Coinfection of Gynura bicolor with a New Strain of Vanilla Distortion Mosaic Virus and a Novel Maculavirus in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. High-Throughput Sequencing and De Novo Transcriptome Assembly
2.3. Molecular Detection, Rapid Amplification of cDNA Ends, and Full Viral Genome Amplification of Viruses
2.4. Detection of Virions by Transmission Electron Microscopy
2.5. Viral Sequence Analysis
2.6. Quantitative RT-PCR of Viral RNA
2.7. Transmission Studies
3. Results
3.1. Identification of Two Novel Viruses in G. Bicolor Through High-Throughput Sequencing Technology
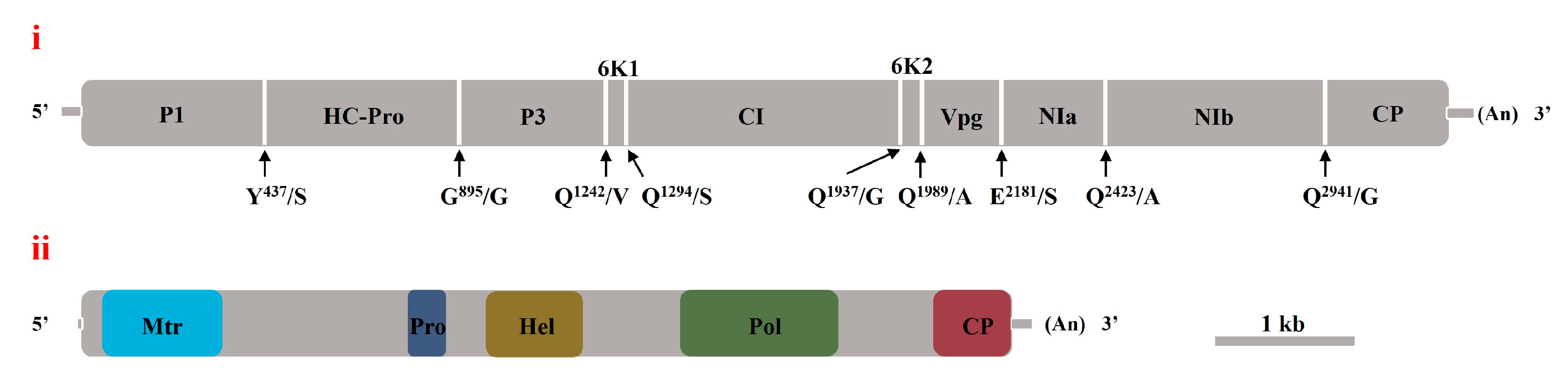
3.2. Sequence Determination and Structural Characterization of the Complete RNA Genomes of Two Viruses
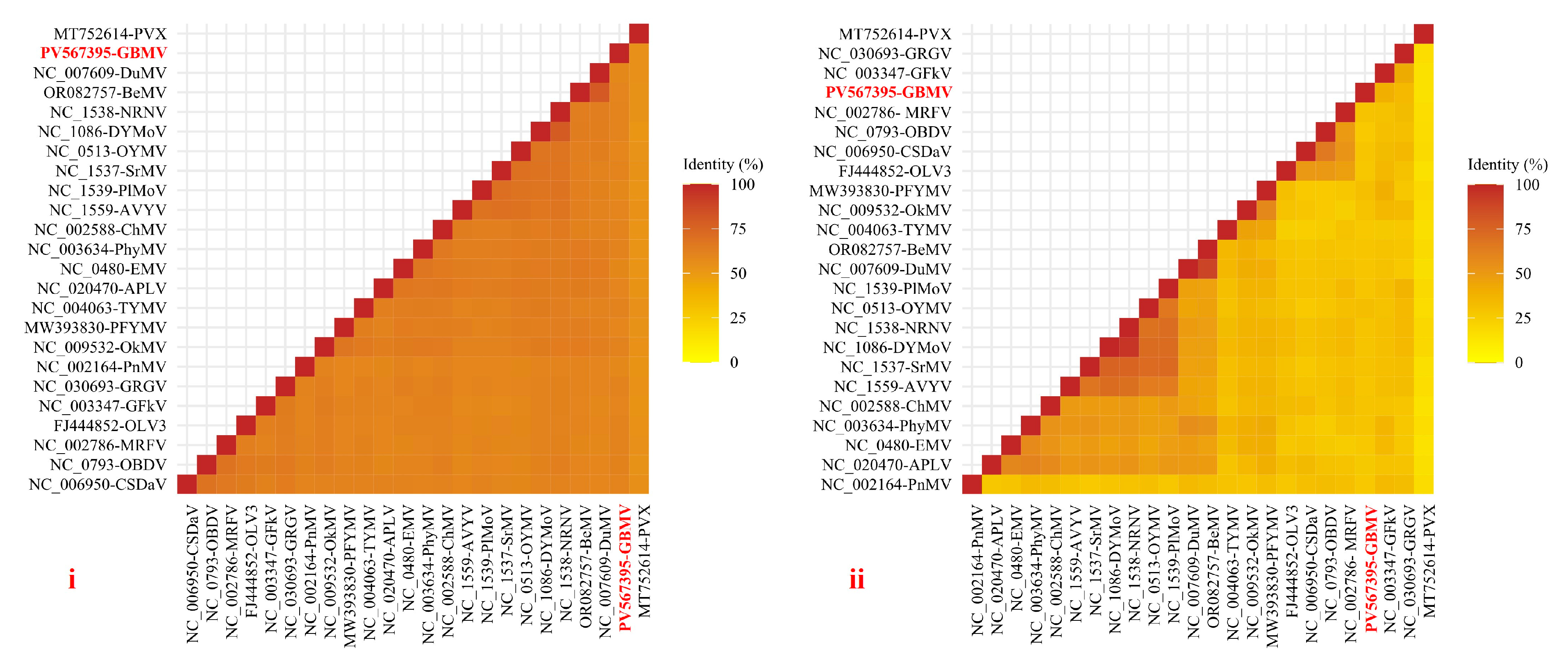
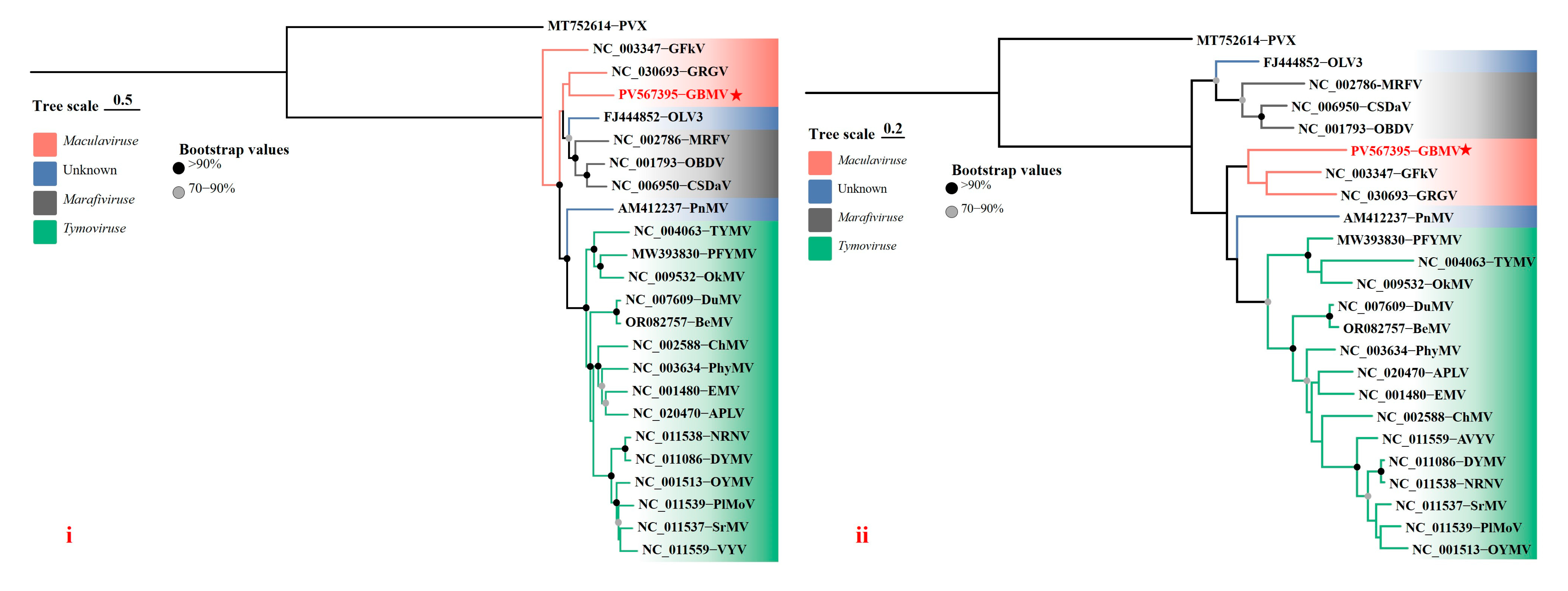
3.3. Sequence Identity Analysis and Phylogenetic Analysis
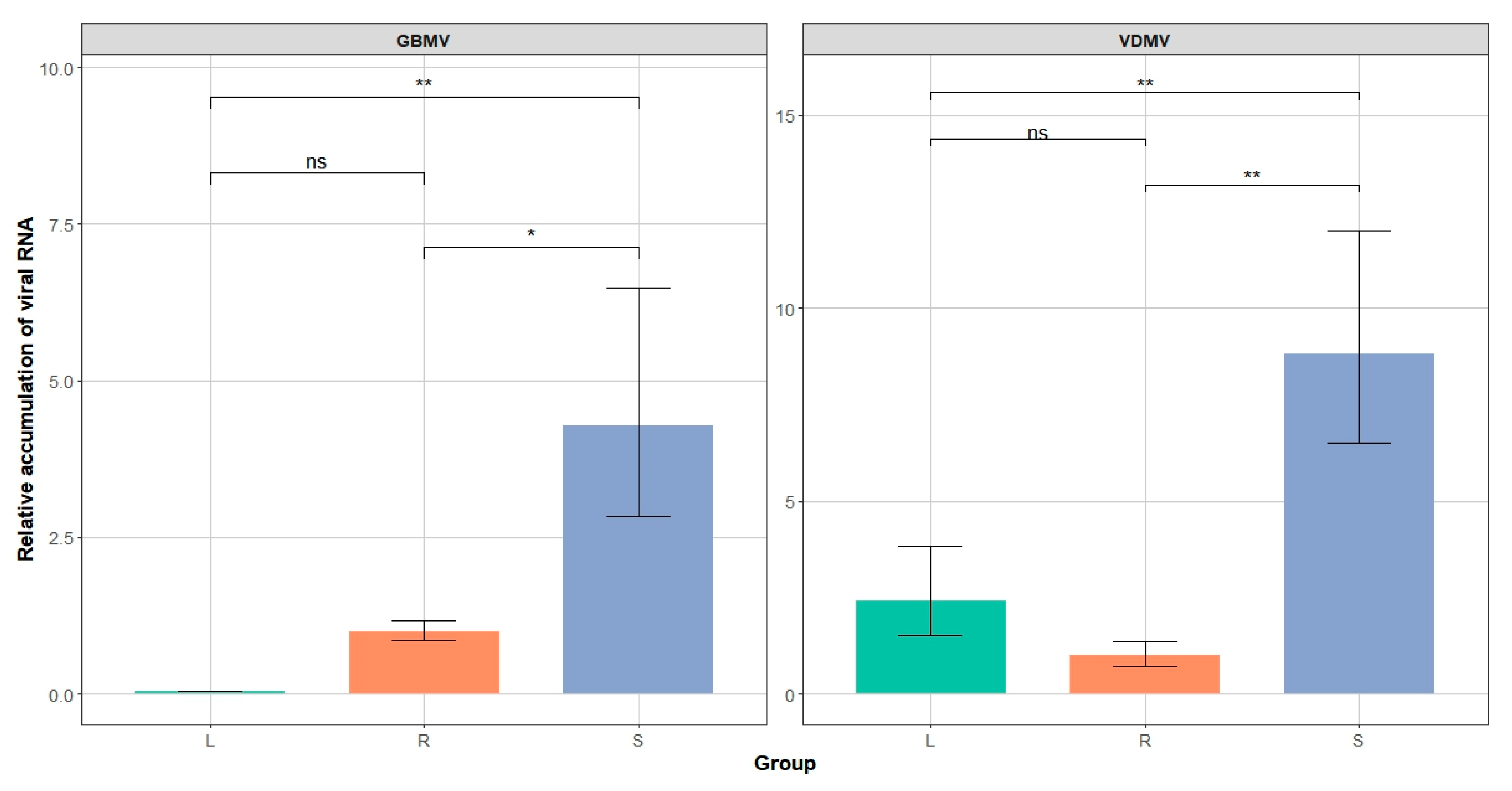
3.4. Transmission Electron Microscopy Observations and Quantification of Two Viruses in Roots, Stems, and Leaves
3.5. Doddertransmission
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Do, T.V.T.; Suhartini, W.; Mutabazi, F.; Mutukumira, A.N. Gynura bicolor DC. (Okinawa spinach): A comprehensive review on nutritional constituents, phytochemical compounds, utilization, health benefits, and toxicological evaluation. Food Res. Int. 2020, 134, 109222. [Google Scholar] [CrossRef]
- Shao, C.; Zhou, W.; Qi, M.; Yuan, Z. Year-round facility cultivation techniques for the medicinal and edible plant Gynura bicolor. Jiangsu Agric. Sci. 2015, 43, 226–227. [Google Scholar]
- Tian, G.; Kang, X.; Zheng, S.; Yao, Z.; Ma, B.; Zhang, J.; Qiu, H. Technical regulations for nutrient film technique hydroponics of Gynura bicolor in facilities. Hebei Agric. Sci. 2020, 24, 28–30. [Google Scholar]
- Krishnan, V.; Ahmad, S.; Mahmood, M. Antioxidant Potential in Different Parts and Callus of Gynura procumbens and Different Parts of Gynura bicolor. Biomed. Res. Int. 2015, 2015, 147909. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Wang, A. Research Advances in Potyviruses: From the Laboratory Bench to the Field. Annu. Rev. Phytopathol. 2021, 59, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ala-Poikela, M.; Rajamäki, M.; Valkonen, J.P.T. A Novel Interaction Network Used by Potyviruses in Virus-Host Interactions at the Protein Level. Viruses 2019, 11, 1158. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef]
- Clark, C.A.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Gibson, R.W.; Mukasa, S.B.; Tugume, A.K.; Tairo, F.D.; et al. Sweetpotato Viruses: 15 Years of Progress on Understanding and Managing Complex Diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.; Wuriyanghan, H.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Revers, F.; García, J.A. Molecular Biology of Potyviruses. Adv. Virus Res. 2015, 92, 101–199. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Tian, Y.; Wang, S.; Sun, X.; Li, S.; Ma, W.; Zhou, T.; Fan, Z. Maize catalases localized in peroxisomes support the replication of maize chlorotic mottle virus. Phytopathol. Res. 2021, 3, 17. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Stewart, L.R. Maize Lethal Necrosis: An Emerging, Synergistic Viral Disease. Annu. Rev. Virol. 2018, 5, 301–322. [Google Scholar] [CrossRef]
- Elbeaino, T.; Digiaro, M.; Martelli, G.P. Complete sequence of Fig fleck-associated virus, a novel member of the family Tymoviridae. Virus Res. 2011, 161, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Innami, K.; Aizawa, T.; Tsukui, T.; Katsuma, S.; Imanishi, S.; Kawasaki, H.; Iwanaga, M. Infection studies of nontarget mammalian cell lines with Bombyx mori macula-like virus. J. Virol. Methods 2016, 229, 24–26. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Mijit, M.; Li, S.; Zhang, Z. Complete nucleotide sequence of a novel strain of fig fleck-associated virus from China. Arch. Virol. 2017, 162, 1145–1148. [Google Scholar] [CrossRef]
- Komínková, M.; Ben Mansour, K.; Komínek, P.; Brožová, J.; Střalková, R. Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines. Viruses 2024, 16, 343. [Google Scholar] [CrossRef]
- Schoonvaere, K.; Smagghe, G.; Francis, F.; de Graaf, D.C. Study of the Metatranscriptome of Eight Social and Solitary Wild Bee Species Reveals Novel Viruses and Bee Parasites. Front. Microbiol. 2018, 9, 177. [Google Scholar] [CrossRef]
- Perry, B.J.; Darestani, M.M.; Ara, M.G.; Hoste, A.; Jandt, J.M.; Dutoit, L.; Holmes, E.C.; Ingram, T.; Geoghegan, J.L. Viromes of Freshwater Fish with Lacustrine and Diadromous Life Histories Differ in Composition. Viruses 2022, 14, 257. [Google Scholar] [CrossRef]
- Martelli, G.P.; Sabanadzovic, S.; Abou Ghanem-Sabanadzovic, N.; Saldarelli, P. Maculavirus, a new genus of plant viruses. Arch. Virol. 2002, 147, 1847–1853. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Abou-Ghanem, N.; Castellano, M.A.; Digiaro, M.; Martelli, G.P. Grapevine fleck virus-like viruses in Vitis. Arch. Virol. 2000, 145, 553–565. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Li, F.; Zhang, X.; Ye, J.; Wei, T.; Li, Z.; Tao, X.; Cui, F.; Wang, X.; et al. Plant virology in the 21st century in China: Recent advances and future directions. J. Integr. Plant Biol. 2024, 66, 579–622. [Google Scholar] [CrossRef]
- Edwards, M.C.; Weiland, J.J. First infectious clone of the propagatively transmitted Oat blue dwarf virus. Arch. Virol. 2010, 155, 463–470. [Google Scholar] [CrossRef]
- Dolja, V.V.; Krupovic, M.; Koonin, E.V. Deep Roots and Splendid Boughs of the Global Plant Virome. Annu. Rev. Phytopathol. 2020, 58, 23–25. [Google Scholar] [CrossRef]
- Adams, I.P.; Rai, S.; Deka, M.; Harju, V.; Hodges, T.; Hayward, G.; Skelton, A.; Fox, A.; Boonham, N. Genome sequence of vanilla distortion mosaic virus infecting Coriandrum sativum. Arch. Virol. 2014, 159, 3463–3465. [Google Scholar] [CrossRef]
- Sadras, V.; Guirao, M.; Moreno, A.; Fereres, A. Inter-virus relationships in mixed infections and virus-drought relationships in plants: A quantitative review. Plant J. 2024, 117, 1786–1799. [Google Scholar] [CrossRef]
- Savenkov, E.I.; Valkonen, J.P. Potyviral helper-component proteinase expressed in transgenic plants enhances titers of Potato leaf roll virus but does not alleviate its phloem limitation. Virology 2001, 283, 285–293. [Google Scholar] [CrossRef]
- Karyeija, R.F.; Kreuze, J.F.; Gibson, R.W.; Valkonen, J.P. Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweet potato plants. Virology 2000, 269, 26–36. [Google Scholar] [CrossRef]
- Kappagantu, M.; Collum, T.D.; Dardick, C.; Culver, J.N. Viral hacks of the plant vasculature: The role of phloem alterations in systemic virus infection. Annu. Rev. Virol. 2020, 7, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mejia, A.; Hajizadeh, M.; Atanda, H.Y.; Tzanetakis, I.E. Overcoming the woody barrier: Dodder enables efficient transfer of infectious clones to woody plants. J. Virol. Methods 2025, 334, 115114. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Guo, M.; Sun, P.; Zhang, L. Coinfection of Gynura bicolor with a New Strain of Vanilla Distortion Mosaic Virus and a Novel Maculavirus in China. Viruses 2025, 17, 1290. https://doi.org/10.3390/v17101290
Li Z, Guo M, Sun P, Zhang L. Coinfection of Gynura bicolor with a New Strain of Vanilla Distortion Mosaic Virus and a Novel Maculavirus in China. Viruses. 2025; 17(10):1290. https://doi.org/10.3390/v17101290
Chicago/Turabian StyleLi, Zhengnan, Mengze Guo, Pingping Sun, and Lei Zhang. 2025. "Coinfection of Gynura bicolor with a New Strain of Vanilla Distortion Mosaic Virus and a Novel Maculavirus in China" Viruses 17, no. 10: 1290. https://doi.org/10.3390/v17101290
APA StyleLi, Z., Guo, M., Sun, P., & Zhang, L. (2025). Coinfection of Gynura bicolor with a New Strain of Vanilla Distortion Mosaic Virus and a Novel Maculavirus in China. Viruses, 17(10), 1290. https://doi.org/10.3390/v17101290





