Anti-SARS-CoV-2 Antibody Development over Four Years in Blood Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Studies of Pre-Pandemic Blood Donor Samples
2.2. Seroprevalence Study over Nearly Four Years
2.2.1. Donor Population
2.2.2. Serological Analysis
2.2.3. Measures Ordered by the Federal Office of Public Health (FOPH)
2.2.4. Vaccination Strategy in Switzerland
2.2.5. Ethics Committee
3. Results
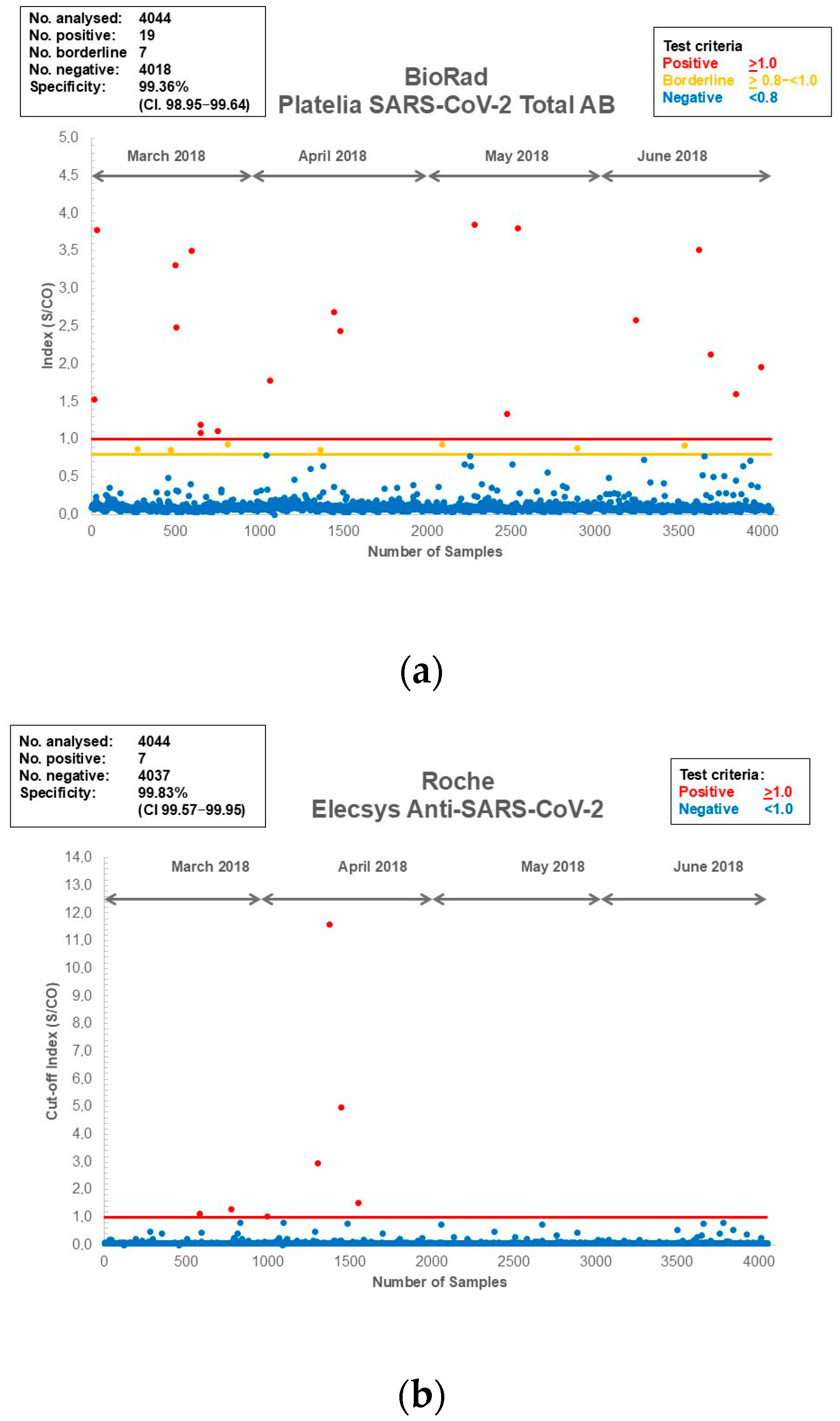
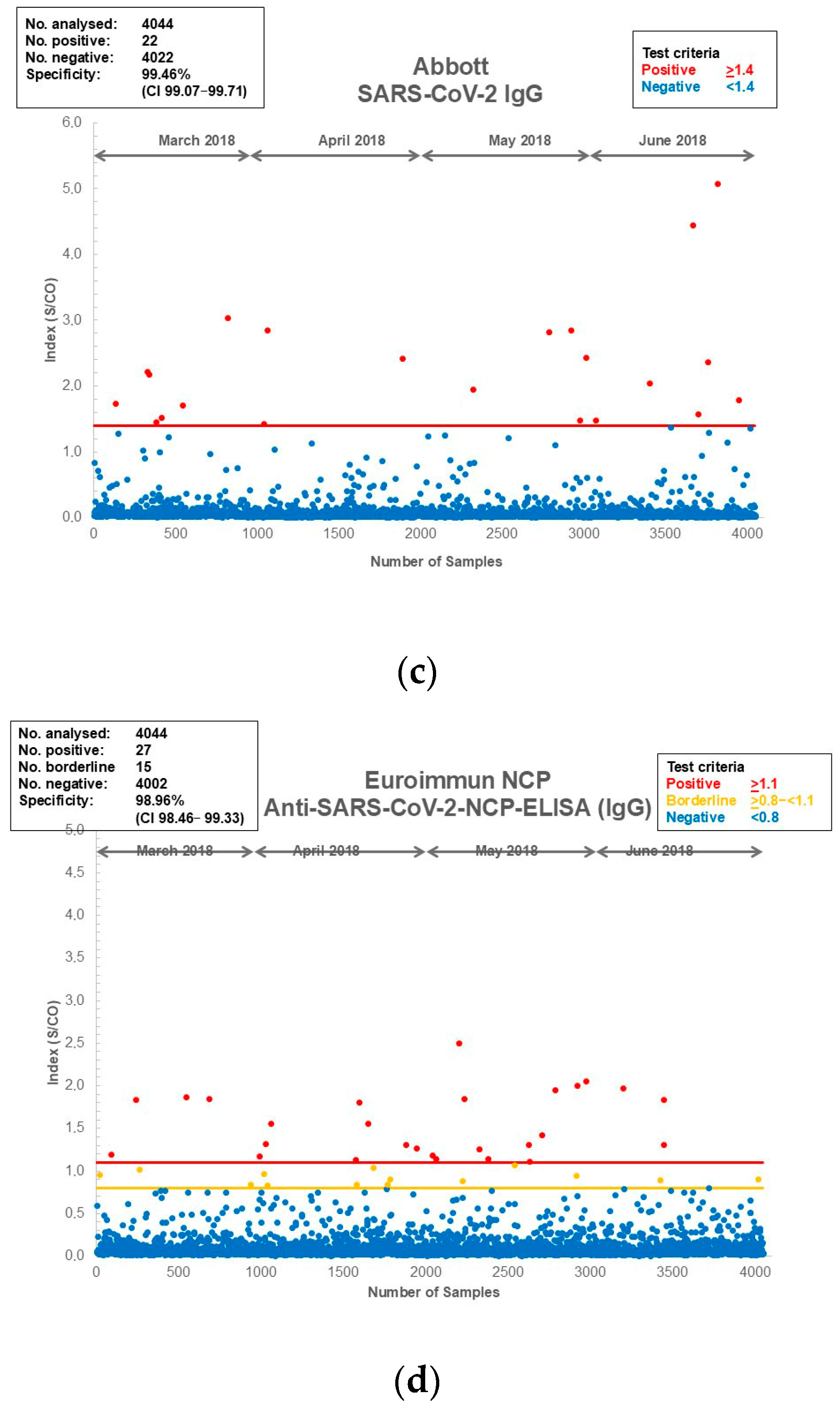
3.1. Assay Specificity and Sensitivity of the Assays Used for the Current Study
3.2. Seroprevalence Study Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Ethics Commission |
| ECLIA | Electrochemiluminescence Immunoassay |
| FOPH | Federal Office of Public Health |
| NCP | Nucleocapsid Protein |
| MOC | Multiple Over Cutoff |
| S | S Protein |
Appendix A

References
- Scire, J.; Nadeau, S.; Vaughan, T.; Brupbacher, G.; Fuchs, S.; Sommer, J.; Koch, K.N.; Misteli, R.; Mundorff, L.; Götz, T.; et al. Reproductive number of the COVID-19 epidemic in Switzerland with a focus on the Cantons of Basel-Stadt and Basel-Landschaft. Swiss Med. Wkly. 2020, 150, w20271. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. COVID-19 Pandemic. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 15 December 2024).
- Riguzzi, M.; Gashi, S. Lessons From the First Wave of COVID-19: Work-Related Consequences, Clinical Knowledge, Emotional Distress, and Safety-Conscious Behavior in Healthcare Workers in Switzerland. Front. Psychol. 2021, 12, 628033. [Google Scholar] [CrossRef]
- Coronavirus: Wichtige Entscheide des Bundesrats. Federal Department of the Enviroment, Transport, Energy and Communications. 2021. Available online: https://www.uvek.admin.ch/de/coronavirus-wichtige-entscheide-des-bundesrats (accessed on 15 December 2024).
- Zaballa, M.E.; Perez-Saez, J.; de Mestral, C.; Pullen, N.; Lamour, J.; Turelli, P.; Raclot, C.; Baysson, H.; Pennacchio, F.; Villers, J.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in Geneva, Switzerland: A population-based study. Lancet Reg. Health Eur. 2023, 24, 100547. [Google Scholar] [CrossRef]
- Zens, K.D.; Llanas-Cornejo, D.; Menges, D.; Fehr, J.S.; Münz, C.; Puhan, M.A.; Frei, A. Longitudinal humoral and cell-mediated immune responses in a population-based cohort in Zurich, Switzerland between March and June 2022—Evidence for protection against Omicron SARS-CoV-2 infection by neutralizing antibodies and spike-specific T-cell responses. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, 133, 18–26. [Google Scholar] [CrossRef]
- Amati, R.; Frei, A.; Kaufmann, M.; Sabatini, S.; Pellaton, C.; Fehr, J.; Albanese, E.; Puhan, M.A. Functional immunity against SARS-CoV-2 in the general population after a booster campaign and the Delta and Omicron waves, Switzerland, March 2022. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 2200561. [Google Scholar] [CrossRef]
- Tancredi, S.; Chiolero, A.; Wagner, C.; Haller, M.L.; Chocano-Bedoya, P.; Ortega, N.; Rodondi, N.; Kaufmann, L.; Lorthe, E.; Baysson, H.; et al. Seroprevalence trends of anti-SARS-CoV-2 antibodies and associated risk factors: A population-based study. Infection 2023, 51, 1453–1465. [Google Scholar] [CrossRef]
- Belloni, G.; Dupraz, J.; Butty, A.; Pasquier, J.; Estoppey, S.; Bochud, M.; Gonseth-Nussle, S.; D’Acremont, V. SARS-CoV-2 Seroprevalence in Employees of Four Essential Non-Health Care Sectors at Moderate/High Risk of Exposure to Coronavirus Infection: Data From the “First Wave”. J. Occup. Environ. Med. 2023, 65, 10–15. [Google Scholar] [CrossRef]
- Sendi, P.; Widmer, N.; Branca, M.; Thierstein, M.; Büchi, A.E.; Güntensperger, D.; Blum, M.R.; Baldan, R.; Tinguely, C.; Heg, D.; et al. Do quantitative levels of antispike-IgG antibodies aid in predicting protection from SARS-CoV-2 infection? Results from a longitudinal study in a police cohort. J. Med. Virol. 2023, 95, e28904. [Google Scholar] [CrossRef] [PubMed]
- Gallian, P.; Pastorino, B.; Morel, P.; Chiaroni, J.; Ninove, L.; de Lamballerie, X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antivir. Res. 2020, 181, 104880. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Hogema, B.M.; Reusken, C.; Reimerink, J.H.; Molier, M.; Karregat, J.H.M.; Ijlst, J.; Novotný, V.M.J.; van Lier, R.A.W.; Zaaijer, H.L. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat. Commun. 2020, 11, 5744. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Knabbe, C.; Vollmer, T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2020, 25, 2001285. [Google Scholar] [CrossRef]
- Valenti, L.; Bergna, A.; Pelusi, S.; Facciotti, F.; Lai, A.; Tarkowski, M.; Lombardi, A.; Berzuini, A.; Caprioli, F.; Santoro, L.; et al. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 outbreak in Milan. Blood Transfus. Trasfus. Del Sangue 2021, 19, 181–189. [Google Scholar] [CrossRef]
- Calò, F.; Di Fraia, A.; Russo, A.; Di Biase, A.; Misso, S.; Coppola, N. Blood donor serological screening for SARS-CoV-2 as a tool to estimate the prevalence of asymptomatic infection in a low-intermediate endemic area of southern Italy after the first wave of the pandemic. Blood Transfus. Trasfus. Del Sangue 2022, 20, 263–264. [Google Scholar] [CrossRef]
- Erikstrup, C.; Laksafoss, A.D.; Gladov, J.; Kaspersen, K.A.; Mikkelsen, S.; Hindhede, L.; Boldsen, J.K.; Jørgensen, S.W.; Ethelberg, S.; Holm, D.K.; et al. Seroprevalence and infection fatality rate of the SARS-CoV-2 Omicron variant in Denmark: A nationwide serosurveillance study. Lancet Reg. Health Eur. 2022, 21, 100479. [Google Scholar] [CrossRef]
- Piron, M.; Jané, M.; Ciruela, P.; Basile, L.; Martínez, A.; Puig, L.; Bes, M.; Sauleda, S. SARS-CoV-2 seroprevalence in blood donors before and after the first wave in Catalonia (Spain). Blood Transfus. Trasfus. Del Sangue 2022, 20, 353–361. [Google Scholar] [CrossRef]
- Offergeld, R.; Preußel, K.; Zeiler, T.; Aurich, K.; Baumann-Baretti, B.I.; Ciesek, S.; Corman, V.M.; Dienst, V.; Drosten, C.; Görg, S.; et al. Monitoring the SARS-CoV-2 Pandemic: Prevalence of Antibodies in a Large, Repetitive Cross-Sectional Study of Blood Donors in Germany-Results from the SeBluCo Study 2020–2022. Pathogens 2023, 12, 551. [Google Scholar] [CrossRef]
- Riester, E.; Majchrzak, M.; Mühlbacher, A.; Tinguely, C.; Findeisen, P.; Hegel, J.K.; Laimighofer, M.; Rank, C.M.; Schönfeld, K.; Langen, F.; et al. Multicentre Performance Evaluation of the Elecsys Anti-SARS-CoV-2 Immunoassay as an Aid in Determining Previous Exposure to SARS-CoV-2. Infect. Dis. Ther. 2021, 10, 2381–2397. [Google Scholar] [CrossRef]
- FOPH. Coronavirus: Federal Council to Lift Measures—Mask Requirement on Public Transport and in Healthcare Institutions and Isolation in the Event of Illness to Remain Until End of March; The Swiss Federal Council: Bern, Switzerland, 2022; pp. 1–5. Available online: https://www.news.admin.ch/en/nsb?id=87216 (accessed on 15 December 2024).
- Coronavirus: Federal Council Lifts Requirements to Quarantine and to Work from Home and Launches Consultation on a Widespread Easing of Measures; Federal Department of the Environment, Transport, Energy and Communications: Bern, Switzerland, 2022; Available online: https://www.uvek.admin.ch/en/nsb?id=87041 (accessed on 15 December 2024).
- Swissmedic Grants Authorisation for the first COVID-19 Vaccine in Switzerland: Vaccine from Pfizer/BioNTech Authorised in the Rolling Review Procedure After Close Scrutiny of the Risks and Benefits; Swiss Agency for Therapeutic Products: Bern, Switzerland, 2020; Available online: https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/covid-19-impfstoff_erstzulassung.html (accessed on 19 December 2020).
- Swissmedic Grants Authorisation for the COVID-19 Vaccine from Moderna; Swiss Agency for Therapeutic Products: Bern, Switzerland, 2021; Available online: https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/zulassung-covid-19-impfstoff-moderna.html#:~:text=The%20establishment%20licence (accessed on 12 January 2021).
- COVID-19 Switzerland: Vaccinations, Switzerland and Liechtenstein; Federal Office of Public Health FOPH: Bern, Switzerland, 2023; Available online: https://www.covid19.admin.ch/en/vaccination/persons?geo=CH (accessed on 15 December 2024).
- Riester, E.; Findeisen, P.; Hegel, J.K.; Kabesch, M.; Ambrosch, A.; Rank, C.M.; Pessl, F.; Laengin, T.; Niederhauser, C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods 2021, 297, 114271. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.; Gay, N.; Hesketh, L.; Morgan-Capner, P.; Miller, E. Ten years of serological surveillance in England and Wales: Methods, results, implications and action. Int. J. Epidemiol. 2000, 29, 362–368. [Google Scholar] [CrossRef]
- Surendra, H.; Supargiyono; Ahmad, R.A.; Kusumasari, R.A.; Rahayujati, T.B.; Damayanti, S.Y.; Tetteh, K.K.A.; Chitnis, C.; Stresman, G.; Cook, J.; et al. Using health facility-based serological surveillance to predict receptive areas at risk of malaria outbreaks in elimination areas. BMC Med. 2020, 18, 9. [Google Scholar] [CrossRef]
- Winter, A.K.; Martinez, M.E.; Cutts, F.T.; Moss, W.J.; Ferrari, M.J.; McKee, A.; Lessler, J.; Hayford, K.; Wallinga, J.; Metcalf, C.J.E. Benefits and Challenges in Using Seroprevalence Data to Inform Models for Measles and Rubella Elimination. J. Infect. Dis. 2018, 218, 355–364. [Google Scholar] [CrossRef]
- Arnold, B.F.; Scobie, H.M.; Priest, J.W.; Lammie, P.J. Integrated Serologic Surveillance of Population Immunity and Disease Transmission. Emerg. Infect. Dis. 2018, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Ramòn, F.J.; Lucia, D.F.; Tommaso, G.; Mariantonietta, D.S.; Giuseppina, F.; Mohamed, F.A.; Maurizio, M.; Michele, C.; and Santantonio, T.A. Increased SARS-CoV-2 seroprevalence in healthy blood donors after the second pandemic wave in South-Eastern Italy: Evidence for asymptomatic young donors as potential virus spreaders. Infect. Dis. 2022, 54, 241–246. [Google Scholar] [CrossRef]
- Amorim Filho, L.; Szwarcwald, C.L.; Mateos, S.O.G.; Leon, A.; Medronho, R.A.; Veloso, V.G.; Lopes, J.I.F.; Porto, L.; Chieppe, A.; Werneck, G.L. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev. De Saude Publica 2020, 54, 69. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Hou, W.; Zhao, L.; Zhang, Y.; Wang, Y.; Wu, L.; Xu, T.; Wang, L.; Wang, J.; Ma, J.; et al. The prevalence of antibodies to SARS-CoV-2 among blood donors in China. Nat. Commun. 2021, 12, 1383. [Google Scholar] [CrossRef]
- Uyoga, S.; Adetifa, I.M.O.; Karanja, H.K.; Nyagwange, J.; Tuju, J.; Wanjiku, P.; Aman, R.; Mwangangi, M.; Amoth, P.; Kasera, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021, 371, 79–82. [Google Scholar] [CrossRef]
- Lewin, A.; Drews, S.J.; Lieshout-Krikke, R.; Erikstrup, C.; Saeed, S.; Fady, H.; Uzicanin, S.; Custer, B.; O’Brien, S.F. An international comparison of anti-SARS-CoV-2 assays used for seroprevalence surveys from blood component providers. Vox Sang. 2021, 116, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Hardick, J.; Morehead, E.; Miller, J.A.; Gaydos, C.A.; Manabe, Y.C. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 130, 104578. [Google Scholar] [CrossRef]
- Emmenegger, M.; De Cecco, E.; Lamparter, D.; Jacquat, R.P.B.; Riou, J.; Menges, D.; Ballouz, T.; Ebner, D.; Schneider, M.M.; Morales, I.C.; et al. Continuous population-level monitoring of SARS-CoV-2 seroprevalence in a large European metropolitan region. iScience 2023, 26, 105928. [Google Scholar] [CrossRef]
- Ahava, M.J.; Jarva, H.; Jääskeläinen, A.J.; Lappalainen, M.; Vapalahti, O.; Kurkela, S. Rapid increase in SARS-CoV-2 seroprevalence during the emergence of Omicron variant, Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 997–999. [Google Scholar] [CrossRef]
- COVID-19 Switzerland: Information on the Current Situation, as of 28 November 2023; Federal Office of Public Health FOPH: Bern, Switzerland, 2023. Available online: https://www.covid19.admin.ch/en/overview (accessed on 15 December 2024).
- Frei, A.; Kaufmann, M.; Amati, R.; Butty Dettwiler, A.; von Wyl, V.; Annoni, A.M.; Vincentini, J.; Pellaton, C.; Pantaleo, G.; Fehr, J.S.; et al. Development of hybrid immunity during a period of high incidence of Omicron infections. Int. J. Epidemiol. 2023, 52, 1696–1707. [Google Scholar] [CrossRef]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Y.; Hou, H.Y.; Wang, F.; Ouyang, Z.Q.; Zhang, Y.; Lai, D.Y.; Banga Ndzouboukou, J.L.; Xu, Z.W.; Zhang, B.; et al. Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy 2021, 76, 551–561. [Google Scholar] [CrossRef]
- Menges, D.; Zens, K.D.; Ballouz, T.; Caduff, N.; Llanas-Cornejo, D.; Aschmann, H.E.; Domenghino, A.; Pellaton, C.; Perreau, M.; Fenwick, C.; et al. Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort. Nat. Commun. 2022, 13, 4855. [Google Scholar] [CrossRef] [PubMed]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Impfquoten-Monitoring in Deutschland (COVIMO); Report No.: Report 6; Robert Koch Institute: Berlin, Germany, 2021; Available online: https://www.rki.de/DE/Themen/Infektionskrankheiten/Impfen/Forschungsprojekte/abgeschlossene-Projekte/COVIMO/Downloads/covimo_studie_bericht_6.pdf?__blob=publicationFile&v=1 (accessed on 15 December 2024).
- Swiss Transfusion Swiss Red Cross (SRC) Guidelines; Updated 2025; Swiss Transfusion Swiss Red Cross (SRC): Bern, Switzerland, 2025. Available online: https://dokuman.sbsc-bsd.ch/de-de/neuevorschriftenbsd/vorschriftenbsd.aspx (accessed on 15 December 2024).
- Lewin, A.; Osiowy, C.; Erikstrup, C.; Custer, B.; Renaud, C.; Tiberghien, P.; Russell, A.; Lieshout-Krikke, R.; O’Brien, S.F. Research partnerships between blood services and public health authorities: An international, cross-sectional survey. Vox Sang. 2022, 117, 1368–1374. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Drews, S.J.; Lewin, A.; Russell, A.; Davison, K.; Goldman, M. How do we decide how representative our donors are for public health surveillance? Transfusion 2022, 62, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.K.; Joseph, A.; Van Wyk, J.; Rocco, S.; Atmaja, A.; May, E.; Yan, T.; Bobrovitz, N.; Chevrier, J.; Cheng, M.P.; et al. SeroTracker: A global SARS-CoV-2 seroprevalence dashboard. Lancet Infect. Dis. 2021, 21, e75–e76. [Google Scholar] [CrossRef] [PubMed]
- SeroTracker: Dashboard and Data Platform for SARS-CoV-2 Serosurveys [Internet]. 2024. Available online: https://www.serotracker.com/pathogen/sarscov2/dashboard (accessed on 15 December 2024).
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef] [PubMed]



| Timepoints | Donor Samples Tested | NCP Antigen % Seroprevalence (95% CI) | S Antigen % Seroprevalence (95% CI) |
|---|---|---|---|
| March 2020 | 1953 | 0.27 (0.05–0.49) | 0.32 (0.08–0.57) |
| June–August 2020 | 2202 | 4.31 (3.46–5.16) | 4.27 (3.42–5.11) |
| January 2021 | 2147 | 16.39 (14.83–17.96) | 16.80 (15.23–18.40) |
| May–June 2021 | 2319 | 21.09 (19.43–22.75) | 55.71 (53.69–57.74) |
| November–December 2021 | 2145 | 22.00 (20.25–23.76) | 90.54 (89.30–91.78) |
| June–August 2022 | 2196 | 74.36 (72.53–76.19) | 98.22 (97.67–98.78) |
| October–December 2022 | 2196 | 83.88 (82.34–85.42) | 99.04 (98.64–99.45) |
| January 2024 | 2171 | 94.98 (94.97–94.99) | 99.77 (99.57–99.97) |
| Assay | Total Number of Samples | Negative Plasma Samples | Positive Plasma Samples | Platform | Antibody/Antigen | Test Principle | Specificity (%) (from PACKAGE Insert) | Specificity (%) (CI) Blood Donors |
|---|---|---|---|---|---|---|---|---|
| Biorad: Platelia SARS-CoV-2 Total Ab | 4044 | 4018 | 26 | Euroimmun Analyzer/Evolis | Total Ab/NCP | ELISA | 99.51 | 99.36 (98.95–99.64) |
| Roche Diagnostics: Elecsys Anti-SARS-CoV-2 | 4044 | 4037 | 7 | cobas e 801 | Total Ab/NCP | ECLIA | 99.81 | 99.83 (99.57–99.95) |
| Abbott: Architect SARS-CoV-2 IgG | 4044 | 4022 | 22 | Architect | IgG/NCP | CMIA | 99.60 | 99.46 (99.07–99.71) |
| Diasorin: LIAISON® SARS-CoV-2 S1/S2 IgG | 4044 | 3991 | 53 | LIAISON XL Analyzer | IgG/S1/S2 | CLIA | 98.50 * | 98.69 (98.14–99.11) |
| Euroimmun Anti-SARS-CoV-2-ELISA-IgG | 4044 | 4007 | 37 | Euroimmun Analyzer/Evolis | IgG/S1 | ELISA | na | 99.09 (98.61–99.43) |
| Euroimmun Anti-SARS-CoV-2-ELISA-NCP IgG | 4044 | 4002 | 42 | Euroimmun Analyzer/Evolis | IgG/NCP truncated | ELISA | 99.40 | 98.96 (98.46–99.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederhauser, C.; Widmer, N.; Tinguely, C.; Suter Riniker, F.; Fontana, S.; Buser, A.; Waldvogel, S.; Thierbach, J.; Züger, M.; Gowland, P. Anti-SARS-CoV-2 Antibody Development over Four Years in Blood Donors. Viruses 2025, 17, 1292. https://doi.org/10.3390/v17101292
Niederhauser C, Widmer N, Tinguely C, Suter Riniker F, Fontana S, Buser A, Waldvogel S, Thierbach J, Züger M, Gowland P. Anti-SARS-CoV-2 Antibody Development over Four Years in Blood Donors. Viruses. 2025; 17(10):1292. https://doi.org/10.3390/v17101292
Chicago/Turabian StyleNiederhauser, Christoph, Nadja Widmer, Caroline Tinguely, Franziska Suter Riniker, Stefano Fontana, Andreas Buser, Sophie Waldvogel, Jutta Thierbach, Max Züger, and Peter Gowland. 2025. "Anti-SARS-CoV-2 Antibody Development over Four Years in Blood Donors" Viruses 17, no. 10: 1292. https://doi.org/10.3390/v17101292
APA StyleNiederhauser, C., Widmer, N., Tinguely, C., Suter Riniker, F., Fontana, S., Buser, A., Waldvogel, S., Thierbach, J., Züger, M., & Gowland, P. (2025). Anti-SARS-CoV-2 Antibody Development over Four Years in Blood Donors. Viruses, 17(10), 1292. https://doi.org/10.3390/v17101292






