Clinical Application of Adenovirus (AdV): A Comprehensive Review
Abstract
1. Introduction
2. Optimization of Adenovirus Vectors
2.1. Replication Competent Adenovirus
2.2. Replication Deficient Adenovirus (Human Ad5, hAd36, Chempanzee)
2.3. Modification Replication Deficient Ad
2.4. Merit and Demerit of Adenovirus Type 5
2.5. Merit and Demerit of Ad 11 and Ad 35
2.6. Merit of Chimera Ad 5/35
2.7. Merit of Fiber Modified (Present Epitope) Ad
3. Application of Adenovirus
3.1. Gene Therapy
3.2. Oncolytic Virus Therapy
3.3. Immunotherapy
3.4. Vaccine Development
| Sl. No. | Generation/Type | Name of the Vector | Specialty | Advantages | Clinical Use | References |
|---|---|---|---|---|---|---|
| 1. | Wild-Type Adenovirus (WTAd) | Adv2, Adv5, Adv11, Adv26 |
| High cloning capacity, short expression time, and comparatively high immune response | Vaccination, oncolytic therapy, virotherapy, and gene therapy | [124,125] |
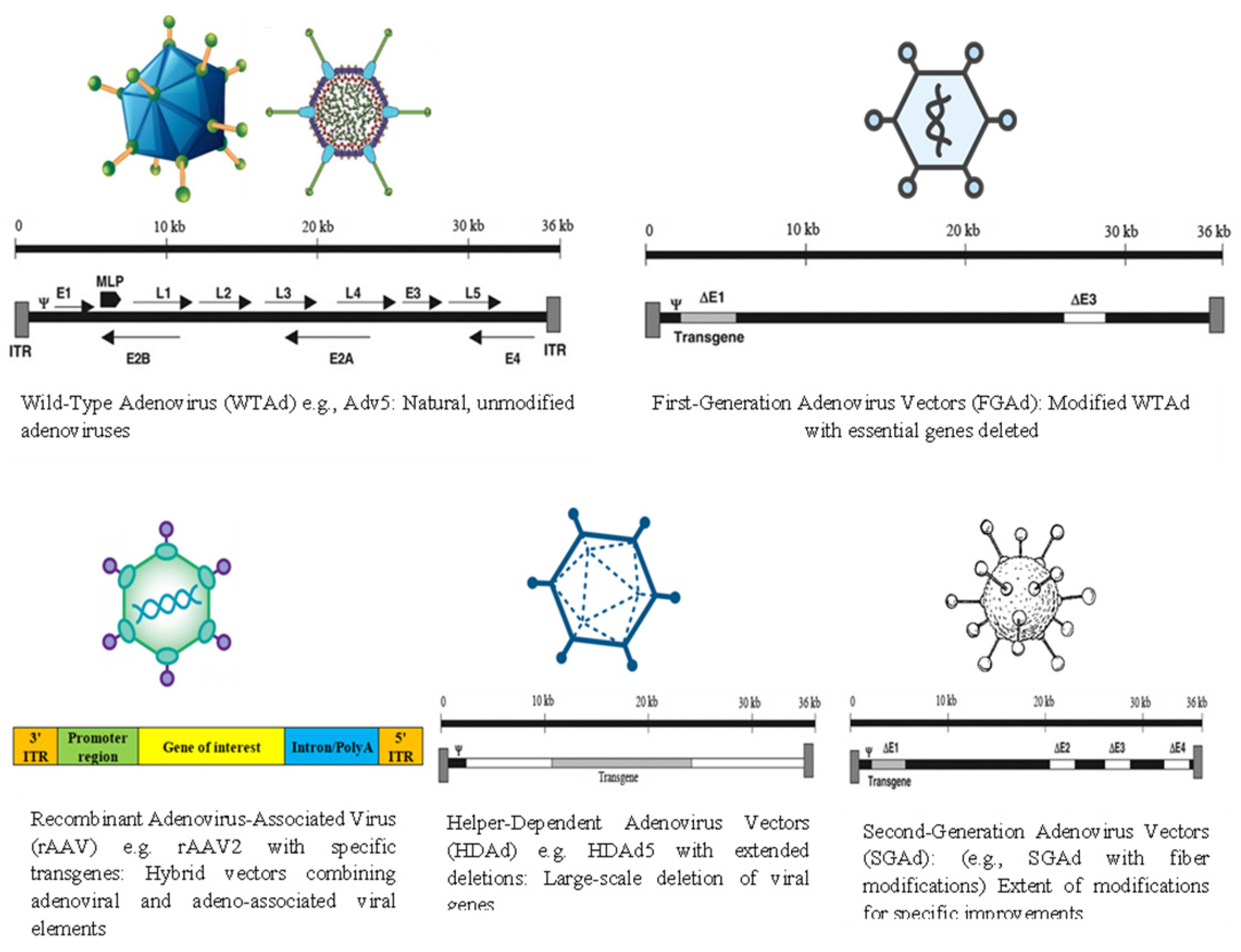
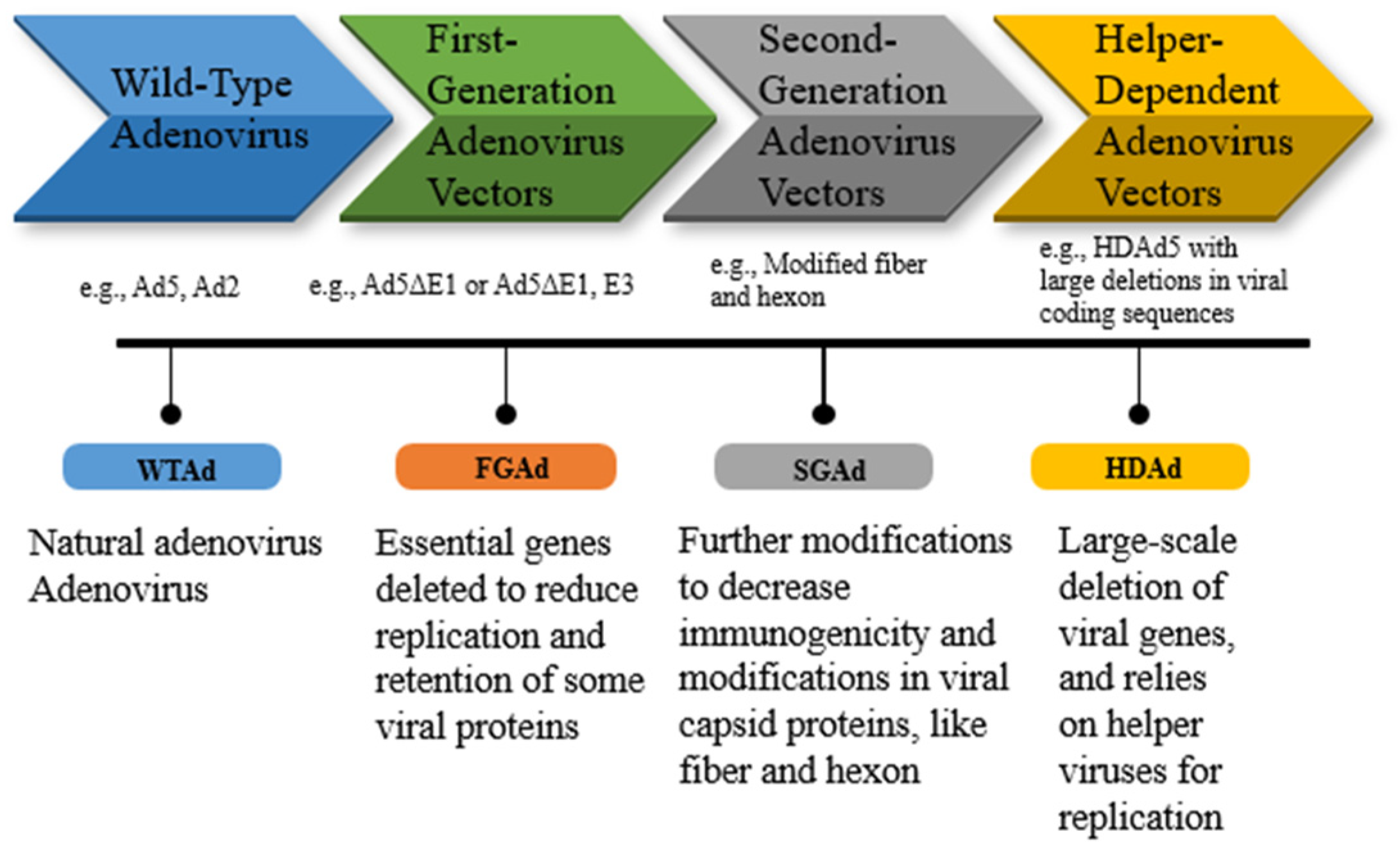
| 2. | First-Generation Adenovirus Vectors (FGAd) | Modified WTAd with essential genes deleted. e.g., Ad5ΔE1 or Ad5ΔE1, E3 |
| High titer level; very efficient transduction of most cells and tissues | Vaccination, and anti-cancer therapy | [74,126] |
| 3. | Second-Generation Adenovirus Vectors (SGAd) | Modified fiber and hexon | Deletion of the E2 and E4 regions from the adenoviral genome | Deletions significantly reduce the synthesis of adenoviral proteins and SGAd still induces host immune responses | Vaccination | [126] |
| 4. | Helper-Dependent Adenovirus Vectors (HDAd) | HDAd5 with extended deletions HDAd5/35++ HDAd6/35++ |
|
|
| [127,128,129,130,131] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subrat Khanal, G.a.A.S.D. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomedicines 2018, 30, 12. [Google Scholar]
- Smith, J.G.; Wiethoff, C.M.; Stewart, P.L.; Nemerow, G.R. Adenovirus. In Cell Entry by Non-Enveloped Viruses; Springer: New York, NY, USA, 2010; pp. 195–224. [Google Scholar]
- Han, G.; Niu, H.; Zhao, S.; Zhu, B.; Wang, C.; Liu, Y.; Zhang, M.; Yang, S.; Liu, F.; Wan, C.; et al. Identification and typing of respiratory adenoviruses in Guangzhou, Southern China using a rapid and simple method. Virol. Sin. 2013, 28, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; An, Y.; Chen, Z. Construction and application of adenoviral vectors. Mol. Ther.—Nucleic Acids 2023, 34, 102027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef] [PubMed]
- Syyam, A.; Nawaz, A.; Ijaz, A.; Sajjad, U.; Fazil, A.; Irfan, S.; Muzaffar, A.; Shahid, M.; Idrees, M.; Malik, K.; et al. Adenovirus vector system: Construction, history and therapeutic applications. BioTechniques 2022, 73, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Zhang, W.; Aydin, M.; Schröer, K.; Ehrhardt, A. The adenovirus vector platform: Novel insights into rational vector design and lessons learned from the COVID-19 vaccine. Viruses 2023, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Yabe, Y.; Trentin, J.J.; Taylor, G. Cancer induction in hamsters by human type 12 adenovirus. Effect of age and of virus dose. Proc. Soc. Exp. Biol. Med. 1962, 111, 343–344. [Google Scholar] [CrossRef]
- Nevels, M.; Täuber, B.; Spruss, T.; Wolf, H.; Dobner, T. “Hit-and-run” transformation by adenovirus oncogenes. J. Virol. 2001, 75, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Dharmapuri, S.; Peruzzi, D.; Aurisicchio, L.D. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin. Biol. Ther. 2009, 9, 1279–1287. [Google Scholar] [CrossRef]
- Wang, W.-C.; Sayedahmed, E.E.; Mittal, S.K. Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy. Viruses 2022, 14, 2727. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Statkute, E.; Wang, E.C.; Stanton, R.J. An Optimized CRISPR/Cas9 Adenovirus Vector (AdZ-CRISPR) for High-Throughput Cloning of sgRNA, Using Enhanced sgRNA and Cas9 Variants. Hum. Gene Ther. 2022, 33, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Baldassarri, S.; Benati, D.; D’alessio, F.; Patrizi, C.; Cattin, E.; Gentile, M.; Raggioli, A.; Recchia, A. Engineered Sleeping Beauty Transposon as Efficient System to Optimize Chimp Adenoviral Production. Int. J. Mol. Sci. 2022, 23, 7538. [Google Scholar] [CrossRef] [PubMed]
- Ziraldo, M.; Bidart, J.E.; Prato, C.A.; Tribulatti, M.V.; Zamorano, P.; Mattion, N.; D’antuono, A.L. Optimized adenoviral vector that enhances the assembly of FMDV O1 virus-like particles in situ increases its potential as vaccine for serotype O viruses. Front. Microbiol. 2020, 11, 591019. [Google Scholar] [CrossRef]
- Goverdhana, S.; Puntel, M.; Xiong, W.; Zirger, J.; Barcia, C.; Curtin, J.; Soffer, E.; Mondkar, S.; King, G.; Hu, J.; et al. Regulatable gene expression systems for gene therapy applications: Progress and future challenges. Mol. Ther. 2005, 12, 189–211. [Google Scholar] [CrossRef]
- Kalafati, E.; Drakopoulou, E.; Anagnou, N.; Pappa, K.I. Developing Oncolytic Viruses for the Treatment of Cervical Cancer. Cells 2023, 12, 1838. [Google Scholar] [CrossRef]
- Schalk, J.A.; de Vries, C.G.; Orzechowski, T.J.; Rots, M.G. A rapid and sensitive assay for detection of replication-competent adenoviruses by a combination of microcarrier cell culture and quantitative PCR. J. Virol. Methods 2007, 145, 89–95. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-capacity adenoviral vectors: Expanding the scope of gene therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current State of Human Gene Therapy: Approved Products and Vectors. Pharmaceuticals 2023, 16, 1416. [Google Scholar] [CrossRef]
- Vrba, S.M.; Kirk, N.M.; Brisse, M.E.; Liang, Y.; Ly, H. Development and applications of viral vectored vaccines to combat zoonotic and emerging public health threats. Vaccines 2020, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; Mccormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Dix, B.R.; Myers, C.J.; Dobson-Le, D.; Huschtscha, L.; Hibma, M.; Royds, J.; Braithwaite, A.W. Evidence that replication of the antitumor adenovirus ONYX-015 is not controlled by the p53 and p14 ARF tumor suppressor genes. J. Virol. 2002, 76, 12483–12490. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Curiel, D.T. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 2010, 18, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Kremer, E.J.; Shayakhmetov, D.M. Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens. Mol. Ther. 2022, 30, 1822–1849. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, S.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, B.; Lama, J.R.; Marmor, M.; del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.; Horwitz, M. Adenoviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.; Jeyanathan, M.; Smieja, M.; Medina, M.F.; Thanthrige-Don, N.; Zganiacz, A.; Yin, C.; Heriazon, A.; Damjanovic, D.; Puri, L.; et al. A human type 5 adenovirus–based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef] [PubMed]
- SMWold, W.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar]
- Koup, R.A.; Lamoreaux, L.; Zarkowsky, D.; Bailer, R.T.; King, C.R.; Gall, J.G.D.; Brough, D.E.; Graham, B.S.; Roederer, M. Replication-defective adenovirus vectors with multiple deletions do not induce measurable vector-specific T cells in human trials. J. Virol. 2009, 83, 6318–6322. [Google Scholar] [CrossRef]
- Doerfler, W. Adenoviral vector DNA-and SARS-CoV-2 mRNA-based Covid-19 vaccines: Possible integration into the human genome-are adenoviral genes expressed in vector-based vaccines? Virus Res. 2021, 302, 198466. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.M.; Rubio, I.G.S.; Toneto, N.A.; Morale, M.G.; Tamura, R.E. The use of adenoviral vectors in gene therapy and vaccine approaches. Genet. Mol. Biol. 2022, 45, e20220079. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mahato, R.I. Gene Therapy. In Pharmaceutical Biotechnology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Xin, K.-Q.; Jounai, N.; Someya, K.; Honma, K.; Mizuguchi, H.; Naganawa, S.; Kitamura, K.; Hayakawa, T.; Saha, S.; Takeshita, F.; et al. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Ther. 2005, 12, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Xin, K.Q.; Sekimoto, Y.; Takahashi, T.; Mizuguchi, H.; Ichino, M.; Yoshida, A.; Okuda, K. Chimeric adenovirus 5/35 vector containing the clade C HIV gag gene induces a cross-reactive immune response against HIV. Vaccine 2007, 25, 3809–3815. [Google Scholar] [CrossRef] [PubMed]
- Someya, K.; Xin, K.Q.; Ami, Y.; Izumi, Y.; Mizuguchi, H.; Ohta, S.; Yamamoto, N.; Honda, M.; Okuda, K. Chimeric adenovirus type 5/35 vector encoding SIV gag and HIV env genes affords protective immunity against the simian/human immunodeficiency virus in monkeys. Virology 2007, 367, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Kostense, S.; Koudstaal, W.; Sprangers, M.; Weverling, G.J.; Penders, G.; Helmus, N.; Vogels, R.; Bakker, M.; Berkhout, B.; Havenga, M.; et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 2004, 18, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Yoshida, A.; Xin, K.; Yoshizaki, S.; Yashima, S.; Abe, S.; Mizuguchi, H.; Okuda, K. Designed recombinant adenovirus type 5 vector induced envelope-specific CD8+ cytotoxic T lymphocytes and cross-reactive neutralizing antibodies against human immunodeficiency virus type 1. J. Gene Med. 2009, 11, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Okuda, K.; Ura, T.; Kondo, A.; Yoshida, A.; Yoshizaki, S.; Mizuguchi, H.; Klinman, D.; Shimada, M. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J. Gene Med. 2009, 11, 570–579. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Hayakawa, T. Adenovirus vectors containing chimeric type 5 and type 35 fiber proteins exhibit altered and expanded tropism and increase the size limit of foreign genes. Gene 2002, 285, 69–77. [Google Scholar] [CrossRef]
- Yang, M.; Yang, C.S.; Guo, W.; Tang, J.; Huang, Q.; Feng, S.; Jiang, A.; Xu, X.; Jiang, G.; Liu, Y.Q. A novel fiber chimeric conditionally replicative adenovirus-Ad5/F35 for tumor therapy. Cancer Biol. Ther. 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Gall, J.G.D.; Kong, W.-p.; Sheets, R.L.; Gomez, L.; King, C.R.; Nabel, G.J. Mechanism of Ad5 vaccine immunity and toxicity: Fiber shaft targeting of dendritic cells. PLoS Pathog. 2007, 3, e25. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.-T.; Chin, K.-T.; Siu, K.-L.; Choy, E.Y.W.; Jeang, K.-T.; Jin, D.-Y. TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J. Virol. 2006, 80, 7052–7059. [Google Scholar] [CrossRef]
- Mittereder, N.; March, K.L.; Trapnell, B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996, 70, 7498–7509. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Papayannopoulou, T.; Stamatoyannopoulos, G.; Lieber, A. Efficient gene transfer into human CD34 + cells by a retargeted adenovirus vector. J. Virol. 2000, 74, 2567. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Gu, M.; Motzel, S.; Zhao, J.; Lin, J.; Su, Q.; Allen, H.; Franlin, L.; Parks, R.J.; Graham, F.L.; et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 1998, 95, 7866–7871. [Google Scholar] [CrossRef]
- Schaack, J. Induction and inhibition of innate inflammatory responses by adenovirus early region proteins. Viral Immunol. 2005, 18, 79–88. [Google Scholar] [CrossRef]
- Wang, Q.; Finer, M.H. Second–generation adenovirus vectors. Nat. Med. 1996, 2, 714–716. [Google Scholar] [CrossRef]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12, S18–S27. [Google Scholar] [CrossRef]
- Nishida, Y.; Kodama, K.; Sengoku, S. The gap between development and manufacturing in gene therapy: Strategic options for overcoming traps. Drug Discov. Today 2023, 28, 103429. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Ni, S.; Li, Z.-Y.; Gaggar, A.; DiPaolo, N.; Feng, Q.; Sandig, V.; Lieber, A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 2005, 79, 5090–5104. [Google Scholar] [CrossRef]
- Shimada, M.; Wang, H.; Ichino, M.; Ura, T.; Mizuki, N.; Okuda, K. Biodistribution and immunity of adenovirus 5/35 and modified vaccinia Ankara vector vaccines against human immunodeficiency virus 1 clade C. Gene Ther. 2022, 29, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, X.; Yin, F.; Zou, X.; Hou, W.; Lu, Z. Fiber modifications enable fowl adenovirus 4 vectors to transduce human cells. J. Gene Med. 2021, 23, e3368. [Google Scholar] [CrossRef]
- Koizumi, N.; Mizuguchi, H.; Utoguchi, N.; Watanabe, Y.; Hayakawa, T. Generation of fiber-modified adenovirus vectors containing heterologous peptides in both the HI loop and C terminus of the fiber knob. J. Gene Med. 2003, 5, 267–276. [Google Scholar] [CrossRef]
- Douglas, J.T. Adenoviral vectors for gene therapy. Mol. Biotechnol. 2007, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Garofalo, M.; Hirvinen, M.; Cerullo, V. The evolution of adenoviral vectors through genetic and chemical surface modifications. Viruses 2014, 6, 832–855. [Google Scholar] [CrossRef]
- Freitag, C.; Kaulfuss, M.; Flühler, L.; Mietz, J.; Weiss, F.; Brücher, D.; Kolibius, J.; Hartmann, K.; Smith, S.N.; Münz, C.; et al. Targeted adenovirus-mediated transduction of human T cells in vitro and in vivo. Mol. Ther.—Methods Clin. Dev. 2023, 29, 120–132. [Google Scholar] [CrossRef]
- Le, T.M.D.; Yoon, A.-R.; Thambi, T.; Yun, C.-O. Polymeric Systems for Cancer Immunotherapy: A Review. Front. Immunol. 2022, 13, 826876. [Google Scholar] [CrossRef]
- Wu, H.; Curiel, D.T. Fiber-modified adenoviruses for targeted gene therapy. In Gene Therapy Protocols: Design and Characterization of Gene Transfer Vectors; Springer: New York, NY, USA, 2008; pp. 113–132. [Google Scholar]
- Carroll, M.W.; Wilkinson, G.W.; Lundstrom, K. Mammalian expression systems and vaccination. In Genetically Engineered Viruses; Springer: New York, NY, USA, 2023; pp. 107–157. [Google Scholar]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Tølbøll Sørensen, A.L.; Rolland, M.; Hartmann, J.; Harboe, Z.B.; Roed, C.; Jensen, T.Ø.; Kolte, L.; El Fassi, D.; Hillingsø, J.; Radziwon-Balicka, A.; et al. A case of thrombocytopenia and multiple thromboses after vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2. Blood Adv. 2021, 5, 2569–2574. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.B.; Lee, S.W.; Lee, M.H.; Koyanagi, A.; Jacob, L.; Tizaoui, K.; Yon, D.K.; Shin, J.I.; Smith, L. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J. Autoimmun. 2021, 122, 102681. [Google Scholar] [CrossRef] [PubMed]
- McCarron, A.; Cmielewski, P.; Drysdale, V.; Parsons, D.; Donnelley, M. Effective viral-mediated lung gene therapy: Is airway surface preparation necessary? Gene Ther. 2023, 30, 469–477. [Google Scholar] [CrossRef]
- Sahu, I.; Haque, A.A.; Weidensee, B.; Weinmann, P.; Kormann, M.S. Recent developments in mRNA-based protein supplementation therapy to target lung diseases. Mol. Ther. 2019, 27, 803–823. [Google Scholar] [CrossRef]
- Martinovich, K.M.; Shaw, N.C.; Kicic, A.; Schultz, A.; Fletcher, S.; Wilton, S.D.; Stick, S.M. The potential of antisense oligonucleotide therapies for inherited childhood lung diseases. Mol. Cell. Pediatr. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, H.; et al. Appraisal for the potential of viral and nonviral vectors in gene therapy: A review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Desfarges, S.; Ciuffi, A. Retroviral Integration Site Selection. Viruses 2010, 2, 111–130. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Ma, N.; Steinhoff, G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013, 14, 46–60. [Google Scholar]
- Mccarty, D.M.; Monahan, E.; Samulski, R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001, 8, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.; Zhu, X.; Xiao, X.; Brook, J.; Housman, D.; Epstein, N.; Hunter, L. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991, 10, 3941–3950. [Google Scholar] [CrossRef] [PubMed]
- Kotin, R.; Linden, R.; Berns, K. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992, 11, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef]
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: Targets, progress, and challenges for treating human diseases. Gene Ther. 2022, 29, 3–12. [Google Scholar] [CrossRef]
- Feola, S.; Russo, S.; Ylösmäki, E.; Cerullo, V. Oncolytic ImmunoViroTherapy: A long history of crosstalk between viruses and immune system for cancer treatment. Pharmacol. Ther. 2022, 236, 108103. [Google Scholar] [CrossRef]
- Jayawardena, N.; Poirier, J.T.; Burga, L.N.; Bostina, M. Virus–Receptor Interactions and Virus Neutralization: Insights for Oncolytic Virus Development. Oncolytic Virotherapy 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Kucan Brlić, P.; Lenac Roviš, T.; Cinamon, G.; Tsukerman, P.; Mandelboim, O.; Jonjić, S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell. Mol. Immunol. 2019, 16, 40–52. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Gilbert, S.M.; Conejo-Garcia, J.; Mulé, J.J. Using oncolytic viruses to ignite the tumour immune microenvironment in bladder cancer. Nat. Rev. Urol. 2021, 18, 543–555. [Google Scholar] [CrossRef]
- Ylösmäki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.-H.; et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013, 73, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.E.; Innocentin, S.; Carr, E.J.; Bradford, B.M.; Lafouresse, F.; Mabbott, N.A.; Mörbe, U.; Ludewig, B.; Groom, J.R.; Good-Jacobson, K.L.; et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J. Exp. Med. 2019, 216, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Heiniö, C.; Clubb, J.; Kudling, T.; Quixabeira, D.; Cervera-Carrascon, V.; Havunen, R.; Grönberg-Vähä-Koskela, S.; Santos, J.M.; Tapper, J.; Kanerva, A.; et al. Effective combination immunotherapy with oncolytic adenovirus and Anti-PD-1 for treatment of human and murine ovarian cancers. Diseases 2022, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Lee, S.; Margolin, K. Cytokines in Cancer Immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral immunotherapy—Update 2019. Oncologist 2020, 25, e423–e438. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves Anti-PD-1 immunotherapy. Cell 2018, 174, 1031–1032. [Google Scholar] [CrossRef]
- Sato-Dahlman, M.; Yamamoto, M. The Development of Oncolytic Adenovirus Therapy in the Past and Future-For the Case of Pancreatic Cancer. Curr. Cancer Drug Targets 2018, 18, 153–161. [Google Scholar] [CrossRef]
- Shenk, T. Adenoviridae-the viruses and their replication. In Fields Virology; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; pp. 2111–2148. [Google Scholar]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological properties and clinical application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.; Zhang, Y.; Siddiqui, F. Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for prostate cancer. Mol. Ther.–Oncolytics 2015, 2, 15006. [Google Scholar] [CrossRef]
- Bortolanza, S.; Bunuales, M.; Otano, I.; Gonzalez-Aseguinolaza, G.; Ortiz-De-Solorzano, C.; Perez, D.; Prieto, J.; Hernandez-Alcoceba, R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in syrian hamsters. Mol. Ther. 2009, 17, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Sato-Dahlman, M.; LaRocca, C.J.; Yanagiba, C.; Yamamoto, M. Adenovirus and immunotherapy: Advancing cancer treatment by combination. Cancers 2020, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Stucchi, A.; Maspes, F.; Montee-Rodrigues, E.; Fousteri, G. Engineered Treg cells: The heir to the throne of immunotherapy. J. Autoimmun. 2023, 144, 102986. [Google Scholar] [CrossRef] [PubMed]
- Gabitzsch, E.S.; Xu, Y.; Balint, J.; Hartman, Z.C.; Lyerly, H.K.; Jones, F.R. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol. Immunother. 2010, 59, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Minor, D. Use of Vaccines to Eradicate Infectious Disease; Els: Cincinnati, OH, USA, 2015; pp. 1–6. [Google Scholar]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Chekhonin, V. Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res. 2018, 257, 40–51. [Google Scholar] [CrossRef]
- Kaufmann, J.K.; Nettelbeck, D.M. Engineering Chimeric Adenoviruses: Exploiting Virus Diversity for Improved Vectors, Vaccines, and Oncolytics, in Adenoviral Vectors for Gene Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 529–549. [Google Scholar]
- Uusi-Kerttula, H.; Hulin-Curtis, S.; Davies, J.; Parker, A.L. Oncolytic adenovirus: Strategies and insights for vector design and immuno-oncolytic applications. Viruses 2015, 7, 6009–6042. [Google Scholar] [CrossRef] [PubMed]
- Majhen, D.; Calderon, H.; Chandra, N.; Fajardo, C.A.; Rajan, A.; Alemany, R.; Custers, J. Adenovirus-based vaccines for fighting infectious diseases and cancer: Progress in the field. Hum. Gene Ther. 2014, 25, 301–317. [Google Scholar] [CrossRef]
- Folegatti, M.; Jenkin, D.; Morris, S.; Gilbert, S.; Kim, D.; Robertson, J.S.; Smith, E.R.; Martin, E.; Gurwith, M.; Chen, R.T. Vaccines based on the replication-deficient simian adenoviral vector ChAdOx1: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2022, 40, 5248–5262. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Kondo, A.; Yoshida, A.; Yoshizaki, S.; Abe, S.; Bao, L.-L.; Mizuki, N.; Ichino, M.; Klinman, D.; Okuda, K.; et al. Partial protection against SIV challenge by vaccination of adenovirus and MVA vectors in rhesus monkeys. Gene Ther. 2009, 17, 4–13. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Li, Z.; Wang, X.; Feng, X.; Wang, Z.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; et al. A Tropism-transformed oncolytic adenovirus with dual capsid modifications for enhanced glioblastoma therapy. J. Cancer 2020, 11, 5713. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yoshida, K.; Nishimoto, T.; Hatanaka, K.; Ohnami, S.; Asaka, M.; Douglas, J.T.; Curiel, D.T.; Yoshida, T.; Aoki, K. Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther. 2007, 14, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Neukirch, L.; Fougeroux, C.; Andersson, A.-M.C.; Holst, J. The potential of adenoviral vaccine vectors with altered antigen presentation capabilities. Expert Rev. Vaccines 2020, 19, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Gabitzsch, E.S.; Morse, M.A.; Lyerly, H.K.; Balint, J.; Jones, F. Immunotherapeutic treatment of metastatic colorectal cancer using ETBX-011. J. Clin. Oncol. 2014, 32, 3093. [Google Scholar] [CrossRef]
- Osada, T.; Yang, X.Y.; Hartman, Z.C.; Glass, O.; Hodges, B.L.; Niedzwiecki, D.; A Morse, M.; Lyerly, H.K.; Amalfitano, A.; Clay, T.M. Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther. 2009, 16, 673–682. [Google Scholar] [CrossRef]
- Elzey, B.D.; Siemens, D.R.; Ratliff, T.L.; Lubaroff, D.M. Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (alvac) cytokine gene delivery induces destruction of established prostate tumors. Int. J. Cancer 2001, 94, 842–849. [Google Scholar] [CrossRef]
- Wieking, B.G.; Vermeer, D.W.; Spanos, W.C.; Lee, K.M.; Vermeer, P.; Lee, W.T.; Xu, Y.; Gabitzsch, E.S.; Balcaitis, S.; Balint, J.P.; et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012, 19, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Stephenson, K.B.; Nikota, J.K.; Hu, Q.N.; Nguyen, A.; Wan, Y.; Lichty, B.D. Preclinical development of peptide vaccination combined with oncolytic MG1-E6E7 for HPV-associated cancer. Vaccine 2018, 36, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.G.; Atherton, M.J.; Bridle, B.W.; Stephenson, K.B.; Le Boeuf, F.; Hummel, J.L.; Martin, C.G.; Pomoransky, J.; Breitbach, C.J.; Diallo, J.-S.; et al. Development and applications of oncolytic Maraba virus vaccines. Oncolytic Virotherapy 2018, 7, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; Hotte, S.J.; Razak, A.R.A.; Renouf, D.J.; Lichty, B.; Bell, J.C.; Powers, J.; Breitbach, C.J.; Stojdl, D.F.; Stephenson, K.B.; et al. Phase I study of oncolytic virus (OV) MG1 maraba/MAGE-A3 (MG1MA3), with and without transgenic MAGE-A3 adenovirus vaccine (AdMA3) in incurable advanced/metastatic MAGE-A3-expressing solid tumours: CCTG IND.214. J. Clin. Oncol. 2017, 35, e14637. [Google Scholar] [CrossRef]
- Pol, J.G.; Acuna, S.A.; Yadollahi, B.; Tang, N.; Stephenson, K.B.; Atherton, M.J.; Hanwell, D.; El-Warrak, A.; Goldstein, A.; Moloo, B.; et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. OncoImmunology 2019, 8, e1512329. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Cawood, R.; Chen, H.H.; Carroll, F.; Bazan-Peregrino, M.; van Rooijen, N.; Seymour, L.W. Use of tissue-specific MicroRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009, 5, e1000440. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.; Byrne, B.J.; Corti, M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses 2023, 15, 2378. [Google Scholar] [CrossRef]
- Liu, J.; Seol, D.-W. Helper virus-free gutless adenovirus (HF-GLAd): A new platform for gene therapy. BMB Rep. 2020, 53, 565–575. [Google Scholar] [CrossRef]
- Bandara, R.A.; Chen, Z.R.; Hu, J. Potential of helper-dependent Adenoviral vectors in CRISPR-cas9-mediated lung gene therapy. Cell Biosci. 2021, 11, 145. [Google Scholar] [CrossRef]
- Toietta, G.; Pastore, L.; Cerullo, V.; Finegold, M.; Beaudet, A.L.; Lee, B. Generation of Helper-Dependent Adenoviral Vectors by Homologous Recombination. Mol. Ther. 2002, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Georgakopoulou, A.; Zhang, W.; Kim, J.; Gil, S.; Ehrhardt, A.; Lieber, A. HDAd6/35++-a new helper-dependent adenovirus vector platform for in vivo transduction of hematopoietic stem cells. Mol. Ther.-Methods Clin. Dev. 2023, 29, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Rosewell, A.; Vetrini, F.N. Helper-dependent adenoviral vectors. J. Genet. Syndr. Gene Ther. 2011, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Ng, P. Helper-dependent adenoviral vectors. In Adenoviral Vectors for Gene Therapy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 423–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salauddin, M.; Saha, S.; Hossain, M.G.; Okuda, K.; Shimada, M. Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses 2024, 16, 1094. https://doi.org/10.3390/v16071094
Salauddin M, Saha S, Hossain MG, Okuda K, Shimada M. Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses. 2024; 16(7):1094. https://doi.org/10.3390/v16071094
Chicago/Turabian StyleSalauddin, Md., Sukumar Saha, Md. Golzar Hossain, Kenji Okuda, and Masaru Shimada. 2024. "Clinical Application of Adenovirus (AdV): A Comprehensive Review" Viruses 16, no. 7: 1094. https://doi.org/10.3390/v16071094
APA StyleSalauddin, M., Saha, S., Hossain, M. G., Okuda, K., & Shimada, M. (2024). Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses, 16(7), 1094. https://doi.org/10.3390/v16071094







