar] [CrossRef]
- Berruex, F.; Gerber, V.; Wohlfender, F.D.; Burger, D.; Koch, C. Clinical course of sarcoids in 61 Franches-Montagnes horses over a 5–7 year period. Vet. Q. 2016, 36, 189–196. [Google Scholar] [CrossRef]
- Assis-Brasil, N.D.; Marcolongo-Pereira, C.; Stigger, A.L.; Fiss, L.; Santos, B.L.; Coelho, A.C.B.; Sallis, E.S.V.; Fernandes, C.G.; Schild, A.L. Equine Dermatopathies in Southern Brazil: A Study of 710 Cases. Ciênc. Rural 2015, 45, 519–524. [Google Scholar] [CrossRef]
- Baust, J.G.; Gage, A.A. The Molecular Basis of Cryosurgery. BJU Int. 2005, 95, 1187–1191. [Google Scholar] [CrossRef]
- Schaffer, P.A.; Wobeser, B.; Martin, L.E.; Dennis, M.M.; Duncan, C.G. Cutaneous Neoplastic Lesions of Equids in the Central United States and Canada: 3,351 Biopsy Specimens from 3,272 Equids (2000–2010). J. Am. Vet. Med. Assoc. 2013, 242, 99–104. [Google Scholar] [CrossRef]
- Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.; Newton, J.R. Preventive Health Care and Owner-Reported Disease Prevalence of Horses and Ponies in Great Britain. Res. Vet. Sci. 2013, 95, 418–424. [Google Scholar] [CrossRef]
- Aminin, D.; Wang, Y.M. Macrophages as a “Weapon” in Anticancer Cellular Immunotherapy. Kaohsiung J. Med. Sci. 2021, 37, 749–758. [Google Scholar] [CrossRef]
- Perez-Penco, M.; Byrdal, M.; Lara de la Torre, L.; Ballester, M.; Khan, S.; Siersbæk, M.; Lecoq, I.; Madsen, C.O.; Kjeldsen, J.W.; Svane, I.M.; et al. The Antitumor Activity of TGFβ-Specific T Cells Is Dependent on IL-6 Signaling. Cell Mol. Immunol. 2025, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Collinet, A.; Grimm, P.; Jacotot, E.; Julliand, V. Biomarkers for monitoring the equine large intestinal inflammatory response to stress-induced dysbiosis and probiotic supplementation. J. Anim. Sci. 2022, 100, skac268. [Google Scholar] [CrossRef] [PubMed]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Okesanya, O.J.; Othman, Z.K.; Ibrahim, A.M.; Adigun, O.A.; Ukoaka, B.M.; Abdi, M.I.; Lucero-Prisno, D.E. Holistic Approaches to Zoonoses: Integrating Public Health, Policy, and One Health in a Dynamic Global Context. Zoonotic Dis. 2025, 5, 5. [Google Scholar] [CrossRef]
- Singh, S.; Saavedra-Avila, N.A.; Tiwari, S.; Porcelli, S.A. A Century of BCG Vaccination: Immune Mechanisms, Animal Models, Non-Traditional Routes and Implications for COVID-19. Front. Immunol. 2022, 13, 959656. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Efficacy | Recurrence | Sessions | Cost (Relative) | Accessibility | Key Limitations |
|---|---|---|---|---|---|---|
| BCG Immunotherapy | 60–70% | 15–30% | 1–3 | Low (10× cheaper than cisplatin) | High (basic training needed) | Adverse reactions (30%) |
| Topical Cisplatin | 70–85% | 20–40% | 4–6 | High | Low (requires biosafety protocols) | Toxicity, logistical complexity |
| Surgical Excision | 50–80% | 20–40% | 1 | Moderate | Moderate (needs infrastructure) | High recurrence, postoperative care |
| Cryotherapy | 60–75% | 15–35% | 2–4 | Moderate | Low (requires specialized equipment) | Limited to accessible lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.M.; de Castro, E.L.A.; Pereira, A.J.M.; Thiesen, R.; Thiesen, R.M.C.; Salvarani, F.M. BCG Immunotherapy in Equine Sarcoid Treatment: Mechanisms, Clinical Efficacy, and Challenges in Veterinary Oncology. Viruses 2025, 17, 1322. https://doi.org/10.3390/v17101322
Monteiro MM, de Castro ELA, Pereira AJM, Thiesen R, Thiesen RMC, Salvarani FM. BCG Immunotherapy in Equine Sarcoid Treatment: Mechanisms, Clinical Efficacy, and Challenges in Veterinary Oncology. Viruses. 2025; 17(10):1322. https://doi.org/10.3390/v17101322
Chicago/Turabian StyleMonteiro, Mariana Martins, Elcidimar Lucas Aleixo de Castro, Ana Júlia Moaraes Pereira, Roberto Thiesen, Roberta Martins Crivelaro Thiesen, and Felipe Masiero Salvarani. 2025. "BCG Immunotherapy in Equine Sarcoid Treatment: Mechanisms, Clinical Efficacy, and Challenges in Veterinary Oncology" Viruses 17, no. 10: 1322. https://doi.org/10.3390/v17101322
APA StyleMonteiro, M. M., de Castro, E. L. A., Pereira, A. J. M., Thiesen, R., Thiesen, R. M. C., & Salvarani, F. M. (2025). BCG Immunotherapy in Equine Sarcoid Treatment: Mechanisms, Clinical Efficacy, and Challenges in Veterinary Oncology. Viruses, 17(10), 1322. https://doi.org/10.3390/v17101322







