Geographic Distribution of Vaccinia Virus, Diagnosis and Demographic Aspects of Affected Populations, Minas Gerais, Brazil, 2000–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Set
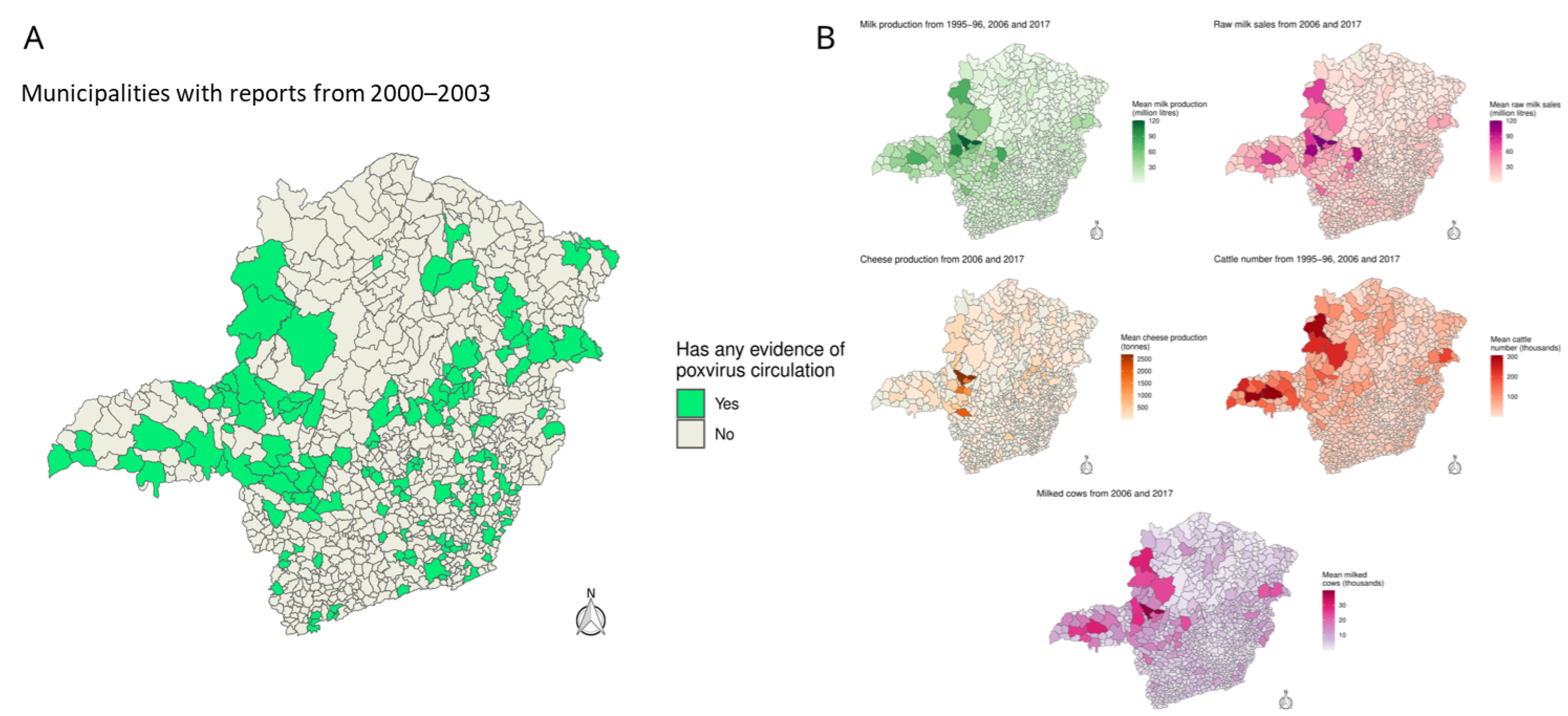
2.2. Geospatial Analysis, Agricultural and Livestock Production
2.3. Clinical Samples
2.4. Serological Assays
2.5. Molecular Assays
3. Results
3.1. Geographical Distribution of BV Cases Across MG State
3.2. Descriptive Analysis of Infected Individuals
3.3. Laboratory Diagnosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D.; WHO. Smallpox and Its Eradication; WHO: Geneva, Switzerland, 1988; Available online: https://apps.who.int/iris/handle/10665/39485 (accessed on 1 August 2023).
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian Vaccinia Viruses and Their Origins. Emerg. Infect. Dis. 2007, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.d.S.; da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.N.; Borges, A.S.; Peiró, J.R.; Pituco, E.M.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Araçatuba Virus: A Vaccinialike Virus Associated with Infection in Humans and Cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; dos Santos, J.R.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; et al. Passatempo Virus, a Vaccinia Virus Strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1941. [Google Scholar] [CrossRef]
- Damaso, C.R.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.S.; Figueiredo, P.D.O.; Costa, G.B.; de Assis, F.L.; Drumond, B.P.; Da Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; Trindade, G.D.S. Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly. Viruses 2017, 9, 340. [Google Scholar] [CrossRef]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses. Viruses 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Monkeypox: An emerging global threat during the COVID-19 pandemic. J. Microbiol. Immunol. Infect. 2022, 55, 787–794. [Google Scholar] [CrossRef]
- Ghebreyesus, T.A. Why the monkeypox outbreak constitutes a public health emergency of international concern. BMJ 2022, 378, o1978. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.T.; Oliveira, G.P.; Afonso, J.A.B.; Souto, R.J.C.; de Mendonça, C.L.; Dantas, A.F.M.; Abrahao, J.S.; Kroon, E.G. An Update on the Known Host Range of the Brazilian Vaccinia Virus: An Outbreak in Buffalo Calves. Front. Microbiol. 2019, 9, 3327. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, M.; Wennink, G.J. Zoonotic risks of pathogens from dairy cattle and their milk-borne transmission. J. Dairy Res. 2023, 90, 325–331. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Oliveira, T.M.L.; Campos, R.K.; Madureira, M.C.; Kroon, E.G.; Lobato, Z.I.P. Bovine Vaccinia Outbreaks: Detection and Isolation of Vaccinia Virus in Milk Samples. Foodborne Pathog. Dis. 2009, 6, 1141–1146. [Google Scholar] [CrossRef]
- Trindade, G.S.; Guedes, M.I.C.; Drumond, B.P.; Mota, B.E.F.; Abrahão, J.S.; Lobato, Z.I.P.; Gomes, J.A.S.; Corrêa-Oliveira, R.; Nogueira, M.L.; Kroon, E.G.; et al. Zoonotic Vaccinia Virus: Clinical and Immunological Characteristics in a Naturally Infected Patient. Clin. Infect. Dis. 2009, 48, e37–e40. [Google Scholar] [CrossRef]
- Trindade, G.d.S.; Drumond, B.P.; Guedes, M.I.M.C.; Leite, J.A.; Mota, B.E.F.; Campos, M.A.; da Fonseca, F.G.; Nogueira, M.L.; Lobato, Z.I.P.; Bonjardim, C.A.; et al. Zoonotic Vaccinia Virus Infection in Brazil: Clinical Description and Implications for Health Professionals. J. Clin. Microbiol. 2007, 45, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.G.; Mota, B.E.F.; Abrahão, J.S.; Fonseca, F.G.; Trindade, G.S. Zoonotic Brazilian Vaccinia virus: From field to therapy. Antivir. Res. 2011, 92, 150–163. [Google Scholar] [CrossRef]
- Ministério da Saúde. Lista Nacional de Notificação Compulsória de Doenças, Agravos e Eventos de Saúde Pública; Ministério da Saúde: Brasília, Brazil, 2022. Available online: https://www.gov.br/saude/pt-br/composicao/svsa/notificacao-compulsoria/lista-nacional-de-notificacao-compulsoria-de-doencas-agravos-e-eventos-de-saude-publica (accessed on 22 May 2024).
- Campos, T.; Cartaz–Lista Atualizada das Doenças de Notificação Compulsória, 2023–Portal da Vigilância em Saúde. Imprensa Nacional. 2023. Available online: http://vigilancia.saude.mg.gov.br/index.php/download/cartaz-lista-atualizadas-das-doencas-de-notificacao-compulsoria/ (accessed on 22 May 2024).
- de Oliveira, J.S.; Costa, G.B.; Luiz, A.P.M.F.; Leite, J.A.; Bonjardim, C.A.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; Trindade, G.d.S. Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281–3285. [Google Scholar] [CrossRef]
- Domingos, I.J.d.S.; de Oliveira, J.S.; Rocha, K.L.S.; de Oliveira, D.B.; Kroon, E.G.; Costa, G.B.; Trindade, G.d.S. Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going? Pathogens 2021, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.S.; DA Fonseca, F.G.; Ferreira, P.C.P.; Leite, J.A.; Kroon, E.G.; Trigueiro, R.C.; DOS Santos, J.R.; Lobato, Z.I.P.; Bonjardim, C.A.; Drumond, B.P.; et al. Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: Implications on the emergence of zoonotic Orthopoxviruses. Am. J. Trop. Med. Hyg. 2006, 75, 486–490. [Google Scholar] [CrossRef]
- Assis, F.L.; Almeida, G.M.F.; Oliveira, D.B.; Franco-Luiz, A.P.M.; Campos, R.K.; Guedes, M.I.M.; Fonseca, F.G.; Trindade, G.S.; Drumond, B.P.; Kroon, E.G.; et al. Characterization of a New Vaccinia virus Isolate Reveals the C23L Gene as a Putative Genetic Marker for Autochthonous Group 1 Brazilian Vaccinia virus. PLoS ONE 2012, 7, e50413. [Google Scholar] [CrossRef]
- Trindade, G.D.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Mota, B.E.F.; Abrahão, J.S.; De Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses 2016, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Reis, B.B.; Ricci Junior, J.E.R.; Fernandes, F.S.; Corrêa, J.F.; Schatzmayr, H.G. Infecção em humanos por varíola bovina na microrregião de Itajubá, Estado de Minas Gerais: Relato de caso. Rev. Soc. Bras. Med. Trop. 2008, 41, 507–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abrahão, J.S.; Guedes, M.I.M.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.P.; Silva-Fernandes, A.T.; Rodrigues, G.O.L.; Lima, L.S.; et al. One More Piece in the VACV Ecological Puzzle: Could Peridomestic Rodents Be the Link between Wildlife and Bovine Vaccinia Outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Campos, R.K.; Trindade, G.S.; Fonseca, F.G.; Ferreira, P.C.P.; Kroon, E.G. Outbreak of Severe Zoonotic Vaccinia Virus Infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Borges, I.; Mccollum, A.; Mehal, J.; Haberling, D.; Dutra, L.; Vieira, F.; Andrade, L.; Kroon, E.; Holman, R.; Reynolds, M.; et al. Dairy production practices and associated risks for bovine vaccinia exposure in cattle, Brazil. New Microbes New Infect. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Assis, F.L.; Borges, I.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Trindade, G.d.S.; Lobato, Z.I.P.; Guedes, M.I.M.; Mesquita, V.; Kroon, E.G.; Abrahão, J.S. Group 2 Vaccinia Virus, Brazil. Emerg. Infect. Dis. 2012, 18, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.B.; Borges, I.A.; Campos, S.P.S.; Vieira, F.N.; De Ázara, T.M.F.; Marques, F.A.; Costa, G.B.; Luis, A.P.M.F.; De Oliveira, J.S.; Ferreira, P.C.P.; et al. Serologic and Molecular Evidence of Vaccinia Virus Circulation among Small Mammals from Different Biomes, Brazil. Emerg. Infect. Dis. 2017, 23, 931–938. [Google Scholar] [CrossRef]
- Rehfeld, I.S.; Fraiha, A.L.S.; Matos, A.C.D.; Costa, A.G.; Gallinari, G.C.; Costa, A.; Guedes, M.I.M.; Lobato, Z.I.P. Short communication: Parapoxvirus and Orthopoxvirus coinfection in milk of naturally infected cows. J. Dairy Sci. 2018, 101, 7801–7803. [Google Scholar] [CrossRef]
- Costa, G.B.; Borges, I.A.; Alves, P.A.; Miranda, J.B.; Luiz, A.P.M.; Ferreira, P.C.; Abrahão, J.S.; Moreno, E.C.; Kroon, E.G.; Trindade, G.d.S. Alternative Routes of Zoonotic Vaccinia Virus Transmission, Brazil. Emerg. Infect. Dis. 2015, 21, 2244–2246. [Google Scholar] [CrossRef]
- Costa, G.B.; Miranda, J.B.; Almeida, G.G.; de Oliveira, J.S.; Pinheiro, M.S.; Gonçalves, S.A.; dos Reis, J.K.P.; Gonçalves, R.; Ferreira, P.C.P.; Bonjardim, C.A.; et al. Detection of Vaccinia Virus in Urban Domestic Cats, Brazil. Emerg. Infect. Dis. 2017, 23, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.A.L.; de Freitas Almeida, G.M.; Oliveira, G.P.; Abrahão, J.S.; Kroon, E.G.; Trindade, G.S. Molecular evidence of Orthopoxvirus DNA in capybara (Hydrochoerus hydrochaeris) stool samples. Arch. Virol. 2016, 162, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; de Almeida, L.R.; Cerqueira, A.G.R.; Mesquita, W.U.; de Oliveira, J.S.; Miranda, J.B.; Saraiva-Silva, A.T.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; et al. Vaccinia Virus among Domestic Dogs and Wild Coatis, Brazil, 2013–2015. Emerg. Infect. Dis. 2018, 24, 2338–2342. [Google Scholar] [CrossRef]
- Lima, M.T.; Oliveira, G.P.; Assis, F.L.; de Oliveira, D.B.; Vaz, S.M.; Trindade, G.d.S.; Abrahão, J.S.; Kroon, E.G. Ocular Vaccinia Infection in Dairy Worker, Brazil. Emerg. Infect. Dis. 2018, 24, 161–162. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Rocha, K.L.S.; Silva-Oliveira, R.; Macedo, M.V.; Silva, T.G.M.; Gonçalves-Dos-Santos, M.E.; de Oliveira, C.H.; Aquino-Teixeira, S.M.; Ottone, V.d.O.; da Silva, A.J.J.; et al. Serological Evidence of Orthopoxvirus Infection in Neotropical Primates in Brazil. Pathogens 2022, 11, 1167. [Google Scholar] [CrossRef]
- Domingos, I.J.S.; Rocha, K.L.S.; Graciano, J.M.; Almeida, L.R.; Doty, J.B.; Paglia, A.P.; Oliveira, D.B.; Nakazawa, Y.J.; Trindade, G.d.S. Orthopoxvirus Circulation in an Endemic Area in Brazil: Investigation of Infections in Small Mammals during an Absence of Outbreaks. Viruses 2023, 15, 842. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.S.; Barbosa Costa, G.; Stoffella Dutra, A.G.; Domingos, I.J.D.S.; Costa, P.S.P.M.D.; Silva, P.H.B.E.; Kroon, E.G.; de Oliveira, D.B.; Trindade, G.S. Low prevalence of anti-Orthopoxvirus neutralizing antibodies in an urban population of Brazil. J. Med. Virol. 2023, 95, e28859. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística (IBGE) Censo 2000, IBGE. 2000. Available online: https://www.ibge.gov.br/estatisticas/sociais/populacao/9663-censo-demografico-2000.html?edicao=9773&t=resultados (accessed on 30 August 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE) Censo 2010, IBGE. 2010. Available online: https://www.ibge.gov.br/estatisticas/sociais/populacao/9662-censo-demografico-2010.html?edicao=9673&t=resultados (accessed on 30 August 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE) Censo 2022, IBGE. 2022. Available online: https://www.ibge.gov.br/estatisticas/sociais/populacao/22827-censo-demografico-2022.html?edicao=37225&t=resultados (accessed on 22 May 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE) Censo Agropecuário 1995–1996, IBGE. 1995. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/20700-1995-1996-censoagro1995.html (accessed on 22 May 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE) Censo Agropecuário 2006, IBGE. 2006. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/2017-np-censo-agropecuario/9827-censo-agropecuario.html (accessed on 22 May 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Censo Agropecuário 2017. IBGE-Censo Agro 2017. 2017. Available online: https://censoagro2017.ibge.gov.br/templates/censo_agro/resultadosagro/index.html (accessed on 22 May 2024).
- R Core Team. R: The R Project for Statistical Computing. R Project. 2023. Available online: https://www.r-project.org/ (accessed on 22 May 2024).
- Wickham, H. Function Reference. ggplot2. 2016. Available online: https://ggplot2.tidyverse.org/reference/index.html (accessed on 22 May 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 22 May 2024).
- Pebesma, E.; Bivand, R. Spatial Data Science; Chapman and Hall/CRC: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439. [Google Scholar] [CrossRef]
- Pedersen, T.L. Package Patchwork. 2023. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 22 October 2024).
- Baquero, O.S. ggsn. Index of /src/contrib/Archive/ggsn. 2019. Available online: https://cran.r-project.org/src/contrib/Archive/ggsn/ (accessed on 22 October 2024).
- ipeaGIT. geobr: Easy Access to Official Spatial Data Sets of Brazil in R and Python. GitHub. 2023. Available online: https://github.com/ipeaGIT/geobr (accessed on 22 May 2024).
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, A., Jr.; Yan, L.; Belshe, R.B. Improved Assay to Detect Neutralizing Antibody following Vaccination with Diluted or Undiluted Vaccinia (Dryvax) Vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef]
- Kroon, E.G.; Abrahão, J.S.; Trindade, G.d.S.; Oliveira, G.P.; Luiz, A.P.M.F.; Costa, G.B.; Lima, M.T.; Calixto, R.S.; de Oliveira, D.B.; Drumond, B.P. Natural Vaccinia Virus Infection: Diagnosis, Isolation, and Characterization. Curr. Protoc. Microbiol. 2016, 42, 14A.5.1–14A.5.43. [Google Scholar] [CrossRef]
- Trindade, G.d.S.; Li, Y.; Olson, V.A.; Emerson, G.; Regnery, R.L.; da Fonseca, F.G.; Kroon, E.G.; Damon, I. Real-time PCR assay to identify variants of Vaccinia virus: Implications for the diagnosis of bovine vaccinia in Brazil. J. Virol. Methods 2008, 152, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Olson, V.A.; Laue, T.; Laker, M.T.; Damon, I.K. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 2006, 36, 194–203. [Google Scholar] [CrossRef]
| Characteristics | Group | (n) | % |
|---|---|---|---|
| Sex (n = 97) | Female | 20 | 20.62 |
| Male | 77 | 79.38 | |
| Age * (n = 97) | 0–10 y/o | 10 | 10.31 |
| 11–20 y/o | 12 | 12.37 | |
| 21–30 y/o | 26 | 26.80 | |
| 31–40 y/o | 22 | 22.68 | |
| 41–50 y/o | 18 | 18.56 | |
| >50 y/o | 9 | 9.28 | |
| Working age ** (n = 97) | Yes | 84 | 86.6 |
| No | 13 | 13.4 | |
| Potentially vaccinated *** (n = 97) | Yes | 31 | 37.11 |
| No | 66 | 62.89 | |
| Ethnicity (n = 63) | White | 30 | 47.62 |
| Mixed | 30 | 47.62 | |
| Black | 3 | 4.76 | |
| Residential area (n = 88) | Rural | 64 | 72.73 |
| Urban | 24 | 27.27 |
| Risk Factor | Yes (n) | % | No (n) | % |
|---|---|---|---|---|
| Contact with bovines (n = 38) | 30 | 100 | 0 | 0 |
| Contact with domestic animals * (n = 37) | 27 | 72.98 | 10 | 27.02 |
| Contact with wild animals ** (n = 37) | 11 | 29.73 | 26 | 70.27 |
| Contact with rodents (n = 37) | 4 | 10.81 | 33 | 89.19 |
| Work-related infection (n = 66) | 50 | 70.76 | 16 | 24.24 |
| Household infection (n = 37) | 8 | 21.62 | 29 | 78.38 |
| Report ID | County | Year * | Age ** | Gender | Potentially Vaccinated | Titer (NU/mL) |
|---|---|---|---|---|---|---|
| 48 | Paracatu | 2017 | 48 | M | Yes | 800 |
| 62 | Salto da Divisa | 2018 | 37 | M | No | 800 |
| 63 | Salto da Divisa | 2018 | 44 | M | Yes | 100 |
| 64 | Salto da Divisa | 2018 | 46 | M | Yes | 50 |
| 83 | Joanésia | 2021 | 48 | M | Yes | 400 |
| 84 | Joanésia | 2021 | 16 | M | No | 100 |
| 85 | Joanésia | 2021 | 20 | M | No | 50 |
| 86 | Joanésia | 2021 | 22 | M | No | 100 |
| 87 | Joanésia | 2021 | 43 | M | No | 50 |
| 88 | Joanésia | 2021 | 60 | M | Yes | 400 |
| 90 | Joanésia | 2021 | 24 | M | No | 200 |
| 91 | Joanésia | 2021 | 72 | M | Yes | 800 |
| 92 | Juiz de Fora | 2021 | 60 | M | Yes | 400 |
| 93 | Papagaios | 2021 | 30 | M | No | 800 |
| 94 | Papagaios | 2021 | 6 | F | No | 100 |
| 95 | Papagaios | 2021 | 28 | F | No | 100 |
| 96 | Papagaios | 2021 | 9 | F | No | 50 |
| 97 | Uberaba | 2021 | 40 | M | No | 400 |
| Report ID | County | Year * | Age ** | Gender | Potentially Vaccinated |
|---|---|---|---|---|---|
| 66 | Teófilo Otoni | 2018 | 30 | M | No |
| 67 | Teófilo Otoni | 2018 | 39 | M | No |
| 68 | Teófilo Otoni | 2018 | 41 | M | Yes |
| 69 | Araçuaí | 2019 | 30 | M | No |
| 74 | Iturama | 2019 | 42 | M | Yes |
| 82 | Joanésia | 2021 | 18 | M | No |
| 83 | Joanésia | 2021 | 48 | M | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
e Silva, P.H.B.; de Oliveira, M.D.; de Almeida, I.M.; Domingos, I.J.S.; Stoffella-Dutra, A.G.; Barbosa Costa, G.; de Oliveira, J.S.; Iani, F.C.M.; de Castro, M.R.; Abrahão, J.S.; et al. Geographic Distribution of Vaccinia Virus, Diagnosis and Demographic Aspects of Affected Populations, Minas Gerais, Brazil, 2000–2023. Viruses 2025, 17, 22. https://doi.org/10.3390/v17010022
e Silva PHB, de Oliveira MD, de Almeida IM, Domingos IJS, Stoffella-Dutra AG, Barbosa Costa G, de Oliveira JS, Iani FCM, de Castro MR, Abrahão JS, et al. Geographic Distribution of Vaccinia Virus, Diagnosis and Demographic Aspects of Affected Populations, Minas Gerais, Brazil, 2000–2023. Viruses. 2025; 17(1):22. https://doi.org/10.3390/v17010022
Chicago/Turabian Stylee Silva, Pedro H. B., Maycon D. de Oliveira, Iara M. de Almeida, Iago J. S. Domingos, Ana G. Stoffella-Dutra, Galileu Barbosa Costa, Jaqueline S. de Oliveira, Felipe C. M. Iani, Márcio R. de Castro, Jonatas S. Abrahão, and et al. 2025. "Geographic Distribution of Vaccinia Virus, Diagnosis and Demographic Aspects of Affected Populations, Minas Gerais, Brazil, 2000–2023" Viruses 17, no. 1: 22. https://doi.org/10.3390/v17010022
APA Stylee Silva, P. H. B., de Oliveira, M. D., de Almeida, I. M., Domingos, I. J. S., Stoffella-Dutra, A. G., Barbosa Costa, G., de Oliveira, J. S., Iani, F. C. M., de Castro, M. R., Abrahão, J. S., Kroon, E. G., & Trindade, G. d. S. (2025). Geographic Distribution of Vaccinia Virus, Diagnosis and Demographic Aspects of Affected Populations, Minas Gerais, Brazil, 2000–2023. Viruses, 17(1), 22. https://doi.org/10.3390/v17010022








