Animal Models, Therapeutics, and Vaccine Approaches to Emerging and Re-Emerging Flaviviruses
Abstract
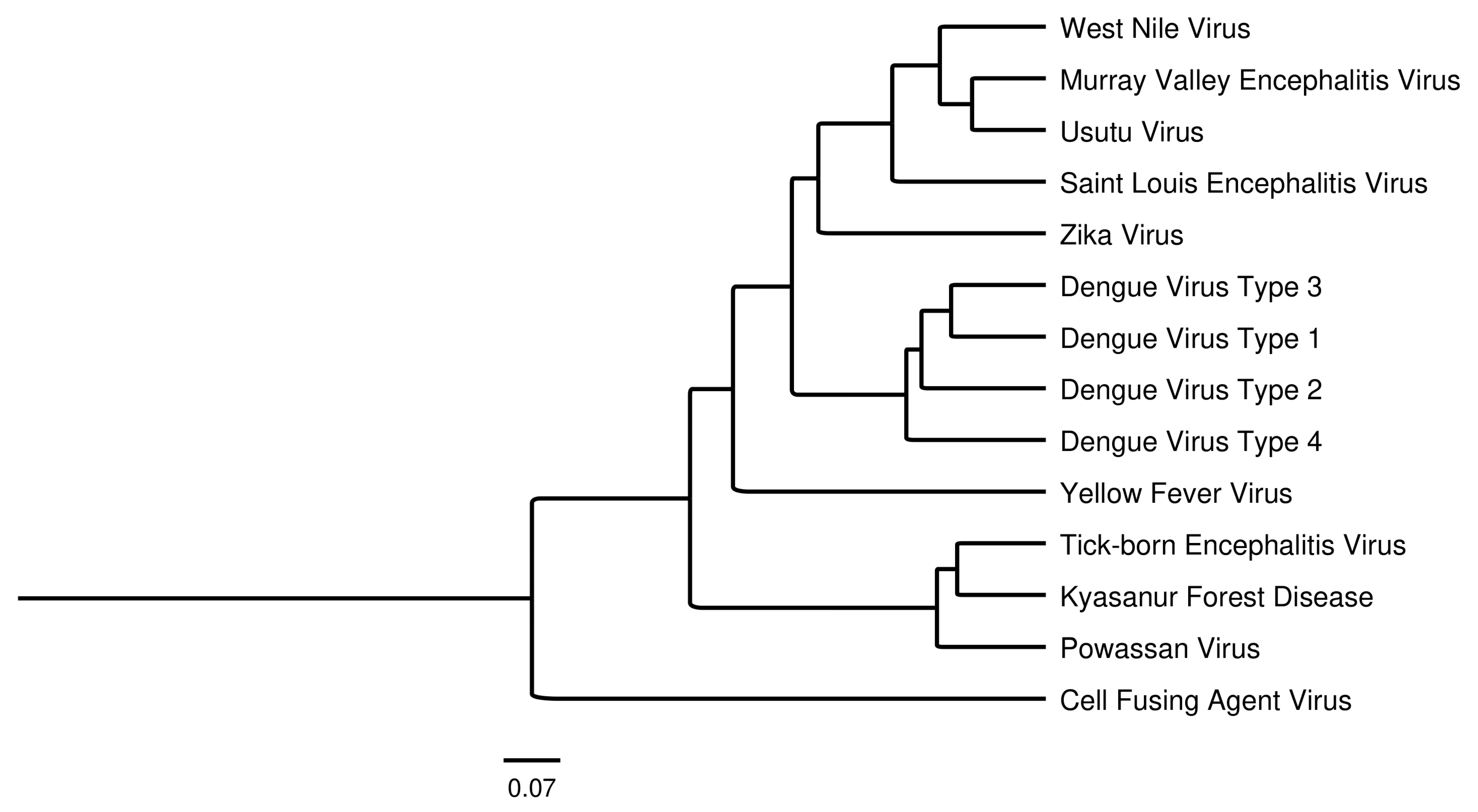
:1. Introduction
| Global Statistics | |||||
|---|---|---|---|---|---|
| Virus | Cases | Severe Infection Chance 7 | Fatality Rate | Vector | Source |
| Dengue Virus | 100–400 m | <5% | 1.10% | Aedes aegypti | [14,15,16] |
| Zika Virus | <1000 | 5–14% 1 | 10% 2 | Aedes aegypti | [17,18] |
| West Nile Virus | - | <1% | 4–14% | Culex spp. | [19,20] |
| Japanese Encephalitis Virus | 30–50 k | <1% | 20–30% | Culex tritaeniorhynchus | [21,22] |
| Tick-borne Encephalitis Virus | 10–12 k | 2–30% | 2 3, 6–8% 4 | I. ricinus 3/I. persulcatus 4 | [23,24,25] |
| Yellow Fever Virus | 84–170 k 5 | 10–15% 6 | 20–50% 6 | Aedes aegypti | [26,27] |
2. Flavivirus Characteristics
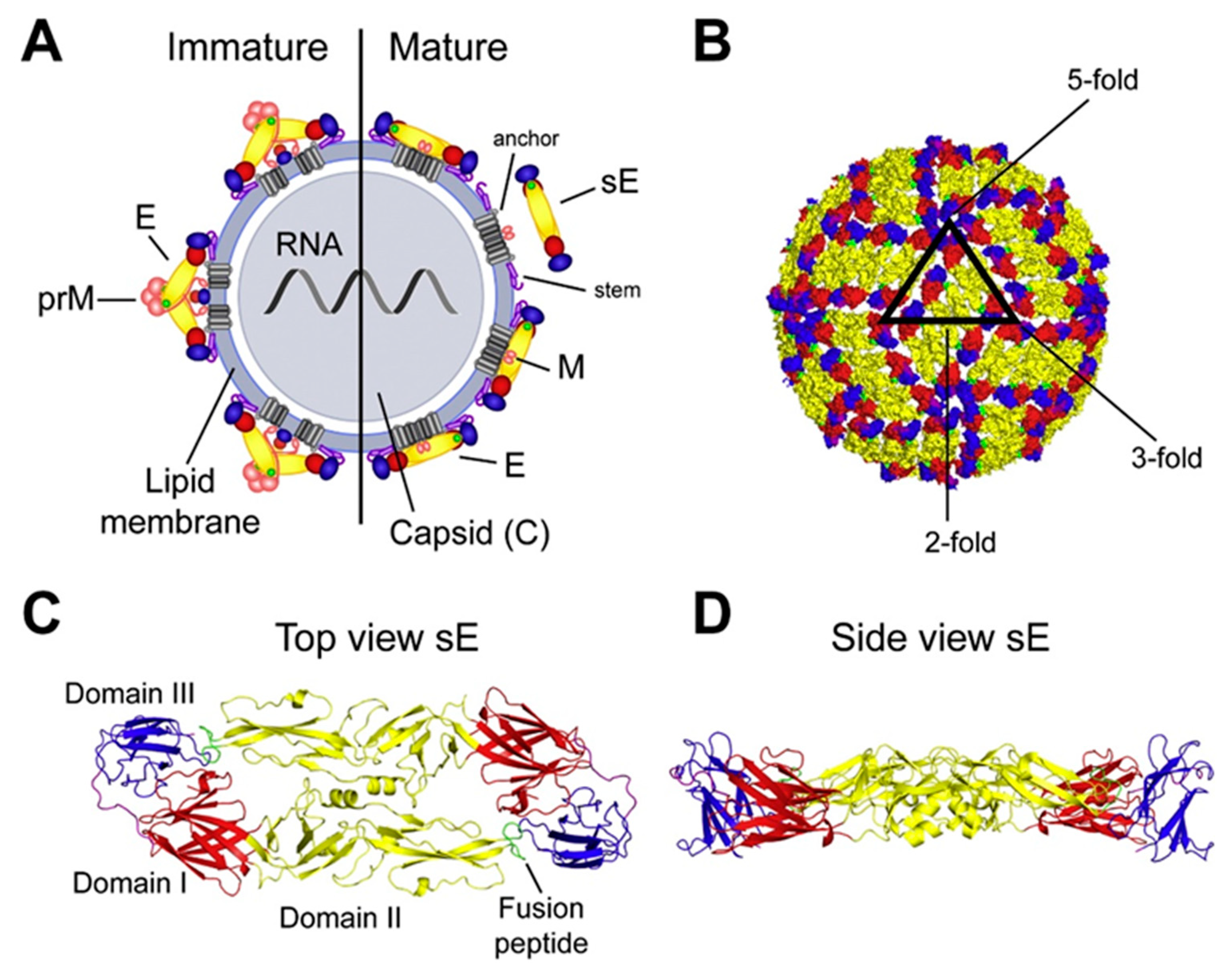
2.1. Virus Structure
2.2. Nonstructural Proteins
2.3. Flavivirus Receptors
2.4. Host Innate Response
2.5. Animal Models
| Virus | Animal Model | Mechanism Addressed | Source |
|---|---|---|---|
| Dengue Virus | BALB/c | Pathogenesis of tissues | [95] |
| Dengue Virus | BALB/c and C57BL/6 | Age-related pathogenesis | [96] |
| Dengue Virus | AG129 | Antibody-dependent enhancement | [97] |
| Zika Virus | C57BL/6 | Neonatal pathogenesis | [98] |
| Zika Virus | C57BL/6 IFNAR-/- | IFN-α/β signaling | [99] |
| Zika Virus | C57BL/6 IFNAR-/- | Insect to mouse transmission | [100] |
| Zika Virus | Anti-IFNR1-treated Rag1-/- | Vertical and sexual transmission | [101] |
| Zika Virus | CC Mice | Genetic factors in pathology | [102] |
| West Nile | Gold Hamster | Encephalitis | [78] |
| West Nile | Outbred Swiss mice [Arc(S)] | Pathogenicity between strains | [79] |
| West Nile | CC RI | Chronic infection | [80] |
| West Nile | BALB/c | Pathogenicity and neuroinvasiveness | [84] |
| Tick-borne Encephalitis Virus | BALB/c/STS/CcS-11 | Genetic factors in pathology | [103] |
| Tick-borne Encephalitis Virus | B6 IL-10KO/TNF-α KO | Cytokines | [104] |
| Tick-borne Encephalitis Virus | C57BL/6j | Systemic inflammatory and stress responses | [105] |
| Japanese Encephalitis Virus | C57BL/6 | Pathogenesis | [106] |
| Japanese Encephalitis Virus | Not Stated | Encephalitis and disease progression | [107] |
| Japanese Encephalitis Virus | BALB/c | Contract transmission | [108] |
| Yellow Fever | C57BL/6 PVR-Tg21 IFNAR-/- | Immunopathogenesis | [109] |
| Yellow Fever | A129 | Viscerotropic infection | [110] |
| Yellow Fever | AG129 | Neurotropic disease | [111] |
| Yellow Fever | AG129 | Neurotropic and viscerotropic disease | [112] |
| Yellow Fever | CD-1 | Fetal development | [113] |
| Yellow Fever | hSTAT2 KI | Immunocompetency | [114] |
3. Therapeutics
3.1. Antiviral Drugs
3.2. Monoclonal Antibodies
4. Vaccine Approaches
New Advances in Flavivirus E glycoprotein Vaccine Design
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Zhan, J.; Chen, L.; Chen, H.; Cheng, S. Global, Regional, and National Dengue Burden from 1990 to 2017: A Systematic Analysis Based on the Global Burden of Disease Study 2017. EClinicalMedicine 2021, 32, 100712. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Harry Stevens Where Mosquito Season Is Getting Longer. Available online: https://www.washingtonpost.com/climate-environment/interactive/2024/mosquito-season-state-change-global-warming/ (accessed on 9 October 2024).
- Carvalho, F.D.; Moreira, L.A. Why Is Aedes Aegypti Linnaeus so Successful as a Species? Neotrop. Entomol. 2017, 46, 243–255. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control Treatment, Prevention and Control Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika Virus-Spread, Epidemiology, Genome, Transmission Cycle, Clinical Manifestation, Associated Challenges, Vaccine and Antiviral Drug Development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Solomon, T. Japanese Encephalitis-the Prospects for New Treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Pustijanac, E.; Buršić, M.; Talapko, J.; Škrlec, I.; Meštrović, T.; Lišnjić, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Antibody-Enhanced Dengue Virus Infection in Primate Leukocytes. Nature 1977, 265, 739–741. [Google Scholar] [CrossRef]
- Yamanaka, A.; Miyazaki, K.; Shimizu, J.; Senju, S. Dengue Virus Susceptibility in Novel Immortalized Myeloid Cells. Heliyon 2020, 6, e05407. [Google Scholar] [CrossRef]
- Selck, F.W.; Adalja, A.A.; Boddie, C.R. An Estimate of the Global Health Care and Lost Productivity Costs of Dengue. Vector-Borne Zoonotic Dis. 2014, 14, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Shepard, D.S.; Bettis, A.A.; Nguyen, H.A.; McBride, A.; Clapham, H.E.; Turner, H.C. Productivity Costs from a Dengue Episode in Asia: A Systematic Literature Review. BMC Infect. Dis. 2020, 20, 393. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Adams, L.; Paz-Bailey, G. Dengue. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/dengue (accessed on 27 October 2024).
- Mallhi, T.H.; Khan, A.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H. Determinants of Mortality and Prolonged Hospital Stay among Dengue Patients Attending Tertiary Care Hospital: A Cross-Sectional Retrospective Analysis. BMJ Open 2017, 7, e016805. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Costa, M.C.N.; Cardim, L.L.; Teixeira, M.G.; Barreto, M.L.; Carvalho-Sauer, R.d.C.O.d.; Barreto, F.R.; Carvalho, M.S.I.; Oliveira, W.K.; França, G.V.A.; Carmo, E.H.; et al. Case Fatality Rate Related to Microcephaly Congenital Zika Syndrome and Associated Factors: A Nationwide Retrospective Study in Brazil. Viruses 2020, 12, 1228. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. West Nile Virus Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile Virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Simon, L.V.; Sandhu, D.S.; Goyal, A.; Kruse, B. Japanese Encephalitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hills, S.; Lindsey, N.; Fischer, M. Japanese Encephalitis. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/japanese-encephalitis#:~:text=Seizures%20are%20common%2C%20especially%20among,%2C%20moderate%20leukocytosis%2C%20and%20hyponatremia (accessed on 27 October 2024).
- Phipps, L.P.; Johnson, N. Tick-Borne Encephalitis Virus. J. Med. Microbiol. 2022, 71, 001492. [Google Scholar]
- Cohen, M.K.; Muntner, P.; Kent, C.K.; Gottardy, A.J.; Leahy, M.A.; Spriggs, S.R.; Velarde, A.; Yang, T.; Doan, Q.M.; King, P.H.; et al. Tick-Borne Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023; Morbidity and Mortality Weekly Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023.
- Tick-Borne Encephalitis. Available online: https://www.who.int/health-topics/tick-borne-encephalitis#tab=tab_2 (accessed on 27 October 2024).
- Song, A.T.W.; D’Albuquerque, L.A.C. An Official Learning Resource of AASLD Review: Acute Liver Failure Secondary to Yellow Fever: A Challenging Scenario. Clin. Liver Dis. 2019, 13, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Yellow Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 27 October 2024).
- de Silva, A. Safety of Dengue Vaccine? Clin. Infect. Dis. 2022, 76, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, I.C.; Allison, S.L.; Heinz, F.X.; Helenius, A. Folding and Dimerization of Tick-Borne Encephalitis Virus Envelope Proteins PrM and E in the Endoplasmic Reticulum. J. Virol. 2002, 76, 5480–5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Corver, J.; Chipman, P.R.; Zhang, W.; Pletnev, S.V.; Sedlak, D.; Baker, T.S.; Strauss, J.H.; Kuhn, R.J.; Rossmann, M.G. Structures of Immature Flavivirus Particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Byk, L.A.; Gamarnik, A.V. Properties and Functions of the Dengue Virus Capsid Protein. Annu. Rev. Virol. 2016, 3, 263–284. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and their antigenic structure. J. Clin. Virol. 2012, 55, 289–295. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the Dengue Virus Envelope Protein after Membrane Fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ge, P.; Yu, X.; Brannan, J.M.; Bi, G.; Zhang, Q.; Schein, S.; Zhou, Z.H. Cryo-EM Structure of the Mature Dengue Virus at 3.5-Å Resolution. Nat. Struct. Mol. Biol. 2013, 20, 105–110. [Google Scholar] [CrossRef]
- Gallichotte, E.N.; Baric, T.J.; Yount, B.L.; Widman, D.G.; Durbin, A.; Whitehead, S.; Baric, R.S.; de Silva, A.M. Human Dengue Virus Serotype 2 Neutralizing Antibodies Target Two Distinct Quaternary Epitopes. PLoS Pathog. 2018, 14, e1006934. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.D.; Austin, S.K.; Sukupolvi-Petty, S.; O’Brien, K.M.; Johnson, S.; Fremont, D.H.; Diamond, M.S. Genotype-Specific Neutralization and Protection by Antibodies against Dengue Virus Type 3. J. Virol. 2010, 84, 10630–10643. [Google Scholar] [CrossRef]
- Muller, D.; Young, P. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a Genetic and Physical Interaction between Nonstructural Proteins NS1 and NS4B That Modulates Replication of West Nile Virus. J. Virol. 2012, 86, 7360–7371. [Google Scholar] [CrossRef]
- Tan, M.J.A.; Brown, N.G.; Chan, K.W.K.; Jin, J.Y.; Zu Kong, S.Y.; Vasudevan, S.G. Mutations in the Cytoplasmic Domain of Dengue Virus NS4A Affect Virus Fitness and Interactions with Other Non-Structural Proteins. J. Gen. Virol. 2020, 101, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism Conceived and Designed the Experiments. Cell Rep. 2019, 26, 1598–1613. [Google Scholar] [CrossRef]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.A.; et al. Endocytosis of Flavivirus NS1 Is Required for NS1-Mediated Endothelial Hyperpermeability and Is Abolished by a Single N-Glycosylation Site Mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the Complement Component C4 by Flavivirus Nonstructural Protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Carpio, K.L.; Barrett, A.D.T. Flavivirus Ns1 and Its Potential in Vaccine Development. Vaccines 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Dubrau, D.; Tortorici, M.A.; Rey, F.A.; Tautz, N. A Positive-Strand RNA Virus Uses Alternative Protein-Protein Interactions within a Viral Protease/Cofactor Complex to Switch between RNA Replication and Virion Morphogenesis. PLoS Pathog. 2017, 13, e1006134. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Hunke, C.; Grüber, G.; Vasudevan, S.G.; Lescar, J. Crystal Structure of the NS3 Protease-Helicase from Dengue Virus. J. Virol. 2008, 82, 173–183. [Google Scholar] [CrossRef]
- Wahaab, A.; Liu, K.; Hameed, M.; Anwar, M.N.; Kang, L.; Li, C.; Ma, X.; Wajid, A.; Yang, Y.; Khan, U.H.; et al. Identification of Cleavage Sites Proteolytically Processed by Ns2b-Ns3 Protease in Polyprotein of Japanese Encephalitis Virus. Pathogens 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Goethals, O.; Kaptein, S.J.F.; Kesteleyn, B.; Bonfanti, J.F.; Van Wesenbeeck, L.; Bardiot, D.; Verschoor, E.J.; Verstrepen, B.E.; Fagrouch, Z.; Putnak, J.R.; et al. Blocking NS3–NS4B Interaction Inhibits Dengue Virus in Non-Human Primates. Nature 2023, 615, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Yao, W.; Lu, J.; Chen, J.; Morrison, J.; Hai, R.; Song, J. A Conformational Selection Mechanism of Flavivirus NS5 for Species-Specific STAT2 Inhibition. Commun. Biol. 2024, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Krejčová, K.; Krafcikova, P.; Klima, M.; Chalupska, D.; Chalupsky, K.; Zilecka, E.; Boura, E. Structural and Functional Insights in Flavivirus NS5 Proteins Gained by the Structure of Ntaya Virus Polymerase and Methyltransferase. Structure 2024, 32, 1099–1109.e3. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, J.; Westaway, E.G.; Khromykh, A.; Mackenzie, J.M. Regulated Cleavages at the West Nile Virus NS4A-2K-NS4B Junctions Play a Major Role in Rearranging Cytoplasmic Membranes and Golgi Trafficking of the NS4A Protein. J. Virol. 2006, 80, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, R.L.; Mackenzie, J.M. A Conserved Peptide in West Nile Virus NS4A Protein Contributes to Proteolytic Processing and Is Essential for Replication. J. Virol. 2011, 85, 11274–11282. [Google Scholar] [CrossRef]
- Muñ Oz-Jordá, J.L.; Sá Nchez-Burgos, G.G.; Laurent-Rolle, M.; García-Sastre, A. Inhibition of Interferon Signaling by Dengue Virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef]
- Ambrose, R.L.; Mackenzie, J.M. West Nile Virus Differentially Modulates the Unfolded Protein Response to Facilitate Replication and Immune Evasion. J. Virol. 2011, 85, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Klaitong, P.; Smith, D.R. Roles of Non-Structural Protein 4a in Flavivirus Infection. Viruses 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, V.P.; Keller, M.; Schmidt, K.; Ulrich, R.G.; Groschup, M.H. AVβ3 Integrin Expression Is Essential for Replication of Mosquito and Tick-Borne Flaviviruses in Murine Fibroblast Cells. Viruses 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. ZIKA Virus Entry Mechanisms in Human Cells. Infect. Genet. Evol. 2019, 69, 22–29. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [PubMed]
- Germi, R.; Crance, J.M.; Garin, D.; Guimet, J.; Lortat-Jacob, H.; Ruigrok, R.W.H.; Zarski, J.P.; Drouet, E. Heparan Sulfate-Mediated Binding of Infectious Dengue Virus Type 2 and Yellow Fever Virus. Virology 2002, 292, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maguire, T.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue Virus Infectivity Depends on Envelope Protein Binding to Target Cell Heparan Sulfate. Nature 1997, 3, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis Virus to BHK Cells Selects for Use of Heparan Sulfate as an Attachment Receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Chee, H.Y.; AbuBakar, S. Identification of a 48 KDa Tubulin or Tubulin-like C6/36 Mosquito Cells Protein That Binds Dengue Virus 2 Using Mass Spectrometry. Biochem. Biophys. Res. Commun. 2004, 320, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vega-Almeida, T.O.; Salas-Benito, M.; De Nova-Ocampo, M.A.; del Angel, R.M.; Salas-Benito, J.S. Surface Proteins of C6/36 Cells Involved in Dengue Virus 4 Binding and Entry. Arch. Virol. 2013, 158, 1189–1207. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.-L.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-Cell-Specific ICAM3-Grabbing Non-Integrin Is Essential for the Productive Infection of Human Dendritic Cells by Mosquito-Cell-Derived Dengue Viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.R.-d.; del Angel, R.M. Isolation of Putative Dengue Virus Receptor Molecules by Affinity Chromatography Using a Recombinant E Protein Ligand. J. Virol. Methods 2004, 116, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Salas-benito, J.S.; Rosa, M. Identification of Two Surface Proteins from C6 / 36 Cells That Bind Dengue Type 4 Virus. J. Virol. 1997, 71, 7246–7252. [Google Scholar] [CrossRef]
- Bournazos, S.; Thi, H.; Vo, M.; Duong, V.; Auerswald, H.; Ly, S.; Sakuntabhai, A.; Dussart, P.; Cantaert, T.; Ravetch, J.V. Antibody Fucosylation Predicts Disease Severity in Secondary Dengue Infection HHS Public Access. Science (1979) 2021, 372, 1102–1105. [Google Scholar]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Nguyen, T. The Interferons and Their Receptors-Distribution and Regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2′-O Methylation of the Viral MRNA Cap Evades Host Restriction by IFIT Family Members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Farzan, M. The Broad-Spectrum Antiviral Functions of IFIT and IFITM Proteins. Nat. Rev. Immunol. 2013, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jordán, J.L.; Laurent-Rolle, M.; Ashour, J.; Martínez-Sobrido, L.; Ashok, M.; Lipkin, W.I.; García-Sastre, A. Inhibition of Alpha/Beta Interferon Signaling by the NS4B Protein of Flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, X.J.; Mokhonov, V.V.; Shi, P.-Y.; Randall, R.; Khromykh, A.A. Inhibition of Interferon Signaling by the New York 99 Strain and Kunjin Subtype of West Nile Virus Involves Blockage of STAT1 and STAT2 Activation by Nonstructural Proteins. J. Virol. 2005, 79, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, e01970-16. [Google Scholar] [CrossRef] [PubMed]
- Miorin, L.; Maestre, A.M.; Fernandez-Sesma, A.; García-Sastre, A. Antagonism of Type I Interferon by Flaviviruses. Biochem. Biophys. Res. Commun. 2017, 492, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Rydell-Törmänen, K.; Johnson, J.R. The Applicability of Mouse Models to the Study of Human Disease. In Mouse Cell Culture; Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2019; Volume 1940, pp. 3–22. [Google Scholar]
- Xiao, S.; Guzman, H.; Zhang, H.; Travassos da Rosa, A.; Tesh, R.B. West Nile Virus Infection in the Golden Hamster (Mesocricetus Auratus): A Model for West Nile Encephalitis. Emerg. Infect. Dis. 2001, 7, 714. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.; Payne, J.; Harper, J.; Frazer, L.; Eastwood, S.; Wilson, S.; Lowther, S.; Lunt, R.; Warner, S.; Carr, M.; et al. Evaluation of a Mouse Model for the West Nile Virus Group for the Purpose of Determining Viral Pathotypes. J. Gen. Virol. 2014, 95, 1221–1232. [Google Scholar] [CrossRef]
- Graham, J.B.; Swarts, J.L.; Wilkins, C.; Thomas, S.; Green, R.; Sekine, A.; Voss, K.M.; Ireton, R.C.; Mooney, M.; Choonoo, G.; et al. A Mouse Model of Chronic West Nile Virus Disease. PLoS Pathog. 2016, 12, e1005996. [Google Scholar] [CrossRef]
- Johnston, L.J.; Halliday, G.M.; King, N.J. Phenotypic Changes in Langerhans’ Cells after Infection with Arboviruses: A Role in the Immune Response to Epidermally Acquired Viral Infection? J. Virol. 1996, 70, 4761–4766. [Google Scholar] [CrossRef]
- McGruder, B.; Saxena, V.; Wang, T. Lessons from the Murine Models of West Nile Virus Infection. In West Nile Virus; Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2016; Volume 1435, pp. 61–69. [Google Scholar]
- Fratkin, J.D.; Leis, A.A.; Stokic, D.S.; Slavinski, S.A.; Geiss, R.W. Spinal Cord Neuropathology in Human West Nile Virus Infection. Arch. Pathol. Lab. Med. 2004, 128, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Bernard, K.A. West Nile Virus Infection in Birds and Mammals. Ann. N. Y. Acad. Sci. 2001, 951, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.D.; Uhrlaub, J.L.; Hirsch, A.; Wiley, C.A.; Nikolich-Žugich, J. Key Role of T Cell Defects in Age-Related Vulnerability to West Nile Virus. J. Exp. Med. 2009, 206, 2735–2745. [Google Scholar] [CrossRef]
- Enis, D.; Ash, N.; Arzad, F.; Ostashari, M.; Nnie, A.; Ine, F.; Ames, J.; Iller, M.; Aniel O’l Eary, D.; Urray, R.M.; et al. The outbreak of West Nile virus infection in the New York city area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar]
- Daffis, S.; Samuel, M.A.; Suthar, M.S.; Keller, B.C.; Gale, M.; Diamond, M.S. Interferon Regulatory Factor IRF-7 Induces the Antiviral Alpha Interferon Response and Protects against Lethal West Nile Virus Infection. J. Virol. 2008, 82, 8465–8475. [Google Scholar] [CrossRef]
- Graham, J.B.; Swarts, J.L.; Lund, J.M. A Mouse Model of West Nile Virus Infection. Curr. Protoc. Mouse Biol. 2017, 7, 221–235. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Alpha/Beta Interferon Protects against Lethal West Nile Virus Infection by Restricting Cellular Tropism and Enhancing Neuronal Survival. J. Virol. 2005, 79, 13350–13361. [Google Scholar] [CrossRef] [PubMed]
- Leist, S.R.; Baric, R.S. Giving the Genes a Shuffle: Using Natural Variation to Understand Host Genetic Contributions to Viral Infections. Trends Genet. 2018, 34, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Leist, S.R.; Gralinski, L.E.; Martinez, D.R.; Winkler, E.S.; Okuda, K.; Hawkins, P.E.; Gully, K.L.; Graham, R.L.; Scobey, D.T.; et al. A Multitrait Locus Regulates Sarbecovirus Pathogenesis. mBio 2022, 13, e0145422. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Thomas, S.; Swarts, J.; McMillan, A.A.; Ferris, M.T.; Suthar, M.S.; Treuting, P.M.; Ireton, R.; Gale, M.; Lund, J.M. Genetic Diversity in the Collaborative Cross Model Recapitulates Human West Nile Virus Disease Outcomes. mBio 2015, 6, e00493-15. [Google Scholar] [CrossRef]
- Green, R.; Wilkins, C.; Thomas, S.; Sekine, A.; Ireton, R.C.; Ferris, M.T.; Hendrick, D.M.; Voss, K.; de Villena, F.P.-M.; Baric, R.; et al. Identifying Protective Host Gene Expression Signatures within the Spleen during West Nile Virus Infection in the Collaborative Cross Model. Genom. Data 2016, 10, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Swarts, J.L.; Thomas, S.; Voss, K.M.; Sekine, A.; Green, R.; Ireton, R.C.; Gale, M.; Lund, J.M. Immune Correlates of Protection from West Nile Virus Neuroinvasion and Disease. J. Infect. Dis. 2019, 219, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Jácome, F.C.; Caldas, G.C.; Rasinhas, A.d.C.; de Almeida, A.L.T.; de Souza, D.D.C.; Paulino, A.C.; da Silva, M.A.N.; Bandeira, D.M.; Barth, O.M.; dos Santos, F.B.; et al. Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle. Microorganisms 2021, 9, 2536. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.B.; García, A.G.; Brahamian, J.M.; Mauri, A.; Ferretti, A.; Polack, F.P.; Talarico, L.B. A Murine Model of Dengue Virus Infection in Suckling C57BL/6 and BALB/c Mice. Anim. Model. Exp. Med. 2021, 4, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Ireland, D.D.C.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T Cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016, 12, e1006004. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Johnson, K.E.E.; Noval, M.G.; Rangel, M.V.; De Jesus, E.; Geber, A.; Schuster, S.; Cadwell, K.; Ghedin, E.; Stapleford, K.A. Mapping the Evolutionary Landscape of Zika Virus Infection in Immunocompromised Mice. Virus Evol. 2020, 6, veaa092. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Woods, T.A.; Rosenke, R.; Scott, D.P.; Best, S.M.; Peterson, K.E. Sexual and Vertical Transmission of Zika Virus in Anti-Interferon Receptor-Treated Rag1-Deficient Mice. Sci. Rep. 2017, 7, 7176. [Google Scholar] [CrossRef]
- Manet, C.; Simon-Lorière, E.; Jouvion, G.; Hardy, D.; Prot, M.; Conquet, L.; Flamand, M.; Panthier, J.-J.; Sakuntabhai, A.; Montagutelli, X. Genetic Diversity of Collaborative Cross Mice Controls Viral Replication, Clinical Severity, and Brain Pathology Induced by Zika Virus Infection, Independently of Oas1b. J. Virol. 2020, 94, 1034–1053. [Google Scholar] [CrossRef] [PubMed]
- Palus, M.; Vojtíšková, J.; Salát, J.; Kopecký, J.; Grubhoffer, L.; Lipoldová, M.; Demant, P.; Růžek, D. Mice with Different Susceptibility to Tick-Borne Encephalitis Virus Infection Show Selective Neutralizing Antibody Response and Inflammatory Reaction in the Central Nervous System. J. Neuroinflamm. 2013, 10, 847. [Google Scholar] [CrossRef]
- Tun, M.M.N.; Aoki, K.; Senba, M.; Buerano, C.C.; Shirai, K.; Suzuki, R.; Morita, K.; Hayasaka, D. Protective Role of TNF-α, IL-10 and IL-2 in Mice Infected with the Oshima Strain of Tick-Borne Encephalitis Virus. Sci. Rep. 2014, 4, 5344. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, D.; Nagata, N.; Fujii, Y.; Hasegawa, H.; Sata, T.; Suzuki, R.; Gould, E.A.; Takashima, I.; Koike, S. Mortality Following Peripheral Infection with Tick-Borne Encephalitis Virus Results from a Combination of Central Nervous System Pathology, Systemic Inflammatory and Stress Responses. Virology 2009, 390, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Banerjee, A.; Vrati, S. Development and Characterization of an Animalmodel of Japanese Encephalitis Virus Infection in Adolescent C57BL/6 Mouse. DMM Dis. Models Mech. 2021, 14, dmm049176. [Google Scholar] [CrossRef]
- Fu, T.L.; Ong, K.C.; Wong, K.T. Pathological Findings in a Mouse Model of Japanese Encephalitis Infected via the Footpad. Neurol. Asia 2015, 20, 349. [Google Scholar]
- Chai, C.; Palinski, R.; Xu, Y.; Wang, Q.; Cao, S.; Geng, Y.; Zhao, Q.; Wen, Y.; Huang, X.; Yan, Q.; et al. Aerosol and Contact Transmission Following Intranasal Infection of Mice with Japanese Encephalitis Virus. Viruses 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.K.; Pfeiffer, J.K. Spectrum of Disease Outcomes in Mice Infected with YFV-17D. General. Virol. 2015, 96, 1328–1339. [Google Scholar] [CrossRef]
- Meier, K.C.; Gardner, C.L.; Khoretonenko, M.V.; Klimstra, W.B.; Ryman, K.D. A Mouse Model for Studying Viscerotropic Disease Caused by Yellow Fever Virus Infection. PLoS Pathog. 2009, 5, e1000614. [Google Scholar] [CrossRef] [PubMed]
- Kum, D.B.; Mishra, N.; Vrancken, B.; Thibaut, H.J.; Wilder-Smith, A.; Lemey, P.; Neyts, J.; Dallmeier, K. Limited Evolution of the Yellow Fever Virus 17d in a Mouse Infection Model. Emerg. Microbes Infect. 2019, 8, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, B.A.; Garbino, N.C.; Liss, N.M.; Piper, J.; Blair, C.D.; Roehrig, J.T. A Small Animal Peripheral Challenge Model of Yellow Fever Using Interferon-Receptor Deficient Mice and the 17D-204 Vaccine Strain. Vaccine 2012, 30, 3180–3187. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; Magaldi, F.M.; Sato, H.K.; Bevilacqua, E. Yellow Fever Vaccination in a Mouse Model Is Associated with Uninterrupted Pregnancies and Viable Neonates Except When Administered at Implantation Period. Front. Microbiol. 2020, 11, 245. [Google Scholar] [CrossRef]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H.; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685.e6. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type i Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Winkler, C.W.; Peterson, K.E. Using Immunocompromised Mice to Identify Mechanisms of Zika Virus Transmission and Pathogenesis _ Enhanced Reader. Immunology 2017, 153, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual Transmission of Zika Virus and Other Flaviviruses: A Living Systematic Review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef]
- Caine, E.A.; Jagger, B.W.; Diamond, M.S. Animal Models of Zika Virus Infection during Pregnancy. Viruses 2018, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika Virus Infection as a Silent Pathology with Loss of Neurogenic Output in the Fetal Brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Ashour, J.; Morrison, J.; Laurent-Rolle, M.; Belicha-Villanueva, A.; Plumlee, C.R.; Bernal-Rubio, D.; Williams, K.L.; Harris, E.; Fernandez-Sesma, A.; Schindler, C.; et al. Mouse STAT2 Restricts Early Dengue Virus Replication. Cell Host Microbe 2010, 8, 410–421. [Google Scholar] [CrossRef]
- Jácome, F.C.; Teixeira De Almeida, A.L.; Coutinho De Souza, D.D.; da Costa Rasinhas, A.; Caldas, G.C.; Nunes Da Silva, M.A.; Barth, O.M.; Barreto-Vieira, D.F. Secondary Dengue Infection in Immunocompetent Murine Model Leads to Heart Tissue Damage. Acta Virol. 2019, 63, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Yount, B.; Pantoja, P.; Henein, S.; Meganck, R.M.; McBride, J.; Munt, J.E.; Baric, T.J.; Zhu, D.; Scobey, T.; et al. A Live Dengue Virus Vaccine Carrying a Chimeric Envelope Glycoprotein Elicits Dual DENV2-DENV4 Serotype-Specific Immunity. Nat. Commun. 2023, 14, 1371. [Google Scholar] [CrossRef] [PubMed]
- Althouse, B.M.; Durbin, A.P.; Hanley, K.A.; Halstead, S.B.; Weaver, S.C.; Cummings, D.A.T. Viral Kinetics of Primary Dengue Virus Infection in Non-Human Primates: A Systematic Review and Individual Pooled Analysis. Virology 2014, 452–453, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Jasperse, B.A.; Mattocks, M.D.; Noll, K.E.; Ferris, M.T.; Heise, M.T.; Lazear, H.M. Neuroinvasive Flavivirus Pathogenesis Is Restricted by Host Genetic Factors in Collaborative Cross Mice, Independently of Oas1b. J. Virol. 2023, 97, e0071523. [Google Scholar] [CrossRef]
- Brown, A.J.; Won, J.J.; Wolfisberg, R.; Fahnøe, U.; Catanzaro, N.; West, A.; Moreira, F.R.; Nogueira Batista, M.; Ferris, M.T.; Linnertz, C.L.; et al. Host Genetic Variation Guides Hepacivirus Clearance, Chronicity, and Liver Fibrosis in Mice. Hepatology 2024, 79, 183–197. [Google Scholar] [CrossRef]
- Bharucha, T.; Cleary, B.; Farmiloe, A.; Sutton, E.; Hayati, H.; Kirkwood, P.; al Hamed, L.; van Ginneken, N.; Subramaniam, K.S.; Zitzmann, N.; et al. Mouse Models of Japanese Encephalitis Virus Infection: A Systematic Review and Metaanalysis Using a Meta-Regression Approach. PLoS Negl. Trop. Dis. 2022, 16, e0010116. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.L.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martínez-Romero, C.; Tenoever, B.R.; et al. The Interferon Signaling Antagonist Function of Yellow Fever Virus NS5 Protein Is Activated by Type i Interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef]
- Chen, R.E.; Diamond, M.S. Dengue Mouse Models for Evaluating Pathogenesis and Countermeasures. Curr. Opin. Virol. 2020, 43, 50–58. [Google Scholar] [CrossRef]
- Da Silveira, L.T.C.; Tura, B.; Santos, M. Systematic Review of Dengue Vaccine Efficacy. BMC Infect. Dis. 2019, 19, 750. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Horton, J.; Barrett, A.D.T.; Demanou, M.; Monath, T.P.; Tomori, O.; Van Herp, M.; Zeller, H.; Fall, I.S.; Cibrelus, L.; et al. Yellow Fever Resurgence: An Avoidable Crisis? NPJ Vaccines 2022, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, H.; Patel, M.A.; Gupta, V.; Bansal, T.; Upadhyay, S.; Shaheen, N.; Jain, R. COVID-19 Vaccine Challenges in Developing and Developed Countries. Cureus 2022, 14, e23951. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Wu, Y.S.; Poh, C.L. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses 2023, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-Spectrum Agents for Flaviviral Infections: Dengue, Zika and Beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.; Li, J.; et al. Mutations in the SARS-CoV-2 RNA-Dependent RNA Polymerase Confer Resistance to Remdesivir by Distinct Mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Sun, Y.; Rao, Z. Current Progress in Antiviral Strategies. Trends Pharmacol. Sci. 2014, 35, 86–102. [Google Scholar] [CrossRef]
- Ji, X.; Li, Z. Medicinal Chemistry Strategies toward Host Targeting Antiviral Agents. Med. Res. Rev. 2020, 40, 1519–1557. [Google Scholar] [CrossRef]
- Martin, A.M.; Nolan, D.; Gaudieri, S.; Phillips, E.; Mallal, S. Pharmacogenetics of Antiretroviral Therapy: Genetic Variation of Response and Toxicity. Pharmacogenomics 2004, 5, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Kilby, J.M.; Hopkins, S.; Venetta, T.M.; Dimassimo, B.; Cloud, G.A.; Lee, J.Y.; Alldredge, L.; Hunter, E.; Lambert, D.; Bolognesi, D.; et al. Potent Suppression of HIV-1 Replication in Humans by T-20, a Peptide Inhibitor of Gp41-Mediated Virus Entry. Nat. Med. 1998, 4, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and Function of Zika Virus NS5 Protein: Perspectives for Drug Design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef]
- Cihlar, T.; Ray, A.S. Nucleoside and Nucleotide HIV Reverse Transcriptase Inhibitors: 25 Years after Zidovudine. Antivir. Res. 2010, 85, 39–58. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Iversen, P.; Lu, X.; Zou, J.; Kaptein, S.J.F.; Stuthman, K.S.; Van Tongeren, S.A.; Steffens, J.; Gong, R.; Truong, H.; et al. Expanded Profiling of Remdesivir as a Broad-Spectrum Antiviral and Low Potential for Interaction with Other Medications in Vitro. Sci. Rep. 2023, 13, 3131. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H.; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad Spectrum Antiviral Remdesivir Inhibits Human Endemic and Zoonotic Deltacoronaviruses with a Highly Divergent RNA Dependent RNA Polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef] [PubMed]
- Ackaert, O.; Vanhoutte, F.; Verpoorten, N.; Buelens, A.; Lachau-Durand, S.; Lammens, L.; Hoetelmans, R.; Van Loock, M.; Herrera-Taracena, G. Safety, Tolerability, and Pharmacokinetics of JNJ-1802, a Pan-Serotype Dengue Direct Antiviral Small Molecule, in a Phase 1, Double-Blind, Randomized, Dose-Escalation Study in Healthy Volunteers. Clin. Infect. Dis. 2023, 77, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The Broad Spectrum Antiviral Ivermectin Targets the Host Nuclear Transport Importin α/Β1 Heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; De Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin Is a Potent Inhibitor of Flavivirus Replication Specifically Targeting NS3 Helicase Activity: New Prospects for an Old Drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A Nuclear Transport Inhibitor That Modulates the Unfolded Protein Response and Provides in Vivo Protection against Lethal Dengue Virus Infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen®-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import. SLAS Discov. 2011, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.C.; Audsley, M.D.; Lieu, K.G.; Marsh, G.A.; Thomas, D.R.; Heaton, S.M.; Paxman, J.J.; Wagstaff, K.M.; Buckle, A.M.; Moseley, G.W.; et al. Recognition by Host Nuclear Transport Proteins Drives Disorder-to-Order Transition in Hendra Virus v. Sci. Rep. 2018, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Fraser, J.E.; Wang, C.; Maher, B.; Roman, N.; Forwood, J.K.; Wagstaff, K.M.; Borg, N.A.; Jans, D.A. Novel Flavivirus Antiviral That Targets the Host Nuclear Transport Importin α/Β1 Heterodimer. Cells 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Naggie, S.; Boulware, D.R.; Lindsell, C.J.; Stewart, T.G.; Gentile, N.; Collins, S.; McCarthy, M.W.; Jayaweera, D.; Castro, M.; Sulkowski, M.; et al. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022, 328, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Campillo, T.J.; Faillie, J.-L. Adverse Drug Reactions Associated with Ivermectin Use For-19 Reported in the World Organization’s Pharmacovigilance Database. Therapies 2022, 77, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Levine, B.; Hardwick, J.M.; Trapp, B.D.; Crawford, T.O.; Bollinger, R.C.; Griffin, D.E. Antibody-Mediated Clearance of Alphavirus Infection from Neurons. Science 1991, 254, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Kashmiri, S.V.S.; De Pascalis, R.; Gonzales, N.R.; Schlom, J. SDR Grafting—A New Approach to Antibody Humanization. Methods 2005, 36, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gunale, B.; Farinola, N.; Kamat, C.D.; Poonawalla, C.S.; Pisal, S.S.; Dhere, R.M.; Miller, C.; Kulkarni, P.S. An Observer-Blind, Randomised, Placebo-Controlled, Phase 1, Single Ascending Dose Study of Dengue Monoclonal Antibody in Healthy Adults in Australia. Lancet Infect. Dis. 2024, 24, 639–649. [Google Scholar] [CrossRef]
- Robbie, G.J.; Criste, R.; Dall’Acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A Novel Investigational Fc-Modified Humanized Monoclonal Antibody, Motavizumab-YTE, Has an Extended Half-Life in Healthy Adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Kafai, N.M.; Burgomaster, K.E.; Jethva, P.N.; Broeckel, R.M.; Meade-White, K.; Nelson, C.A.; Himansu, S.; Wang, D.; et al. Broadly Neutralizing Monoclonal Antibodies Protect against Multiple Tick-Borne Flaviviruses. J. Exp. Med. 2021, 218, e20210174. [Google Scholar] [CrossRef]
- Wahala, W.M.P.B.; Kraus, A.A.; Haymore, L.B.; Accavitti-Loper, M.A.; de Silva, A.M. Dengue Virus Neutralization by Human Immune Sera: Role of Envelope Protein Domain III-Reactive Antibody. Virology 2009, 392, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Carnahan, R.H.; Andrade, D.V.; Kose, N.; Nargi, R.S.; Fritch, E.J.; Munt, J.E.; Doyle, M.P.; White, L.; Baric, T.J.; et al. Identification of Dengue Virus Serotype 3 Specific Antigenic Sites Targeted by Neutralizing Human Antibodies. Cell Host Microbe 2020, 27, 710–724.e7. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinsky, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A New Class of Highly Potent, Broadly Neutralizing Antibodies Isolated from Viremic Patients Infected with Dengue Virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef]
- Sharma, A.; Zhang, X.; Dejnirattisai, W.; Dai, X.; Gong, D.; Wongwiwat, W.; Duquerroy, S.; Rouvinski, A.; Vaney, M.C.; Guardado-Calvo, P.; et al. The Epitope Arrangement on Flavivirus Particles Contributes to Mab C10’s Extraordinary Neutralization Breadth across Zika and Dengue Viruses. Cell 2021, 184, 6052–6066.e18. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, Cross-Reactivity, and Function of Antibodies Elicited by Zika Virus Infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Nybakken, G.E.; Oliphant, T.; Johnson, S.; Burke, S.; Diamond, M.S.; Fremont, D.H. Structural Basis of West Nile Virus Neutralization by a Therapeutic Antibody. Nature 2005, 437, 764–769. [Google Scholar] [CrossRef]
- Bailey, M.J.; Broecker, F.; Duehr, J.; Arumemi, F.; Krammer, F.; Palese, P.; Tan, G.S. Antibodies Elicited by an NS1-Based Vaccine Protect Mice against Zika Virus. mBio 2019, 10, e02861-18. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.M.; Summers, P.L.; Dubois, D.R.; Cohen, W.H.; Gentry, M.K.; Timchak, R.L.; Burke, D.S.; Eckels, K.H. Monoclonal Antibodies for Dengue Virus PrM Glycoprotein Protect Mice against Lethal Dengue Infection. Am. J. Trop. Med. Hyg. 1989, 41, 576–580. [Google Scholar] [CrossRef]
- Oliphant, T.; Engle, M.; Nybakken, G.E.; Doane, C.; Johnson, S.; Huang, L.; Gorlatov, S.; Mehlhop, E.; Marri, A.; Chung, K.M.; et al. Development of a Humanized Monoclonal Antibody with Therapeutic Potential against West Nile Virus. Nat. Med. 2005, 11, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Goo, L.; Debbink, K.; Kose, N.; Sapparapu, G.; Doyle, M.P.; Wessel, A.W.; Richner, J.M.; Burgomaster, K.E.; Larman, B.C.; Dowd, K.A.; et al. A Protective Human Monoclonal Antibody Targeting the West Nile Virus E Protein Preferentially Recognizes Mature Virions. Nat. Microbiol. 2019, 4, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sapparapu, G.; Fernandez, E.; Kose, N.; Cao, B.; Fox, J.M.; Bombardi, R.G.; Zhao, H.; Nelson, C.A.; Bryan, A.L.; Barnes, T.; et al. Neutralizing Human Antibodies Prevent Zika Virus Replication and Fetal Disease in Mice. Nature 2016, 540, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Erra, E.O.; Askling, H.H.; Yoksan, S.; Rombo, L.; Riutta, J.; Vene, S.; Lindquist, L.; Vapalahti, O.; Kantele, A. Cross-Protective Capacity of Japanese Encephalitis (JE) Vaccines against Circulating Heterologous JE Virus Genotypes. Clin. Infect. Dis. 2013, 56, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Orlinger, K.K.; Hofmeister, Y.; Fritz, R.; Holzer, G.W.; Falkner, F.G.; Unger, B.; Loew-Baselli, A.; Poellabauer, E.M.; Ehrlich, H.J.; Barrett, P.N.; et al. A Tick-Borne Encephalitis Virus Vaccine Based on the European Prototype Strain Induces Broadly Reactive Cross-Neutralizing Antibodies in Humans. J. Infect. Dis. 2011, 203, 1556–1564. [Google Scholar] [CrossRef]
- Martinez, D.R.; Yount, B.; Nivarthi, U.; Munt, J.E.; Delacruz, M.J.; Whitehead, S.S.; Durbin, A.P.; de Silva, A.M.; Baric, R.S. Antigenic Variation of the Dengue Virus 2 Genotypes Impacts the Neutralization Activity of Human Antibodies in Vaccinees. Cell Rep. 2020, 33, 108226. [Google Scholar] [CrossRef]
- Gallichotte, E.N.; Henein, S.; Nivarthi, U.; Delacruz, M.; Scobey, T.; Bonaparte, M.; Moser, J.; Munteanu, A.; Baric, R.; de Silva, A.M. Vaccine-Induced Antibodies to Contemporary Strains of Dengue Virus Type 4 Show a Mechanistic Correlate of Protective Immunity. Cell Rep. 2022, 39, 110930. [Google Scholar] [CrossRef]
- Henein, S.; Adams, C.; Bonaparte, M.; Moser, J.M.; Munteanu, A.; Baric, R.; De Silva, A.M. Dengue Vaccine Breakthrough Infections Reveal Properties of Neutralizing Antibodies Linked to Protection. J. Clin. Investig. 2021, 131, e147066. [Google Scholar] [CrossRef] [PubMed]
- Juraska, M.; Magaret, C.A.; Shao, J.; Carpp, L.N.; Fiore-Gartland, A.J.; Benkeser, D.; Girerd-Chambaz, Y.; Langevin, E.; Frago, C.; Guy, B.; et al. Viral Genetic Diversity and Protective Efficacy of a Tetravalent Dengue Vaccine in Two Phase 3 Trials. Proc. Natl. Acad. Sci. USA 2018, 115, E8378–E8387. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The Human CD8+ T Cell Responses Induced by a Live Attenuated Tetravalent Dengue Vaccine Are Directed against Highly Conserved Epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Phillips, E.; Mallal, S.; Diehl, S.A.; et al. Human CD4+ T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity. J. Virol. 2017, 91, e02147-16. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Engle, R.E.; St. Claire, M.; Purcell, R.H.; Lai, C.J. Monoclonal Antibody-Mediated Enhancement of Dengue Virus Infection in Vitro and in Vivo and Strategies for Prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, L.; Zhang, C.; Wang, X.; Hong, W.; Sun, J.; Liu, R.; Yu, L.; Wang, J.; Zhang, F.; et al. Dengue Immune Sera Enhance Zika Virus Infection in Human Peripheral Blood Monocytes through Fc Gamma Receptors. PLoS ONE 2018, 13, e0200478. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Zambrana, J.V.; Elizondo, D.; Collado, D.; Garcia, N.; Arguello, S.; Mercado, J.C.; Miranda, T.; Ampie, O.; Mercado, B.L.; et al. Dengue and Zika Virus Infections in Children Elicit Cross-Reactive Protective and Enhancing Antibodies That Persist Long Term. Sci. Transl. Med. 2021, 13, eabg9478. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Singh, G.; Acklin, J.A.; Lee, S.; Duehr, J.E.; Chokola, A.N.; Frere, J.J.; Hoffman, K.W.; Foster, G.A.; Krysztof, D.; et al. Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy Provided Critical Reagents and Intellectual Input HHS Public Access. Immunity 2019, 50, 751–762. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.R.; Montes-Gómez, A.E.; García-Cordero, J.; Corzo-Gómez, J.; Vivanco-Cid, H.; Mellado-Sánchez, G.; Muñoz-Medina, J.E.; Gutiérrez-Castañeda, B.; Santos-Argumedo, L.; González-Bonilla, C.; et al. Cross-Reaction, Enhancement, and Neutralization Activity of Dengue Virus Antibodies against Zika Virus: A Study in the Mexican Population. J. Immunol. Res. 2019, 2019, 7239347. [Google Scholar] [CrossRef]
- Xu, M.; Züst, R.; Toh, Y.X.; Pfaff, J.M.; Kahle, K.M.; Davidson, E.; Doranz, B.J.; Velumani, S.; Tukijan, F.; Wang, C.-I.; et al. Protective Capacity of the Human Anamnestic Antibody Response during Acute Dengue Virus Infection. J. Virol. 2016, 90, 11122–11131. [Google Scholar] [CrossRef]
- Huerta, V.; Chinea, G.; Fleitas, N.; Sarría, M.; Sánchez, J.; Toledo, P.; Padrón, G. Characterization of the Interaction of Domain III of the Envelope Protein of Dengue Virus with Putative Receptors from CHO Cells. Virus Res. 2008, 137, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Munt, J.E.; Henein, S.; Adams, C.; Young, E.; Hou, Y.J.; Conrad, H.; Zhu, D.; Dong, S.; Kose, N.; Yount, B.; et al. Homotypic Antibodies Target Novel E Glycoprotein Domains after Natural DENV 3 Infection/Vaccination. Cell Host Microbe 2023, 31, 1850–1865.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.R.; Rajesh, A.J.; Meganck, R.M.; Young, E.F.; Munt, J.E.; Tse, V.L.; Yount, B.; Conrad, H.; White, L.; Henein, S.; et al. Dengue Virus 4/2 Envelope Domain Chimeric Virus Panel Maps Type-Specific Responses against Dengue Serotype 2. mBio 2023, 14, e00818-23. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Arroyo, J.; Pugachev, K.V.; Miller, C.; Zhang, Z.-X.; Weltzin, R.; Georgakopoulos, K.; Catalan, J.; Ocran, S.; Soike, K.; et al. Construction, Safety, and Immunogenicity in Nonhuman Primates of a Chimeric Yellow Fever-Dengue Virus Tetravalent Vaccine. J. Virol. 2001, 75, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Hj Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ylade, M.; Crisostomo, M.V.; Daag, J.V.; Agrupis, K.A.; Cuachin, A.M.; Sy, A.K.; Kim, D.R.; Ahn, H.S.; Escoto, A.C.; Katzelnick, L.C.; et al. Effect of Single-Dose, Live, Attenuated Dengue Vaccine in Children with or without Previous Dengue on Risk of Subsequent, Virologically Confirmed Dengue in Cebu, the Philippines: A Longitudinal, Prospective, Population-Based Cohort Study. Lancet Infect. Dis. 2024, 24, 737–745. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2016; Volume 91, pp. 346–348. [Google Scholar]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Zika Vaccine Development: Current Status. Mayo Clin. Proc. 2019, 94, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- al-Haddad, B.J.S.; Oler, E.; Armistead, B.; Elsayed, N.A.; Weinberger, D.R.; Bernier, R.; Burd, I.; Kapur, R.; Jacobsson, B.; Wang, C.; et al. The Fetal Origins of Mental Illness. Am. J. Obstet. Gynecol. 2019, 221, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid Development of a DNA Vaccine for Zika Virus. Science (1979) 2016, 354, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; Maclaren, G.; Brodie, D. Safety, Tolerability, and Immunogenicity of Two Zika Virus DNA Vaccine Candidates in Healthy Adults: Randomised, Open-Label, Phase 1 Clinical Trials. Lancet 2018, 391, 552–562. [Google Scholar]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika Virus Protection by a Single Low-Dose Nucleoside-Modified MRNA Vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.W.; Chu, H.J. Equine Vaccine for West Nile Virus. Dev. Biol. 2003, 114, 221–227. [Google Scholar]
- el Garch, H.; Minke, J.M.; Rehder, J.; Richard, S.; Edlund Toulemonde, C.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V. A West Nile Virus (WNV) Recombinant Canarypox Virus Vaccine Elicits WNV-Specific Neutralizing Antibodies and Cell-Mediated Immune Responses in the Horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Atmar, R.L.; Cate, T.R.; Petersen, N.J.; Greenberg, S.B.; Ruben, F.; Couch, R.B. Safety of High Doses of Influenza Vaccine and Effect on Antibody Responses in Elderly Persons. Arch. Intern. Med. 2006, 166, 1121–1127. [Google Scholar] [CrossRef]
- Danet, L.; Beauclair, G.; Berthet, M.; Moratorio, G.; Gracias, S.; Tangy, F.; Choumet, V.; Jouvenet, N. Midgut Barriers Prevent the Replication and Dissemination of the Yellow Fever Vaccine in Aedes Aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007299. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, L.; Nie, K.; Zhao, D.; Na, R.; Xu, H.; Cheng, G.; Wang, J.; Yu, Y.; Li, Y. Evaluation of Environment Safety of a Japanese Encephalitis Live Attenuated Vaccine. Biologicals 2019, 60, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Liu, J.; Kanesa-Thasan, N.; Myers, G.A.; Nichols, R.; Deary, A.; McCarthy, K.; Johnson, C.; Ermak, T.; Shin, S.; et al. A Live, Attenuated Recombinant West Nile Virus Vaccine. Proc. Natl. Acad. Sci. USA 2006, 103, 6694–6699. [Google Scholar] [CrossRef]
- Dayan, G.H.; Bevilacqua, J.; Coleman, D.; Buldo, A.; Risi, G. Phase II, Dose Ranging Study of the Safety and Immunogenicity of Single Dose West Nile Vaccine in Healthy Adults ≥ 50 Years of Age. Vaccine 2012, 30, 6656–6664. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Wright, P.F.; Cox, A.; Kagucia, W.; Elwood, D.; Henderson, S.; Wanionek, K.; Speicher, J.; Whitehead, S.S.; Pletnev, A.G. The Live Attenuated Chimeric Vaccine RWN/DEN4Δ30 Is Well-Tolerated and Immunogenic in Healthy Flavivirus-Naïve Adult Volunteers. Vaccine 2013, 31, 5772–5777. [Google Scholar] [CrossRef]
- Woods, C.W.; Sanchez, A.M.; Swamy, G.K.; McClain, M.T.; Harrington, L.; Freeman, D.; Poore, E.A.; Slifka, D.K.; Poer DeRaad, D.E.; Amanna, I.J.; et al. An Observer Blinded, Randomized, Placebo-Controlled, Phase I Dose Escalation Trial to Evaluate the Safety and Immunogenicity of an Inactivated West Nile Virus Vaccine, HydroVax-001, in Healthy Adults. Vaccine 2019, 37, 4222–4230. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A West Nile Virus DNA Vaccine Utilizing a Modified Promoter Induces Neutralizing Antibody in Younger and Older Healthy Adults in a Phase I Clinical Trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- De Filette, M.; Ulbert, S.; Diamond, M.S.; Sanders, N.N. Recent Progress in West Nile Virus Diagnosis and Vaccination. Vet. Res. 2012, 43, 16. [Google Scholar] [CrossRef]
- Lindquist, L.; Vapalahti, O. Tick-Borne Encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Yun, S.I.; Lee, Y.M. Japanese Encephalitis the Virus and Vacci. Hum. Vaccin. Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.T. Yellow Fever Live Attenuated Vaccine: A Very Successful Live Attenuated Vaccine but Still We Have Problems Controlling the Disease. Vaccine 2017, 35, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Wollner, C.J.; Richner, J.M. MRNA Vaccines against Flaviviruses. Vaccines 2021, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Geng, J.; Ah Yi, H.; Gogia, S.; Neelamegham, S.; Jacobs, A.; Lovell, J.F. Functionalization of Cobalt Porphyrin-Phospholipid Bilayers with His-Tagged Ligands and Antigens. Nat. Chem. 2015, 7, 438–446. [Google Scholar] [CrossRef]
- Sevvana, M.; Kuhn, R.J. Mapping the Diverse Structural Landscape of the Flavivirus Antibody Repertoire. Curr. Opin. Virol. 2020, 45, 51–64. [Google Scholar] [CrossRef]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition Determinants of Broadly Neutralizing Human Antibodies against Dengue Viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef]
- Kudlacek, S.T.; Metz, S.; Thiono, D.; Payne, A.M.; Phan, T.T.N.; Tian, S.; Forsberg, L.J.; Maguire, J.; Seim, I.; Zhang, S.; et al. Designed, Highly Expressing, Thermostable Dengue Virus 2 Envelope Protein Dimers Elicit Quaternary Epitope Antibodies. Sci. Adv. 2021, 7, eabg4084. [Google Scholar] [CrossRef]
- De Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.P.B.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E.; et al. Identification of Human Neutralizing Antibodies That Bind to Complex Epitopes on Dengue Virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Manoff, S.B.; Sausser, M.; Falk Russell, A.; Martin, J.; Radley, D.; Hyatt, D.; Roberts, C.C.; Lickliter, J.; Krishnarajah, J.; Bett, A.; et al. Immunogenicity and Safety of an Investigational Tetravalent Recombinant Subunit Vaccine for Dengue: Results of a Phase I Randomized Clinical Trial in Flavivirus-Naïve Adults. Hum. Vaccin. Immunother. 2019, 15, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.A.G.; Clements, D.E.; Bett, A.J.; Sagar, S.L.; Ter Meulen, J.H. The Development of Recombinant Subunit Envelope-Based Vaccines to Protect against Dengue Virus Induced Disease. Vaccine 2011, 29, 7267–7275. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and Structures of a Rationally Designed Prefusion MERS-CoV Spike Antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Antanasijevic, A.; Fallas, J.A.; Sheffler, W.; Copps, J.; Ellis, D.; Hutchinson, G.B.; Moyer, A.; Yasmeen, A.; Tsybovsky, Y.; et al. Tailored Design of Protein Nanoparticle Scaffolds for Multivalent Presentation of Viral Glycoprotein Antigens. eLife 2020, 9, e57659. [Google Scholar] [CrossRef]
- Hutchinson, G.B.; Abiona, O.M.; Ziwawo, C.T.; Werner, A.P.; Ellis, D.; Tsybovsky, Y.; Leist, S.R.; Palandjian, C.; West, A.; Fritch, E.J.; et al. Nanoparticle Display of Prefusion Coronavirus Spike Elicits S1-Focused Cross-Reactive Antibody Response against Diverse Coronavirus Subgenera. Nat. Commun. 2023, 14, 6195. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Hvasta, M.G.; Kudlacek, S.T.; Thiono, D.J.; Tripathy, A.; Nicely, N.I.; de Silva, A.M.; Kuhlman, B. A Conserved Set of Mutations for Stabilizing Soluble Envelope Protein Dimers from Dengue and Zika Viruses to Advance the Development of Subunit Vaccines. J. Biol. Chem. 2022, 298, 102079. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Dejnirattisai, W.; Guardado-Calvo, P.; Vaney, M.C.; Sharma, A.; Duquerroy, S.; Supasa, P.; Wongwiwat, W.; Haouz, A.; Barba-Spaeth, G.; et al. Covalently Linked Dengue Virus Envelope Glycoprotein Dimers Reduce Exposure of the Immunodominant Fusion Loop Epitope. Nat. Commun. 2017, 8, 15411. [Google Scholar] [CrossRef] [PubMed]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; López-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A Protective Zika Virus E-Dimer-Based Subunit Vaccine Engineered to Abrogate Antibody-Dependent Enhancement of Dengue Infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Meganck, R.M.; Zhu, D.; Dong, S.; Snoderly-Foster, L.J.; Dalben, Y.R.; Thiono, D.; White, L.J.; Desilva, A.M.; Baric, R.S.; Tse, L.V. Evolution of a Functionally Intact but Antigenically Distinct DENV Fusion Loop. eLife 2023, 12, RP87555. [Google Scholar] [CrossRef] [PubMed]
- Mateu Ferrando, R.; Lay, L.; Polito, L. Gold Nanoparticle-Based Platforms for Vaccine Development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kaushal, A.; Gupta, S. Vaccine Development: Current Trends and Technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified MRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baric, T.J.; Reneer, Z.B. Animal Models, Therapeutics, and Vaccine Approaches to Emerging and Re-Emerging Flaviviruses. Viruses 2025, 17, 1. https://doi.org/10.3390/v17010001
Baric TJ, Reneer ZB. Animal Models, Therapeutics, and Vaccine Approaches to Emerging and Re-Emerging Flaviviruses. Viruses. 2025; 17(1):1. https://doi.org/10.3390/v17010001
Chicago/Turabian StyleBaric, Thomas J., and Z. Beau Reneer. 2025. "Animal Models, Therapeutics, and Vaccine Approaches to Emerging and Re-Emerging Flaviviruses" Viruses 17, no. 1: 1. https://doi.org/10.3390/v17010001
APA StyleBaric, T. J., & Reneer, Z. B. (2025). Animal Models, Therapeutics, and Vaccine Approaches to Emerging and Re-Emerging Flaviviruses. Viruses, 17(1), 1. https://doi.org/10.3390/v17010001







