Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Virus Materials
2.2. Virus Inoculation
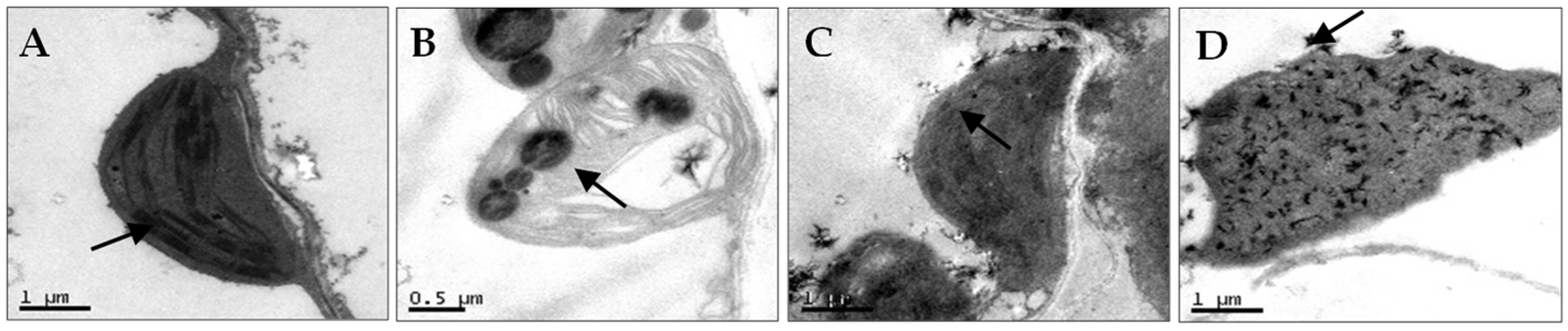
2.3. Chloroplast Ultrastructure Observations
2.4. RT-qPCR Detection
2.5. Production and Quantitative Analysis of Standard Curves of Target Gene and Internal Gene
2.6. Chlorophyll Extraction and Photosynthetic Pigment Measurement
2.7. Photosynthetic Gas Exchange Measurements
2.8. Chlorophyll Fluorescence Parameters Measurement
2.9. Data Analysis
3. Results
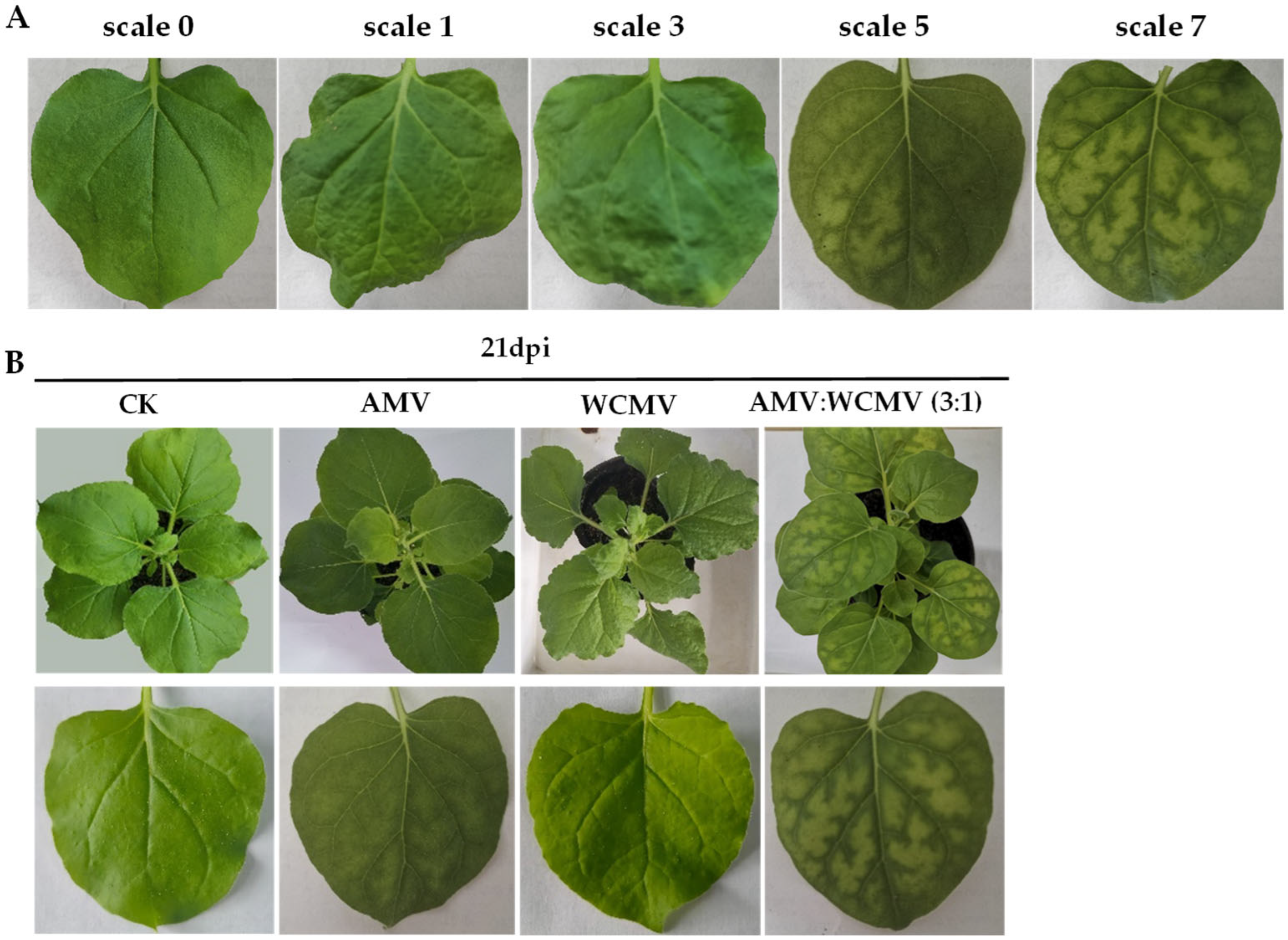
3.1. Diseased Symptoms and Chloroplast Ultrastructural Changes of N. benthamiana After AMV and WCMV Co-Infection
3.2. Production of Standard Curves and Relative Accumulation of AMV CP and WCMV CP after AMV and WCMV Co-Infection
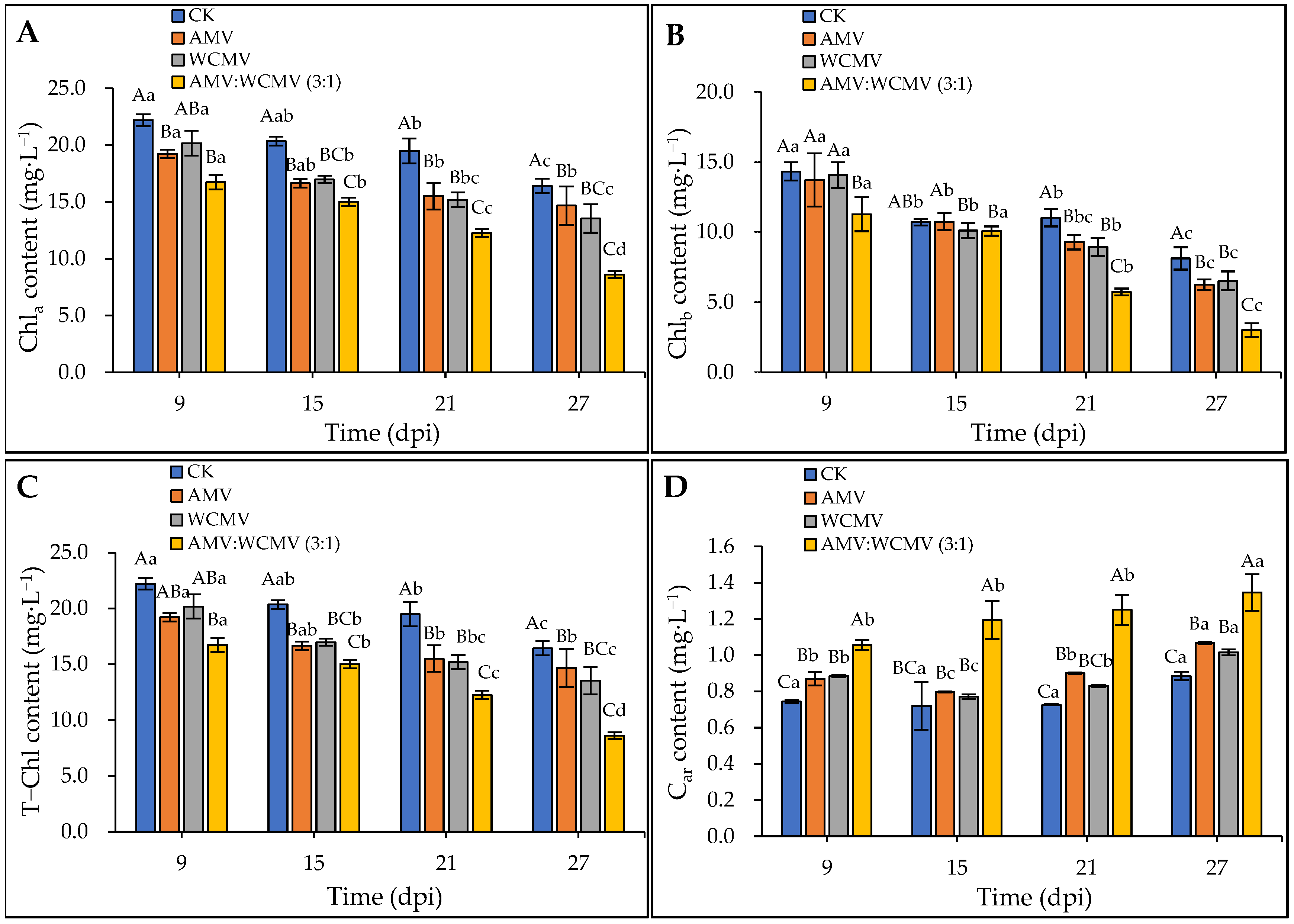
3.3. Changes in the Contents of Photosynthetic Pigments in N. benthamiana after AMV and WCMV Co-Infection
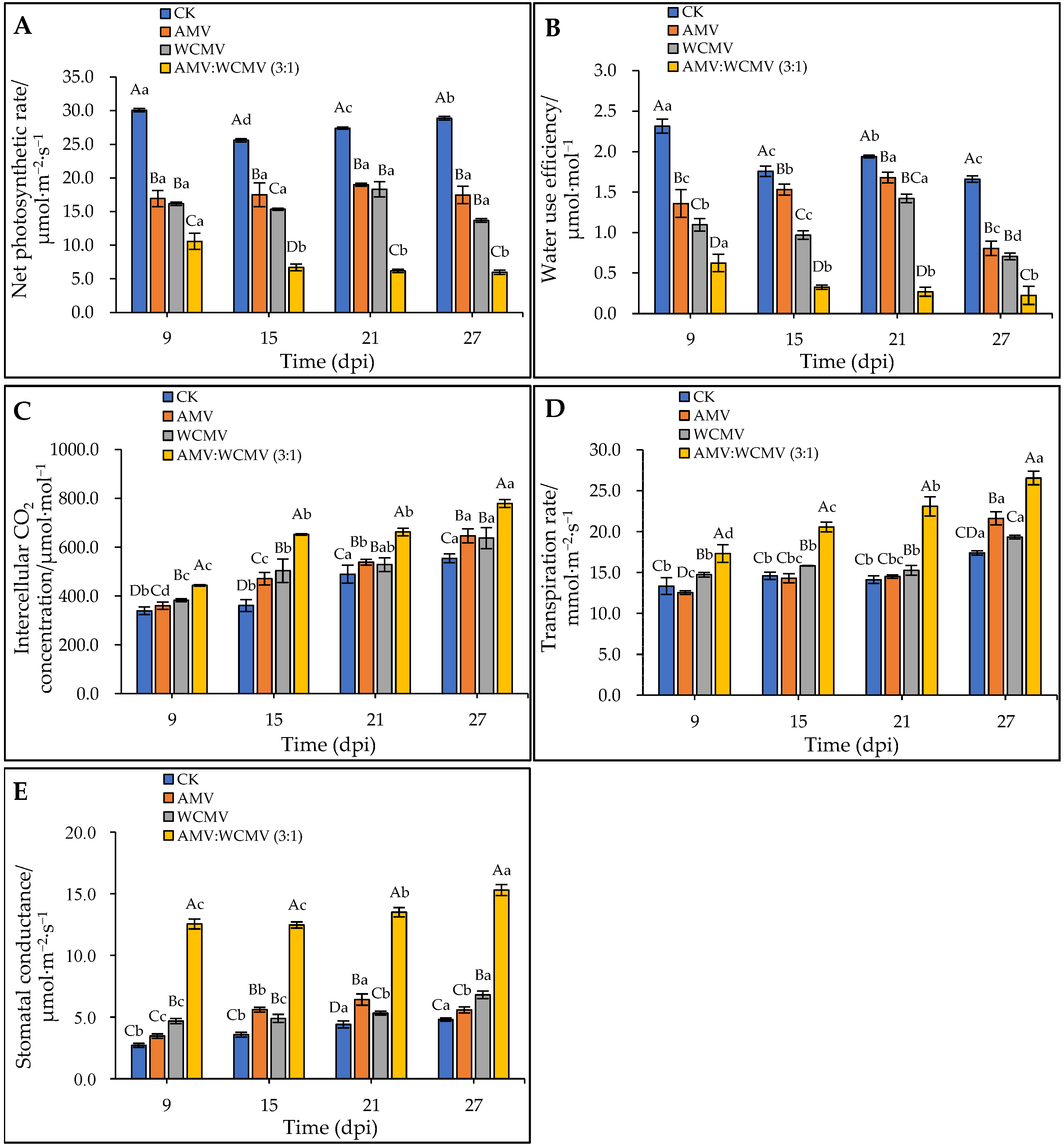
3.4. The Changes in Gas Exchange During Photosynthesis in N. benthamiana after AMV and WCMV Co-Infection
3.5. The Changes in Chlorophyll Fluorescence Parameters in N. benthamiana after AMV and WCMV Co-Infection
4. Discussion
4.1. Synergistic Effect of AMV and WCMV Co-Infection
4.2. Effect on Photosynthetic Characteristics of N. benthamiana after AMV and WCMV Co-Infection
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, S.; Singh, K.; Singh, D.; Sahu, S.K.; Chakraborty, S. Role of viral suppressors governing asymmetric synergism between tomato-infecting be gomoviruses. Appl. Microbiol. Biotechnol. 2021, 105, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.N.; Padmanabhan, M.S. Virus-induced disease: Altering host physiology one interaction at a time. Annu. Rev. Phytopathol. 2007, 45, 221–243. [Google Scholar] [CrossRef]
- Bellah, H.; Seiler, N.F.; Croll, D. Divergent outcomes of direct conspecific pathogen strain interaction and plant co-infection suggest consequences for disease dynamics. Microbiol. Spectr. 2023, 11, e0444322. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, T.V.M.; Peiró, A.; Pallás, V.; Sánchez-Navarro, J. Systemic transport of alfalfa mosaic virus can be mediated by the movement proteins of several viruses assigned to five genera of the 30K family. J. Gen. Virol. 2013, 94, 677–681. [Google Scholar] [CrossRef]
- Janeczko, A.; Dziurka, M.; Gullner, G.; Kocurek, M.; Rys, M.; Saja, D.; Skoczowski, A.; T’obi´as, I.; Kornas, A.; Barna, B. Comparative studies of compatible and incompatible pepper–Tobamovirus interactions and the evaluation of effects of 24-epibrassinolide. Photosynthetica 2018, 56, 763–775. [Google Scholar] [CrossRef]
- Crescenzi, A.; Viggiano, A.; Fanigliulo, A. Resistance breaking tomato spotted wilt virus isolates on resistant pepper varieties in Italy. Commun. Agric. Appl. Biol. Sci. 2013, 78, 609–612. [Google Scholar] [PubMed]
- Bhat, S.; Folimonova, S.Y.; Cole, A.B.; Ballard, K.D.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Nelson, R.S. Influence of host chloroplast proteins on Tobacco mosaic virus accumulation and intercellular movement. Plant Physiol. 2013, 161, 134–147. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef]
- Hallik, L.; Niinemets, U.; Kull, O. Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biol. 2012, 14, 88–99. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, T.; Chen, Z.; Niu, J.; Cui, X.; Mao, Y.; Hassan, M.U.; Kareem, H.A.; Xu, N.; Sui, X.; et al. Occurrence, distribution, and genetic diversity of alfalfa (Medicago sativa L.) viruses in four major alfalfa producing provinces of China. Front. Microbiol. 2022, 12, 771361. [Google Scholar] [CrossRef]
- Li, J.; Shang, Q.; Liu, Y.; Dai, W.; Li, X.; Wei, S.; Hu, G.; McNeill, M.R.; Ban, L. Occurrence, distribution, and transmission of alfalfa viruses in China. Viruses 2022, 14, 1519. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liang, Q.L.; Wei, L.X.; Chen, Y.E.; Cheng, S.F. Effects of Alfalfa Mosaic Virus (AMV) and White Clover Mosaic Virus (WCMV) Co-infection on Four Endogenous Hormones in Nicotiana benthamiana. Chin. J. Grassl. 2023, 45, 99–108. [Google Scholar]
- Piñeyro, M.J.; Albrecht, K.A.; Mondjana, A.M.; Grau, C.R. First Report of Alfalfa mosaic virus in Kura Clover (Trifolium amgibuum) in Wisconsin. Plant Dis. 2002, 86, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, O.A.; Ali, A. First Report of Alfalfa mosaic virus Associated with Severe Mosaic and Mottling of Pepper (Capsicum annuum) and White Clover (Trifolium repens) in Oklahoma. Plant Dis. 2012, 96, 1705. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C.; Congdon, B.S. Australian Cool-Season Pulse Seed-Borne Virus Research: 1. Alfalfa and Cucumber Mosaic Viruses and Less Important Viruses. Viruses 2024, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Liang, Q.L.; Wei, L.X.; Wang, D.; Tian, L.; Tong, F.Y.; Zhang, G.Y.; Cheng, S.F. Study on the epidemic factors of alfalfa mosaic virus and white clover mosaic virus co-infection Nicotiana benthamiana. Pratacultural Sci. 2023, 40, 90–100. [Google Scholar]
- Cheng, S.F.; Liang, Q.L.; Wei, L.X.; Sang, X.W.; Jiang, Y.L. Detection of alfalfa mosaic virus and white clover mosaic virus in alfalfa and their effects on physiological and biochemical characteristics of alfalfa plants. Acta Prataculturae Sin. 2020, 29, 140–149. [Google Scholar]
- Jang, C.; Seo, E.Y.; Nam, J.; Bae, H.; Gim, Y.G.; Kim, H.G.; Cho, I.S.; Lee, Z.W.; Bauchan, G.R.; Hammond, J.; et al. Insights into Alternanthera mosaic virus TGB3 functions: Interactions with Nicotiana benthamiana PsbO correlate with chloroplast vesiculation and veinal necrosis caused by TGB3 over-expression. Front. Plant Sci. 2013, 4, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L.; Wei, L.X.; Xu, B.L.; Calderón-Urrea, A.; Xiang, D. Study of viruses co-infecting white clover (Trifolium repens) in China. J. Integr. Agric. 2017, 16, 1990–1998. [Google Scholar] [CrossRef]
- Livak, K.J.; Wills, Q.F.; Tipping, A.J.; Datta, K.; Mittal, R.; Goldson, A.J.; Sexton, D.W.; Holmes, C.C. Methods for qPCR gene expression profiling applied to 1440 lymphoblastoid single cells. Methods 2013, 59, 71–79. [Google Scholar] [CrossRef]
- Song, X.S.; Wang, Y.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Nogués, S.; Yu, J.Q. Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiol. Plant. 2009, 135, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Tobar, M.; Fiore, N.; Pérez-Donoso, A.G.; León, R.; Rosales, I.M.; Gambardella, M. Divergent molecular and growth responses of young “Cabernet Sauvignon” (Vitis vinifera) plants to simple and mixed infections with Grapevine rupestris stem pitting-associated virus. Hortic. Res. 2020, 7, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzac-i, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Liu, D.; Zhou, X.; Yang, Q. Tomato Yellow Leaf Curl China Virus Impairs Photosynthesis in the Infected Nicotiana benthamiana with βC1 as an Aggravating Factor. Plant Pathol. J. 2019, 35, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Niu, E.; Liu, H.; Zhou, H.; Luo, L.; Wu, Y.; Andika, I.B.; Sun, L. Autophagy Inhibits Intercellular Transport of Citrus Leaf Blotch Virus by Targeting Viral Movement Protein. Viruses 2021, 13, 2189. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.C.; Kim, M.K.; Kwak, H.R.; Choi, H.S.; Kim, J.S.; Park, C.Y.; Cha, B.J. First report of clover yellow vein virus on glycine max in Korea. Plant Dis. 2014, 98, 1283. [Google Scholar] [CrossRef] [PubMed]
- Trębicki, P.; Dáder, B.; Vassiliadis, S.; Fereres, A. Insect-plant-pathogen interactions as shaped by future climate: Effects on biology, distribution, and implications for agriculture. Insect Sci. 2017, 24, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, G.Q.; Chai, J.K.; Wang, M.M.; Jia, X.J.; Guo, Z.F.; Sun, L.L.; Nie, X.M. Effect of barley yellow dwarf virus infection on photosynthesis and chlorophyll fluorescence parameters of oat. Acta Agrestia Sin. 2020, 28, 923–931. [Google Scholar]
- Tseliou, E.; Chondrogiannis, C.; Kalachanis, D.; Goudoudaki, S.; Manoussopoulos, Y.; Grammatikopoulos, G. Integration of biophysical photosynthetic parameters into one photochemical index for early detection of tobacco mosaic virus infection in pepper plants. J. Plant Physiol. 2021, 267, 153542. [Google Scholar] [CrossRef]
- Li, D.X.; Yuan, H.Y.; Guo, Y.X.; Mu, F.; Gong, X.Y.; Zhang, M. Mixture solution soaking extraction efficiencies of chlorophyll from maize. J. Maize Sci. 2006, 14, 117–119. [Google Scholar]
- Lehto, K.; Tikkanen, M.; Hiriart, J.B.; Paakkarinen, V.; Aro, E.M. Depletion of the photosystem II core complex in mature tobacco leaves infected by the flavum strain of tobacco mosaic virus. Mol. Plant-Microbe Interact. 2003, 16, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.A.; Kaur, H.; Ray, I.; Yu, L.X. Strategies to increase prediction accuracy in genomic selection of complex traits in Alfalfa (Medicago sativa L.). Cells 2021, 10, 3372. [Google Scholar] [CrossRef]
- Otulak, K.; Chouda, M.; Bujarski, J.; Garbaczewska, G. The evidence of tobacco rattle virus impact on host plant organelles ultrastructure. Micron 2015, 70, 7–20. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef]
- Torrance, L.; Talianksy, M.E. Potato virus Y emergence and evolution from the andes of south america to become a major destructive pathogen of potato and other solanaceous crops worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Romero, J.M.; Lagorio, M.G. Effects of sub-optimal illumination in plants. Comprehensive chlorophyll fluorescence analysis. J. Photochem. Photobiol. B Biol. 2021, 218, 112182. [Google Scholar] [CrossRef]
- Wilhelmová, N.; Procházková, D.; Sindelarova, M.; Sindelar, L. Photosynthesis in leaves of Nicotiana tabacum L. infected with tobacco mosaic virus. Photosynthetica 2005, 43, 597–602. [Google Scholar] [CrossRef]
- Wall, S.; Vialet-Chabrand, S.; Davey, P.; Van Rie, J.; Galle, A.; Cockram, J.; Lawson, T. Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytol. 2022, 235, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Zhang, X.; Hong, Y.G.; Liu, Y.L. Chloroplast in Plant-Virus Interaction. Front. Microbiol. 2016, 59, 71565. [Google Scholar] [CrossRef]
- Zanini, A.A.; Liliana, D.F.; Luna, D.F.; Paccioretti, P.; Collavino, A.; Rodriguez, M.S. Cassava common mosaic virus infection causes alterations in chloroplast ultrastructure, function, and carbohydrate metabolism of cassava plants. Plant Pathol. 2020, 70, 195–205. [Google Scholar] [CrossRef]
- Tatineni, S.; Alexander, J.; Qu, F. Differential synergistic interactions among four different wheat infecting viruses. Front. Microbiol. 2022, 12, 800318. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, K.; Yamori, W.; Groszmann, M.; Evans, J.R. Stomatal, mesophyll conductance, and biochemical limitations to photosynthesis during induction. Plant Physiol. 2021, 185, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Lv, Y.; Li, T.; Tang, J.; Yang, X.; Bai, J.; Jin, X.; Zhou, H. Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of Naked oat (Avena nuda L.). Sci. Rep. 2022, 12, 11199. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, S.R.; Zhu, W.M.; Yan, J.; Hei, Y.X. Effects of tomato yellow leaf curl virus on photosynthetic characteristics and chloroplast ultra-structure of the tomato leaves. Acta Bot. Boreali-Occident. Sin. 2011, 31, 1355–1359. [Google Scholar]
- Pradhan, G.P.; Xue, Q.; Jessup, K.E.; Hao, B.; Price, J.A.; Rush, C.M. Physiological responses of hard red winter wheat to infection by wheat streak mosaic virus. Phytopathology 2015, 105, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, I.; López-Moya, J.J.; Díaz-Pendón, J.A. Coinfection of tomato plants with tomato yellow leaf curl virus and tomato chlorosis virus affects the interaction with host and white flies. Phytopathology 2022, 112, 944–952. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]



| Primer Name | Formula | R2 | Amplification Efficiency (E) |
|---|---|---|---|
| AMV CP | y = −3.3586x + 33.941 | R2 = 0.9939 | 0.9849 |
| WCMV CP | y = −3.3452x + 32.064 | R2 = 0.9898 | 0.9904 |
| 25S rRNA | y = −3.3252x + 27.819 | R2 = 0.9882 | 0.9986 |
| β-Actin | y = −3.2386x + 26.667 | R2 = 0.9885 | 1.0360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liang, Q.; Wei, L.; Zhou, X. Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana. Viruses 2024, 16, 1255. https://doi.org/10.3390/v16081255
Chen Y, Liang Q, Wei L, Zhou X. Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana. Viruses. 2024; 16(8):1255. https://doi.org/10.3390/v16081255
Chicago/Turabian StyleChen, Yinge, Qiaolan Liang, Liexin Wei, and Xin Zhou. 2024. "Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana" Viruses 16, no. 8: 1255. https://doi.org/10.3390/v16081255
APA StyleChen, Y., Liang, Q., Wei, L., & Zhou, X. (2024). Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana. Viruses, 16(8), 1255. https://doi.org/10.3390/v16081255







