Host Cell Proteases Involved in Human Respiratory Viral Infections and Their Inhibitors: A Review
Abstract
1. Introduction
2. Respiratory Proteases
3. Serine Proteases
3.1. Furin
3.2. Coagulation Cascade Proteases (Plasmin, Thrombin, Factor Xa)
3.3. Elastase
3.4. Type II Transmembrane Serine Proteases (TTSPs)
3.5. TMPRSS2
4. Cysteine Proteases–Cathepsins
5. Metalloproteases
ACE2
6. Protease Inhibitors
7. Current Therapeutic Strategies Targeting Coronaviruses, Influenza Viruses, and Para-Myxoviruses
- Inhibition of furin as an antiviral strategy
- Inhibition of TTSPs as an antiviral strategy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schechter, I.; Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef]
- Majchrzak, M.; Poręba, M. The roles of cellular protease interactions in viral infections and programmed cell death: A lesson learned from the SARS-CoV-2 outbreak and COVID-19 pandemic. Pharmacol. Rep. 2022, 74, 1149–1165. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Reid, T.J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2021, 68, 1850–1867. [Google Scholar] [CrossRef]
- Carossino, M.; Izadmehr, S.; Trujillo, J.D.; Gaudreault, N.N.; Dittmar, W.; Morozov, I.; Balasuriya, U.B.R.; Cordon-Cardo, C.; García-Sastre, A.; Richt, J.A. ACE2 and TMPRSS2 distribution in the respiratory tract of different animal species and its correlation with SARS-CoV-2 tissue tropism. Microbiol. Spectr. 2024, 12, e03270-23. [Google Scholar] [CrossRef]
- Lean, F.Z.X.; Núñez, A.; Spiro, S.; Priestnall, S.L.; Vreman, S.; Bailey, D.; James, J.; Wrigglesworth, E.; Suarez-Bonnet, A.; Conceivao, C.; et al. Differential susceptibility of SARS-CoV-2 in animals: Evidence of ACE2 host receptor distribution in companion animals, livestock and wildlife by immunohistochemical characterisation. Transbound Emerg Dis. 2022, 69, 2275–2286. [Google Scholar] [CrossRef]
- Martins, M.; Boggiatto, P.M.; Buckley, A.; Cassmann, E.D.; Falkenberg, S.; Caserta, L.C.; Fernandes, M.H.V.; Kanipe, C.; Lager, K.; Palmer, M.V.; et al. From Deer-to-Deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer. PLoS Pathog. 2022, 18, e1010197. [Google Scholar] [CrossRef]
- Klestova, Z. Possible spread of SARS-CoV-2 in domestic and wild animals and body temperature role. Virus Res. 2023, 327, 199066. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Barua, A.; Grot, N.; Plawski, A. The basis of mink susceptibility to SARS-CoV-2 infection. J. Appl. Genet. 2022, 63, 543–555. [Google Scholar] [CrossRef]
- Pandey, R.K.; Srivastava, A.; Mishra, R.K.; Singh, P.P.; Chaubey, G. Novel genetic association of the Furin gene polymorphism rs1981458 with COVID-19 severity among Indian populations. Sci. Rep. 2024, 14, 7822. [Google Scholar] [CrossRef]
- Al-Mulla, F.; Mohammad, A.; Al Madhoun, A.; Haddad, D.; Ali, H.; Eaaswarkhanth, M.; John, S.E.; Nizam, R.; Channanath, A.; Abu-Farha, M.; et al. ACE2 and FURIN variants are potential predictors of SARS-CoV-2 outcome: A time to implement precision medicine against COVID-19. Heliyon 2021, 7, e06133. [Google Scholar] [CrossRef]
- Sienko, J.; Marczak, I.; Kotowski, M.; Bogacz, A.; Tejchman, K.; Sienko, M.; Kotifs, K. Association of ACE2 Gene Variants with the Severity of COVID-19 Disease—A Prospective Observational Study. Int. J. Environ. Res. Public Health 2022, 19, 12622. [Google Scholar] [CrossRef]
- David, A.; Parkinson, N.; Peacock, T.P.; Pairo-Castineira, E.; Khanna, T.; Cobat, A.; Tenesa, A.; Sancho-Shmizu, V.; Casanova, J.L.; Abel, L.; et al. A common TMPRSS2 variant has a protective effect against severe COVID-19. Curr. Res. Transl. Med. 2022, 70, 103333. [Google Scholar] [CrossRef]
- Coto, E.; Albaiceta, G.M.; Amado-Rodríguez, L.; García-Clemente, M.; Cuesta-Llavona, E.; Vázquez-Coto, D.; Alonso, B.; Iglesias, S.; Melón, S.; Alvarez-Argüelles, M.E.; et al. FURIN gene variants (rs6224/rs4702) as potential markers of death and cardiovascular traits in severe COVID-19. J. Med. Virol. 2022, 94, 3589–3595. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Chapter 559—Introduction: Serine Peptidases and Their Clans. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2491–2523. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192005597 (accessed on 13 July 2023).
- Andersson, M.K.; Enoksson, M.; Gallwitz, M.; Hellman, L. The extended substrate specificity of the human mast cell chymase reveals a serine protease with well-defined substrate recognition profile. Int. Immunol. 2009, 21, 95–104. [Google Scholar] [CrossRef]
- Nygaard, R.M.; Golden, J.W.; Schiff, L.A. Impact of Host Proteases on Reovirus Infection in the Respiratory Tract. J. Virol. 2012, 86, 1238–1243. [Google Scholar] [CrossRef]
- Brown, M.A.; Stenberg, L.M.; Stenflo, J. Coagulation Factor Xa. In Handbook of Proteolytic Enzymes; Academic Press: Cambridge, MA, USA, 2013; pp. 2908–2915. [Google Scholar]
- Du, L.; Kao, R.Y.; Zhou, Y.; He, Y.; Zhao, G.; Wong, C.; Jiang, S.; Yuen, K.Y.; Jin, D.Y.; Zheng, B.J. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem. Biophys. Res. Commun. 2007, 359, 174–179. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Mercadante, M.; Nilsson-Payant, B.; Johnson, J.L.; Jaimes, J.A.; Muecksch, F.; Weisblum, Y.; Bram, Y.; Chandar, V.; Whittaker, G.R.; et al. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. van der Meer JW, editor. eLife 2022, 11, e77444. [Google Scholar] [CrossRef]
- Hosaka, M.; Nagahama, M.; Kim, W.S.; Watanabe, T.; Hatsuzawa, K.; Ikemizu, J.; Murakami, K.; Nakayama, K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J. Biol. Chem. 1991, 266, 12127–12130. [Google Scholar] [CrossRef]
- Kawaoka, Y.; Webster, R.G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc. Natl. Acad. Sci. USA 1988, 85, 324–328. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Millet, J.K.; Whittaker, G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA 2014, 111, 15214–15219. [Google Scholar] [CrossRef]
- Collins, P.L.; Huang, Y.T.; Wertz, G.W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 1984, 81, 7683–7687. [Google Scholar] [CrossRef]
- Ortmann, D.; Ohuchi, M.; Angliker, H.; Shaw, E.; Garten, W.; Klenk, H.D. Proteolytic cleavage of wild type and mutants of the F protein of human parainfluenza virus type 3 by two subtilisin-like endoproteases, furin and Kex2. J. Virol. 1994, 68, 2772–2776. [Google Scholar] [CrossRef]
- Watanabe, M.; Hirano, A.; Stenglein, S.; Nelson, J.; Thomas, G.; Wong, T.C. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J. Virol. 1995, 69, 3206–3210. [Google Scholar] [CrossRef]
- Ueo, A.; Kubota, M.; Shirogane, Y.; Ohno, S.; Hashiguchi, T.; Yanagi, Y. Lysosome-Associated Membrane Proteins Support the Furin-Mediated Processing of the Mumps Virus Fusion Protein. J. Virol. 2020, 94, e00050-20. [Google Scholar] [CrossRef]
- Borgoño, C.A.; Gavigan, J.A.; Alves, J.; Bowles, B.; Harris, J.L.; Sotiropoulou, G.; Diamandis, E.P. Defining the extended substrate specificity of kallikrein 1-related peptidases. Biol. Chem. 2007, 388, 1215–1225. [Google Scholar] [CrossRef]
- Milewska, A.; Falkowski, K.; Kulczycka, M.; Bielecka, E.; Naskalska, A.; Mak, P.; Lesner, A.; Ochman, M.; Urlik, M.; Diamandis, E.; et al. Kallikrein 13 serves as a priming protease during infection by the human coronavirus HKU1. Sci. Signal. 2020, 13, eaba9902. [Google Scholar] [CrossRef]
- Li, H.X.; Hwang, B.Y.; Laxmikanthan, G.; Blaber, S.I.; Blaber, M.; Golubkov, P.A.; Ren, P.; Iverson, B.I.; Georgiou, G. Substrate specificity of human kallikreins 1 and 6 determined by phage display. Protein Sci. 2008, 17, 664–672. [Google Scholar] [CrossRef]
- Hamilton, B.S.; Whittaker, G.R. Cleavage Activation of Human-adapted Influenza Virus Subtypes by Kallikrein-related Peptidases 5 and 12. J. Biol. Chem. 2013, 288, 17399–17407. [Google Scholar] [CrossRef]
- Harris, J.L.; Backes, B.J.; Leonetti, F.; Mahrus, S.; Ellman, J.A.; Craik, C.S. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc. Natl. Acad. Sci. USA 2000, 97, 7754–7759. [Google Scholar] [CrossRef]
- Golden, J.W.; Schiff, L.A. Neutrophil elastase, an acid-independent serine protease, facilitates reovirus uncoating and infection in U937 promonocyte cells. Virol. J. 2005, 2, 48. [Google Scholar] [CrossRef]
- Belouzard, S.; Madu, I.; Whittaker, G.R. Elastase-mediated Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein at Discrete Sites within the S2 Domain. J. Biol. Chem. 2010, 285, 22758–22763. [Google Scholar] [CrossRef]
- Matsuyama, S.; Ujike, M.; Morikawa, S.; Tashiro, M.; Taguchi, F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. USA 2005, 102, 12543–12547. [Google Scholar] [CrossRef]
- Seidah, N.G. Chapter 730—Proprotein Convertase 5. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3305–3310. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192007304 (accessed on 4 May 2024).
- Basak, A.; Zhong, M.; Munzer, J.S.; Chrétien, M.; Seidah, N.G. Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses: A comparative analysis with fluorogenic peptides. Biochem. J. 2001, 353 Pt 3, 537–545. [Google Scholar] [CrossRef]
- Castellino, F.J. Chapter 648—Plasmin. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2958–2968. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192006487 (accessed on 13 July 2023).
- Xue, F.; Seto, C.T. Selective Inhibitors of the Serine Protease Plasmin: Probing the S3 and S3‘ Subsites Using a Combinatorial Library. J. Med. Chem. 2005, 48, 6908–6917. [Google Scholar] [CrossRef]
- Tse, L.V.; Marcano, V.C.; Huang, W.; Pocwierz, M.S.; Whittaker, G.R. Plasmin-Mediated Activation of Pandemic H1N1 Influenza Virus Hemagglutinin Is Independent of the Viral Neuraminidase. J. Virol. 2013, 87, 5161–5169. [Google Scholar] [CrossRef]
- Dubovi, E.J.; Geratz, J.D.; Tidwell, R.R. Enhancement of respiratory syncytial virus-induced cytopathology by trypsin, thrombin, and plasmin. Infect Immun. 1983, 40, 351–358. [Google Scholar] [CrossRef]
- Kam, Y.W.; Okumura, Y.; Kido, H.; Ng, L.F.P.; Bruzzone, R.; Altmeyer, R. Cleavage of the SARS Coronavirus Spike Glycoprotein by Airway Proteases Enhances Virus Entry into Human Bronchial Epithelial Cells In Vitro. PLoS ONE 2009, 4, e7870. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, T.; Wang, T.; Ding, Y.; Cui, Y.; Nie, H. Competitive cleavage of SARS-CoV-2 spike protein and epithelial sodium channel by plasmin as a potential mechanism for COVID-19 infection. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2022, 323, L569–L577. [Google Scholar] [CrossRef]
- Gallwitz, M.; Enoksson, M.; Thorpe, M.; Hellman, L. The Extended Cleavage Specificity of Human Thrombin. PLoS ONE 2012, 7, e31756. [Google Scholar] [CrossRef]
- Rodriguez, T.; Dobrovolny, H.M. Quantifying the effect of trypsin and elastase on in vitro SARS-CoV infections. Virus Res. 2021, 299, 198423. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Wang, N.; Pallesen, J.; Wrapp, D.; Turner, H.L.; Cottrell, C.A.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018, 8, 15701. [Google Scholar] [CrossRef]
- Schowalter, R.M.; Smith, S.E.; Dutch, R.E. Characterization of Human Metapneumovirus F Protein-Promoted Membrane Fusion: Critical Roles for Proteolytic Processing and Low pH. J. Virol. 2006, 80, 10931–10941. [Google Scholar] [CrossRef]
- Henrickson, K.J. Parainfluenza Viruses. Clin. Microbiol. Rev. 2003, 16, 242–264. [Google Scholar] [CrossRef]
- Thorpe, M.; Fu, Z.; Chahal, G.; Akula, S.; Kervinen, J.; De Garavilla, L.; Hellman, L. Extended cleavage specificity of human neutrophil cathepsin G: A low activity protease with dual chymase and tryptase-type specificities. PLoS ONE 2018, 13, e0195077. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Taggart, C.C.; Dabo, A.J.; Weldon, S.; Cummins, N.; Geraghty, P. Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I-like receptors. Mucosal Immunol. 2015, 8, 161–175. [Google Scholar] [CrossRef]
- Tian, S. A 20 Residues Motif Delineates the Furin Cleavage Site and its Physical Properties May Influence Viral Fusion. Biochem. Insights 2009, 2, BCI.S2049. [Google Scholar] [CrossRef]
- Choi, A.; Kots, E.D.; Singleton, D.T.; Weinstein, H.; Whittaker, G.R. Analysis of the molecular determinants for furin cleavage of the spike protein S1/S2 site in defined strains of the prototype coronavirus murine hepatitis virus (MHV). Virus Res. 2024, 340, 199283. [Google Scholar] [CrossRef]
- Gram Schjoldager, K.T.B.; Vester-Christensen, M.B.; Goth, C.K.; Petersen, T.N.; Brunak, S.; Bennett, E.P.; Levery, S.B.; Clausen, H. A Systematic Study of Site-specific GalNAc-type O-Glycosylation Modulating Proprotein Convertase Processing. J. Biol. Chem. 2011, 286, 40122–40132. [Google Scholar] [CrossRef]
- Molloy, S.S.; Bresnahan, P.A.; Leppla, S.H.; Klimpel, K.R.; Thomas, G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 1992, 267, 16396–16402. [Google Scholar] [CrossRef]
- Böttcher-Friebertshäuser, E.; Klenk, H.D.; Garten, W. Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog. Dis. 2013, 69, 87–100. [Google Scholar] [CrossRef]
- Lu, X.; Shi, Y.; Gao, F.; Xiao, H.; Wang, M.; Qi, J.; Gao, G.F. Insights into Avian Influenza Virus Pathogenicity: The Hemagglutinin Precursor HA0 of Subtype H16 Has an Alpha-Helix Structure in Its Cleavage Site with Inefficient HA1/HA2 Cleavage. J. Virol. 2012, 86, 12861–12870. [Google Scholar] [CrossRef][Green Version]
- Mayer, G.; Boileau, G.; Bendayan, M. Sorting of Furin in Polarized Epithelial and Endothelial Cells: Expression Beyond the Golgi Apparatus. J. Histochem. Cytochem. 2004, 52, 567–579. [Google Scholar] [CrossRef]
- Luczo, J.M.; Stambas, J.; Durr, P.A.; Michalski, W.P.; Bingham, J. Molecular pathogenesis of H5 highly pathogenic avian influenza: The role of the haemagglutinin cleavage site motif. Rev. Med. Virol. 2015, 25, 406–430. [Google Scholar] [CrossRef]
- Schrauwen, E.J.A.; Herfst, S.; Leijten, L.M.; Van Run, P.; Bestebroer, T.M.; Linster, M.; Bodewes, R.; Kreijtz, J.H.C.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; et al. The Multibasic Cleavage Site in H5N1 Virus Is Critical for Systemic Spread along the Olfactory and Hematogenous Routes in Ferrets. J. Virol. 2012, 86, 3975–3984. [Google Scholar] [CrossRef]
- Krzyzaniak, M.A.; Zumstein, M.T.; Gerez, J.A.; Picotti, P.; Helenius, A. Host Cell Entry of Respiratory Syncytial Virus Involves Macropinocytosis Followed by Proteolytic Activation of the F Protein. PLoS Pathog. 2013, 9, e1003309. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Gobeil, S.M.C.; Janowska, K.; McDowell, S.; Mansouri, K.; Parks, R.; Manne, K.; Stalls, V.; Kopp, M.F.; Henderson, R.; Edwards, R.J.; et al. D614G Mutation Alters SARS-CoV-2 Spike Conformation and Enhances Protease Cleavage at the S1/S2 Junction. Cell Rep. 2021, 34, 108630. [Google Scholar] [CrossRef]
- Rajah, M.M.; Bernier, A.; Buchrieser, J.; Schwartz, O. The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation. J. Mol. Biol. 2022, 434, 167280. [Google Scholar] [CrossRef]
- Sasaki, M.; Uemura, K.; Sato, A.; Toba, S.; Sanaki, T.; Maenaka, K.; Hall, W.W.; Orba, Y.; Sawa, H. SARS-CoV-2 variants with mutations at the S1/S2 cleavage site are generated in vitro during propagation in TMPRSS2-deficient cells. PLoS Pathog. 2021, 17, e1009233. [Google Scholar] [CrossRef]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Lubinski, B.; Frazier, L.E.; Phan, M.V.T.; Bugembe, D.L.; Cunningham, J.L.; Tang, T.; Daniel, S.; Cotton, M.; Jaimes, J.A.; Whittaker, G.R. Spike Protein Cleavage-Activation in the Context of the SARS-CoV-2 P681R Mutation: An Analysis from Its First Appearance in Lineage A.23.1 Identified in Uganda. Microbiol. Spectr. 2022, 10, e01514-22. [Google Scholar] [CrossRef]
- Lubinski, B.; Fernandes, M.H.V.; Frazier, L.; Tang, T.; Daniel, S.; Diel, D.G.; Jaimes, J.A.; Whittaker, G.R. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B.1.1.7 (Alpha) spike. iScience 2022, 25, 103589. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menchery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef]
- Zhang, L.; Mann, M.; Syed, Z.A.; Reynolds, H.M.; Tian, E.; Samara, N.L.; Zeldin, D.C.; Tabak, L.A.; Ten Hagen, K.G. Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2109905118. [Google Scholar] [CrossRef]
- Gellenoncourt, S.; Saunders, N.; Robinot, R.; Auguste, L.; Rajah, M.M.; Kervevan, J.; Jeger-Madiot, R.; Staropoli, I.; Planchais, C.; Mouquet, H.; et al. The Spike-Stabilizing D614G Mutation Interacts with S1/S2 Cleavage Site Mutations To Promote the Infectious Potential of SARS-CoV-2 Variants. J. Virol. 2022, 96, e01301-22. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Li, K.S.M.; Li, X.; Tsang, K.Y.; Sridhar, S.; Woo, P.C.Y. Fatal Pneumonia Associated with a Novel Genotype of Human Coronavirus OC43. Front. Microbiol. 2022, 12, 795449. [Google Scholar] [CrossRef]
- Stodola, J.K.; Dubois, G.; Le Coupanec, A.; Desforges, M.; Talbot, P.J. The OC43 human coronavirus envelope protein is critical for infectious virus production and propagation in neuronal cells and is a determinant of neurovirulence and CNS pathology. Virology 2018, 515, 134–149. [Google Scholar] [CrossRef]
- Lee, J.E.; Fusco, M.L.; Hessell, A.J.; Oswald, W.B.; Burton, D.R.; Saphire, E.O. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008, 454, 177–182. [Google Scholar] [CrossRef]
- Murray, J.M.; Aaskov, J.G.; Wright, P.J. Processing of the dengue virus type 2 proteins prM and C-prM. J. Gen. Virol. 1993, 74, 175–182. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Feng, J.J.; Zhou, J.M.; Yu, Z.Z.; Fang, D.Y.; Yan, H.J.; Zeng, G.C.; Jiang, L.F. Identification of a novel infection-enhancing epitope on dengue prM using a dengue cross-reacting monoclonal antibody. BMC Microbiol. 2013, 13, 194. [Google Scholar] [CrossRef]
- Schreuder, H.; Matter, H. Serine Proteinases from the Blood Coagulation Cascade. In Structural Biology in Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 395–422. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118681121.ch17 (accessed on 9 April 2024).
- Franczuk, P.; Tkaczyszyn, M.; Kulak, M.; Domenico, E.; Ponikowski, P.; Jankowska, E.A. Cardiovascular Complications of Viral Respiratory Infections and COVID-19. Biomedicines 2022, 11, 71. [Google Scholar] [CrossRef]
- Lazarowitz, S.G.; Choppin, P.W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 1975, 68, 440–454. [Google Scholar] [CrossRef]
- Sun, X.; Tse, L.V.; Ferguson, A.D.; Whittaker, G.R. Modifications to the Hemagglutinin Cleavage Site Control the Virulence of a Neurotropic H1N1 Influenza Virus. J. Virol. 2010, 84, 8683–8690. [Google Scholar] [CrossRef]
- Gotoh, B.; Ogasawara, T.; Toyoda, T.; Inocencio, N.M.; Hamaguchi, M.; Nagai, Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990, 9, 4189–4195. [Google Scholar] [CrossRef]
- Kummarapurugu, A.B.; Hawkridge, A.M.; Ma, J.; Osei, S.; Martin, R.K.; Zheng, S.; Voynow, J.A. Neutrophil elastase decreases SARS-CoV-2 spike protein binding to human bronchial epithelia by clipping ACE-2 ectodomain from the epithelial surface. J. Biol. Chem. 2023, 299, 104820. [Google Scholar] [CrossRef]
- Masic, A.; Booth, J.S.; Mutwiri, G.K.; Babiuk, L.A.; Zhou, Y. Elastase-Dependent Live Attenuated Swine Influenza A Viruses Are Immunogenic and Confer Protection against Swine Influenza A Virus Infection in Pigs. J. Virol. 2009, 83, 10198–10210. [Google Scholar] [CrossRef]
- Bugge, T.H.; Antalis, T.M.; Wu, Q. Type II Transmembrane Serine Proteases. J. Biol. Chem. 2009, 284, 23177–23181. [Google Scholar] [CrossRef]
- Béliveau, F.; Désilets, A.; Leduc, R. Probing the substrate specificities of matriptase, matriptase-2, hepsin and DESC1 with internally quenched fluorescent peptides. FEBS J. 2009, 276, 2213–2226. [Google Scholar] [CrossRef]
- Zmora, P.; Blazejewska, P.; Moldenhauer, A.S.; Welsch, K.; Nehlmeier, I.; Wu, Q.; Schneider, H.; Pöhlmann, S.; Bertram, S. DESC1 and MSPL Activate Influenza A Viruses and Emerging Coronaviruses for Host Cell Entry. J. Virol. 2014, 88, 12087–12097. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ohnishi, T.; Kawano, S.; Tsuchihashi, S.; Ogawara, M.; Masuda, K.; Yamaoka, K.; Takahashi, M.; Sano, T. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am. J. Respir. Cell Mol. Biol. 1997, 16, 300–308. [Google Scholar] [CrossRef]
- Wysocka, M.; Spichalska, B.; Lesner, A.; Jaros, M.; Brzozowski, K.; Łęgowska, A.; Rolka, K. Substrate specificity and inhibitory study of human airway trypsin-like protease. Bioorganic Med. Chem. 2010, 18, 5504–5509. [Google Scholar] [CrossRef]
- Böttcher, E.; Matrosovich, T.; Beyerle, M.; Klenk, H.D.; Garten, W.; Matrosovich, M. Proteolytic Activation of Influenza Viruses by Serine Proteases TMPRSS2 and HAT from Human Airway Epithelium. J. Virol. 2006, 80, 9896–9898. [Google Scholar] [CrossRef]
- Bertram, S.; Dijkman, R.; Habjan, M.; Heurich, A.; Gierer, S.; Glowacka, I.; Welsch, K.; Winkler, M.; Schneider, H.; Hofmann-Winkler, H.; et al. TMPRSS2 Activates the Human Coronavirus 229E for Cathepsin-Independent Host Cell Entry and Is Expressed in Viral Target Cells in the Respiratory Epithelium. J. Virol. 2013, 87, 6150–6160. [Google Scholar] [CrossRef]
- Bertram, S.; Glowacka, I.; Müller, M.A.; Lavender, H.; Gnirss, K.; Nehlmeier, I.; Niemeyer, D.; He, Y.; Simmons, G.; Drosten, C.; et al. Cleavage and Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by Human Airway Trypsin-Like Protease. J. Virol. 2011, 85, 13363–13372. [Google Scholar] [CrossRef]
- Beaulieu, A.; Gravel, É.; Cloutier, A.; Marois, I.; Colombo, É.; Désilets, A.; Verreault, C.; Leduc, R.; Marsault, É.; Richter, M.V. Matriptase Proteolytically Activates Influenza Virus and Promotes Multicycle Replication in the Human Airway Epithelium. J. Virol. 2013, 87, 4237–4251. [Google Scholar] [CrossRef]
- Whittaker, G.R.; Straus, M.R. Human matriptase/ST 14 proteolytically cleaves H7N9 hemagglutinin and facilitates the activation of influenza A/Shanghai/2/2013 virus in cell culture. Influenza Other Respir. Viruses 2020, 14, 189–195. [Google Scholar] [CrossRef]
- Zmora, P.; Hoffmann, M.; Kollmus, H.; Moldenhauer, A.S.; Danov, O.; Braun, A.; Winkler, M.; Schughart, K.; Pöhlmann, S. TMPRSS11A activates the influenza A virus hemagglutinin and the MERS coronavirus spike protein and is insensitive against blockade by HAI-1. J. Biol. Chem. 2018, 293, 13863–13873. [Google Scholar] [CrossRef]
- Kido, H.; Okumura, Y. MSPL/TMPRSS13. Front. Biosci. 2008, 13, 754–758. [Google Scholar] [CrossRef]
- Kishimoto, M.; Uemura, K.; Sanaki, T.; Sato, A.; Hall, W.W.; Kariwa, H.; Orba, Y.; Sawa, H.; Sasaki, M. TMPRSS11D and TMPRSS13 Activate the SARS-CoV-2 Spike Protein. Viruses 2021, 13, 384. [Google Scholar] [CrossRef]
- Yun Kim, S.; Park, D.; Oh, M.; Sellamuthu, S.; Park, W.J. Detection of site-specific proteolysis in secretory pathways. Biochem. Biophys. Res. Commun. 2002, 296, 419–424. [Google Scholar] [CrossRef]
- Mahmoud, I.S.; Jarrar, Y.B.; Alshaer, W.; Ismail, S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie 2020, 175, 93–98. [Google Scholar] [CrossRef]
- Shirogane, Y.; Takeda, M.; Iwasaki, M.; Ishiguro, N.; Takeuchi, H.; Nakatsu, Y.; Tahara, M.; Kikuta, H.; Yanagi, Y. Efficient Multiplication of Human Metapneumovirus in Vero Cells Expressing the Transmembrane Serine Protease TMPRSS2. J. Virol. 2008, 82, 8942–8946. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93, e01815-18. [Google Scholar] [CrossRef]
- Abe, M.; Tahara, M.; Sakai, K.; Yamaguchi, H.; Kanou, K.; Shirato, K.; Kawase, M.; Noda, M.; Kimura, H.; Matsuyama, S.; et al. TMPRSS2 Is an Activating Protease for Respiratory Parainfluenza Viruses. J. Virol. 2013, 87, 11930–11935. [Google Scholar] [CrossRef]
- Esram, P.; Arumugam, P. Development and Validation of an Enzymatic Assay for TMPRSS4: Evaluation of Molecular Inhibitors. J. Adv. Zool. 2023, 44, 309–321. [Google Scholar] [CrossRef]
- Kühn, N.; Bergmann, S.; Kösterke, N.; Lambertz, R.L.O.; Keppner, A.; Van den Brand, J.M.A.; Pöhlmann, S.; Weiß, S.; Hummler, E.; Hatesuer, B.; et al. The Proteolytic Activation of (H3N2) Influenza A Virus Hemagglutinin Is Facilitated by Different Type II Transmembrane Serine Proteases. J. Virol. 2016, 90, 4298–4307. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Epstein, R.J. The secret identities of TMPRSS2: Fertility factor, virus trafficker, inflammation moderator, prostate protector and tumor suppressor. Tumor Biol. 2021, 43, 159–176. [Google Scholar] [CrossRef]
- Murza, A.; Dion, S.P.; Boudreault, P.L.; Désilets, A.; Leduc, R.; Marsault, É. Inhibitors of type II transmembrane serine proteases in the treatment of diseases of the respiratory tract – A review of patent literature. Expert Opin. Ther. Pat. 2020, 30, 807–824. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Beaudoin, C.A.; Pandurangan, A.P.; Kim, S.Y.; Hamaia, S.W.; Huang, C.L.H.; Blundell, T.L.; Chaitanya Vedithis, S.; Jackson, A.P. In silico analysis of mutations near S1/S2 cleavage site in SARS-CoV-2 spike protein reveals increased propensity of glycosylation in Omicron strain. J. Med. Virol. 2022, 94, 4181–4192. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, L.; Duan, Y.; Tang, X.; Lu, J. Molecular insights into the adaptive evolution of SARS-CoV-2 spike protein. J. Infect. 2024, 88, 106121. [Google Scholar] [CrossRef]
- Kirschke, H. Chapter 410—Cathepsin L. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1808–1817. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192004105 (accessed on 13 July 2023).
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef]
- Du, X.; Tang, H.; Gao, L.; Wu, Z.; Meng, F.; Yan, R.; Qiao, S.; An, J.; Wang, C.; Qin, F.X.F. Omicron adopts a different strategy from Delta and other variants to adapt to host. Sig. Transduct. Target. Ther. 2022, 7, 45. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Mykytyn, A.Z.; Breugem, T.I.; Geurts, M.H.; Beumer, J.; Schipper, D.; Van Acker, R.; Van den Doel, P.B.; Van Royen, M.E.; Zhang, J.; Clevers, H.; et al. SARS-CoV-2 Omicron entry is type II transmembrane serine protease-mediated in human airway and intestinal organoid models. J. Virol. 2023, 97, e00851-23. [Google Scholar] [CrossRef]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef]
- Mainou, B.A. The Orchestra of Reovirus Cell Entry. Curr. Clin. Micro. Rpt. 2017, 4, 142–149. [Google Scholar] [CrossRef]
- Baer, G.S.; Dermody, T.S. Mutations in reovirus outer-capsid protein sigma3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 1997, 71, 4921–4928. [Google Scholar] [CrossRef]
- Wilson, G.J.; Nason, E.L.; Hardy, C.S.; Ebert, D.H.; Wetzel, J.D.; Venkataram Prasad, B.V.; Dermody, T.S. A Single Mutation in the Carboxy Terminus of Reovirus Outer-Capsid Protein σ3 Confers Enhanced Kinetics of σ3 Proteolysis, Resistance to Inhibitors of Viral Disassembly, and Alterations in σ3 Structure. J. Virol. 2002, 76, 9832–9843. [Google Scholar] [CrossRef]
- Doyle, J.D.; Danthi, P.; Kendall, E.A.; Ooms, L.S.; Wetzel, J.D.; Dermody, T.S. Molecular Determinants of Proteolytic Disassembly of the Reovirus Outer Capsid. J. Biol. Chem. 2012, 287, 8029–8038. [Google Scholar] [CrossRef]
- Mainou, B.A.; Dermody, T.S. In Search of Cathepsins: How Reovirus Enters Host Cells. DNA Cell Biol. 2012, 31, 1646–1649. [Google Scholar] [CrossRef]
- Schornberg, K.; Matsuyama, S.; Kabsch, K.; Delos, S.; Bouton, A.; White, J. Role of Endosomal Cathepsins in Entry Mediated by the Ebola Virus Glycoprotein. J. Virol. 2006, 80, 4174–4178. [Google Scholar] [CrossRef]
- Diederich, S.; Sauerhering, L.; Weis, M.; Altmeppen, H.; Schaschke, N.; Reinheckel, T.; Erbar, S.; Maisner, A. Activation of the Nipah virus fusion protein in MDCK cells is mediated by cathepsin B within the endosome-recycling compartment. J. Virol. 2012, 86, 3736–3745. [Google Scholar] [CrossRef]
- Vogt, C.; Eickmann, M.; Diederich, S.; Moll, M.; Maisner, A. Endocytosis of the Nipah Virus Glycoproteins. J. Virol. 2005, 79, 3865–3872. [Google Scholar] [CrossRef]
- Corry, J.; Johnson, S.M.; Cornwell, J.; Peeples, M.E. Preventing Cleavage of the Respiratory Syncytial Virus Attachment Protein in Vero Cells Rescues the Infectivity of Progeny Virus for Primary Human Airway Cultures. J. Virol. 2016, 90, 1311–1320. [Google Scholar] [CrossRef]
- Biniossek, M.L.; Nägler, D.K.; Becker-Pauly, C.; Schilling, O. Proteomic Identification of Protease Cleavage Sites Characterizes Prime and Non-prime Specificity of Cysteine Cathepsins B., L., and S. J. Proteome Res. 2011, 10, 5363–5373. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Kawase, M.; Shirato, K.; Van der Hoek, L.; Taguchi, F.; Matsuyama, S. Simultaneous Treatment of Human Bronchial Epithelial Cells with Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry. J. Virol. 2012, 86, 6537–6545. [Google Scholar] [CrossRef]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Sig. Transduct. Target Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Ebert, D.H.; Deussing, J.; Peters, C.; Dermody, T.S. Cathepsin L and Cathepsin B Mediate Reovirus Disassembly in Murine Fibroblast Cells. J. Biol. Chem. 2002, 277, 24609–24617. [Google Scholar] [CrossRef]
- Kleine-Weber, H.; Elzayat, M.T.; Hoffmann, M.; Pöhlmann, S. Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci. Rep. 2018, 8, 16597. [Google Scholar] [CrossRef]
- Pager, C.T.; Craft, W.W.; Patch, J.; Dutch, R.E. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology 2006, 346, 251–257. [Google Scholar] [CrossRef]
- Coleman, M.D.; Ha, S.D.; Haeryfar, S.M.M.; Barr, S.D.; Kim, S.O. Cathepsin B plays a key role in optimal production of the influenza A virus. J. Virol. Antivir. Res. 2018, 7, 1–20. [Google Scholar] [CrossRef]
- Bollavaram, K.; Leeman, T.H.; Lee, M.W.; Kulkarni, A.; Upshaw, S.G.; Yang, J.; Song, H.; Platt, M.O. Multiple sites on SARS-CoV-2 spike protein are susceptible to proteolysis by cathepsins B, K, L, S, and V. Protein Sci. 2021, 30, 1131–1143. [Google Scholar] [CrossRef]
- Golden, J.W.; Bahe, J.A.; Lucas, W.T.; Nibert, M.L.; Schiff, L.A. Cathepsin S Supports Acid-independent Infection by Some Reoviruses. J. Biol. Chem. 2004, 279, 8547–8557. [Google Scholar] [CrossRef]
- Brinkworth, R.I.; Tort, J.F.; Brindley, P.J.; Dalton, J.P. Phylogenetic relationships and theoretical model of human cathepsin W (lymphopain), a cysteine proteinase from cytotoxic T lymphocytes. Int. J. Biochem. Cell Biol. 2000, 32, 373–384. [Google Scholar] [CrossRef]
- Edinger, T.O.; Pohl, M.O.; Yángüez, E.; Stertz, S. Cathepsin W Is Required for Escape of Influenza A Virus from Late Endosomes. mBio 2015, 6, e00297. [Google Scholar] [CrossRef]
- Brömme, D. Chapter 409—Cathepsin K. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1801–1807. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192004099 (accessed on 4 May 2024).
- Brömme, D. Chapter 414—Cathepsin V. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1831–1834. Available online: https://www.sciencedirect.com/science/article/pii/B9780123822192004130 (accessed on 4 May 2024).
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Yeo, S.J.; Yun, Y.J.; Lyu, M.A.; Woo, S.Y.; Woo, E.R.; Kim, S.J.; Lee, H.J.; Park, H.K.; Kook, Y.H. Respiratory syncytial virus infection induces matrix metalloproteinase-9 expression in epithelial cells. Arch. Virol. 2002, 147, 229–242. [Google Scholar] [CrossRef]
- Rojas-Quintero, J.; Wang, X.; Tipper, J.; Burkett, P.R.; Zuñiga, J.; Ashtekar, A.R.; Polverino, F.; Rout, A.; Yambayev, I.; Hernández, C.; et al. Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection. JCI Insight 2018, 3, e99022. [Google Scholar] [CrossRef]
- Tacon, C.E.; Wiehler, S.; Holden, N.S.; Newton, R.; Proud, D.; Leigh, R. Human Rhinovirus Infection Up-Regulates MMP-9 Production in Airway Epithelial Cells via NF-κB. Am. J. Respir. Cell Mol. Biol. 2010, 43, 201–209. [Google Scholar] [CrossRef]
- Elliott, M.B.; Welliver, R.C.; Laughlin, T.S.; Pryharski, K.S.; LaPierre, N.A.; Chen, T.; Souza, V.; Terio, N.B.; Hancock, G.E. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in the respiratory tracts of human infants following paramyxovirus infection. J. Med. Virol. 2007, 79, 447–456. [Google Scholar] [CrossRef]
- Phillips, J.M.; Gallagher, T.; Weiss, S.R. Neurovirulent Murine Coronavirus JHM.SD Uses Cellular Zinc Metalloproteases for Virus Entry and Cell-Cell Fusion. J. Virol. 2017, 91, e01564-16. [Google Scholar] [CrossRef]
- Yamamoto, M.; Gohda, J.; Kobayashi, A.; Tomita, K.; Hirayama, Y.; Koshikawa, N.; Seiki, M.; Semba, K.; Akiyama, T.; Kawaguchi, Y.; et al. Metalloproteinase-Dependent and TMPRSS2-Independent Cell Surface Entry Pathway of SARS-CoV-2 Requires the Furin Cleavage Site and the S2 Domain of Spike Protein. mBio 2022, 13, e0051922. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Huang, X.; Hu, B.; Chai, Y.; Shi, H.; Zhu, T.; Yuen, T.T.T.; Liu, Y.; Liu, H.; Shi, J.; et al. Altered host protease determinants for SARS-CoV-2 Omicron. Sci. Adv. 2023, 9, eadd3867. [Google Scholar] [CrossRef]
- Fernandez-Patron, C.; Hardy, E. Matrix Metalloproteinases in Health and Disease in the Times of COVID-19. Biomolecules 2022, 12, 692. [Google Scholar] [CrossRef]
- Syed, F.; Li, W.; Relich, R.F.; Russell, P.M.; Zhang, S.; Zimmerman, M.K.; Yu, Q. Excessive Matrix Metalloproteinase-1 and Hyperactivation of Endothelial Cells Occurred in COVID-19 Patients and Were Associated With the Severity of COVID-19. J. Infect. Dis. 2021, 224, 60–69. [Google Scholar] [CrossRef]
- Safont, B.; Tarraso, J.; Rodriguez-Borja, E.; Fernández-Fabrellas, E.; Sancho-Chust, J.N.; Molina, V.; Lopez-Ramirez, C.; Lopez-Martinez, A.; Cabanes, L.; Andreu, A.L.; et al. Lung Function, Radiological Findings and Biomarkers of Fibrogenesis in a Cohort of COVID-19 Patients Six Months After Hospital Discharge. Arch. Bronconeumol. 2022, 58, 142–149. [Google Scholar] [CrossRef]
- Blascke de Mello, M.M.; Parente, J.M.; Schulz, R.; Castro, M.M. Matrix metalloproteinase (MMP)-2 activation by oxidative stress decreases aortic calponin-1 levels during hypertrophic remodeling in early hypertension. Vasc. Pharmacol. 2019, 116, 36–44. [Google Scholar] [CrossRef]
- D`Avila-Mesquita, C.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellinini-Diab, M.J.; Cuoto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar] [CrossRef]
- Salomão, R.; Assis, V.; De Sousa Neto, I.V.; Petriz, B.; Babault, N.; Durigan, J.L.Q.; De Cássia Marqueti, R. Involvement of Matrix Metalloproteinases in COVID-19: Molecular Targets, Mechanisms, and Insights for Therapeutic Interventions. Biology 2023, 12, 843. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, X.; Zhou, B.; Li, J.; Chen, M.; Deng, R.; Wong, G.; Lavillette, D.; Meng, G. SARS-CoV-2 spike engagement of ACE2 primes S2′ site cleavage and fusion initiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2111199119. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, P.; Jiang, J.; Liu, Y.; Yan, R.; Shu, S.; Hu, B.; Xiao, H.; Cai, K.; Yuan, S.; et al. Advances in developing ACE2 derivatives against SARS-CoV-2. Lancet Microbe 2023, 4, e369-78. [Google Scholar] [CrossRef]
- Heindl, M.R.; Rupp, A.L.; Schwerdtner, M.; Bestle, D.; Harbig, A.; De Rocher, A.; Schmacke, L.C.; Staker, B.; Steinmetzer, T.; Stein, D.A.; et al. ACE2 acts as a novel regulator of TMPRSS2-catalyzed proteolytic activation of influenza A virus in airway cells. J. Virol. 2024, 98, e00102-24. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, S.; Gu, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; Van der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Funk, C.J.; Wang, J.; Ito, Y.; Travanty, E.A.; Voelker, D.R.; Holmes, K.V.; Mason, R.J. Infection of human alveolar macrophages by human coronavirus strain 229E. J. Gen. Virol. 2012, 93 Pt 3, 494–503. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Moosavi-Movahedi, Z.; Sheibani, N.; Moosavi-Movahedi, A.A. An outlook on suicide enzyme inhibition and drug design. J. Iran. Chem. Soc. 2022, 19, 1575–1592. [Google Scholar] [CrossRef]
- Ben-Tal, A.; Nir, K. Introduction to Proteins: Structure, Function, and Motion, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2018; 988p. [Google Scholar]
- Rajendran, L.; Knölker, H.J.; Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010, 9, 29–42. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Lotke, R.; Petersen, M.; Sauter, D. Restriction of Viral Glycoprotein Maturation by Cellular Protease Inhibitors. Viruses 2024, 16, 332. [Google Scholar] [CrossRef]
- Stein, P.; Chothia, C. Serpin tertiary structure transformation. J. Mol. Biol. 1991, 221, 615–621. [Google Scholar] [CrossRef]
- Nichols, D.B.; De Martini, W.; Cottrell, J. Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis. Viruses 2017, 9, 215. [Google Scholar] [CrossRef]
- Varkoly, K.; Beladi, R.; Hamada, M.; McFadden, G.; Irving, J.; Lucas, A.R. Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins. Biomolecules 2023, 13, 1393. [Google Scholar] [CrossRef]
- Viswanathan, K.; Liu, L.; Vaziri, S.; Dai, E.; Richardson, J.; Togonu-Bickersteth, B.; Vatsya, P.; Christov, A.; Lucas, A.R. Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 2006, 95, 499–510. [Google Scholar] [CrossRef]
- Lomas, D.A.; Evans, D.L.; Upton, C.; McFadden, G.; Carrell, R.W. Inhibition of plasmin, urokinase, tissue plasminogen activator, and C1S by a myxoma virus serine proteinase inhibitor. J. Biol. Chem. 1993, 268, 516–521. [Google Scholar] [CrossRef]
- Guo, Q.; Yaron, J.R.; Wallen, J.W.; Browder, K.F.; Boyd, R.; Olson, T.L.; Burgin, M.; Ulrich, P.; Aliskevich, E.; Schutz, L.N.; et al. PEGylated Serp-1 Markedly Reduces Pristane-Induced Experimental Diffuse Alveolar Hemorrhage, Altering uPAR Distribution, and Macrophage Invasion. Front. Cardiovasc. Med. 2021, 8, 633212. [Google Scholar] [CrossRef]
- Tarighi, P.; Eftekhari, S.; Chizari, M.; Sabernavaei, M.; Jafari, D.; Mirzabeigi, P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021, 895, 173890. [Google Scholar] [CrossRef]
- Wyde, P.R.; Chetty, S.N.; Jewell, A.M.; Boivin, G.; Piedra, P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antivir. Res. 2003, 60, 51–59. [Google Scholar] [CrossRef]
- Thomas, G.; Couture, F.; Kwiatkowska, A. The Path to Therapeutic Furin Inhibitors: From Yeast Pheromones to SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3435. [Google Scholar] [CrossRef]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A.; et al. Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity. J. Virol. 2022, 96, e00128-22. [Google Scholar] [CrossRef]
- Someya, A.; Tanaka, N.; Okuyama, A. Inhibition of influenza virus AWSN replication by a trypsin inhibitor, 6-amidino-2-naphthyl p-guanidinobenzoate. Biochem. Biophys. Res. Commun. 1990, 169, 148–152. [Google Scholar] [CrossRef]
- Hosoya, M.; Matsuyama, S.; Baba, M.; Suzuki, H.; Shigeta, S. Effects of protease inhibitors on replication of various myxoviruses. Antimicrob. Agents Chemother. 1992, 36, 1432–1436. [Google Scholar] [CrossRef]
- Hosoya, M.; Shigeta, S.; Ishii, T.; Suzuki, H.; Clercq, E.D. Comparative Inhibitory Effects of Various Nucleoside and Nonnucleoside Analogues on Replication of Influenza Virus Types A and B In Vitro and In Ovo. J. Infect. Dis. 1993, 168, 641–646. [Google Scholar] [CrossRef]
- Gunst, J.D.; Staerke, N.B.; Pahus, M.H.; Kristensen, L.H.; Bodilsen, J.; Lohse, N.; Dalgaard, L.S.; Brønnum, D.; Fröbert, O.; Hønge, B.; et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial. EClinicalMedicine 2021, 35, 100849. [Google Scholar] [CrossRef]
- Zhirnov, O.P.; Klenk, H.D.; Wright, P.F. Aprotinin and similar protease inhibitors as drugs against influenza. Antivir. Res. 2011, 92, 27–36. [Google Scholar] [CrossRef]
- Grocott, H.P.; Sheng, H.; Miura, Y.; Sarraf-Yazdi, S.; Mackensen, G.B.; Pearlstein, R.D.; Warner, D.S. The effects of aprotinin on outcome from cerebral ischemia in the rat. Anesth. Analg. 1999, 88, 1–7. [Google Scholar] [CrossRef]
- Mangano, D.T.; Tudor, I.C.; Dietzel, C. The risk associated with aprotinin in cardiac surgery. N. Engl. J. Med. 2006, 354, 353–365. [Google Scholar] [CrossRef]
- Hamilton, B.S.; Chung, C.; Cyphers, S.Y.; Rinaldi, V.D.; Marcano, V.C.; Whittaker, G.R. Inhibition of influenza virus infection and hemagglutinin cleavage by the protease inhibitor HAI-2. Biochem. Biophys. Res. Commun. 2014, 450, 1070–1075. [Google Scholar] [CrossRef][Green Version]
- Straus, M.R.; Kinder, J.T.; Segall, M.; Dutch, R.E.; Whittaker, G.R. SPINT2 inhibits proteases involved in activation of both influenza viruses and metapneumoviruses. Virology 2020, 543, 43–53. [Google Scholar] [CrossRef]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340–348. [Google Scholar] [CrossRef]
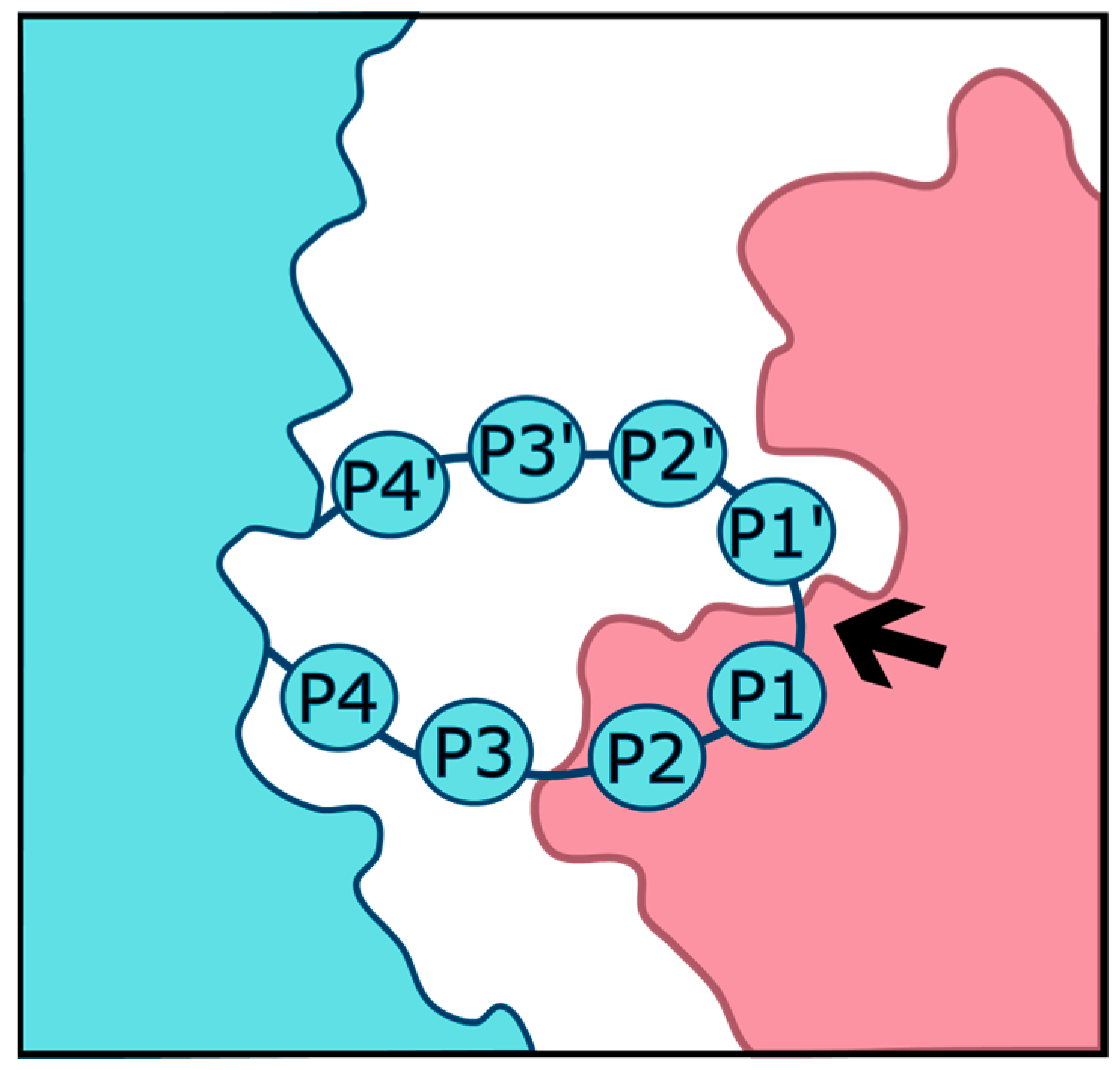
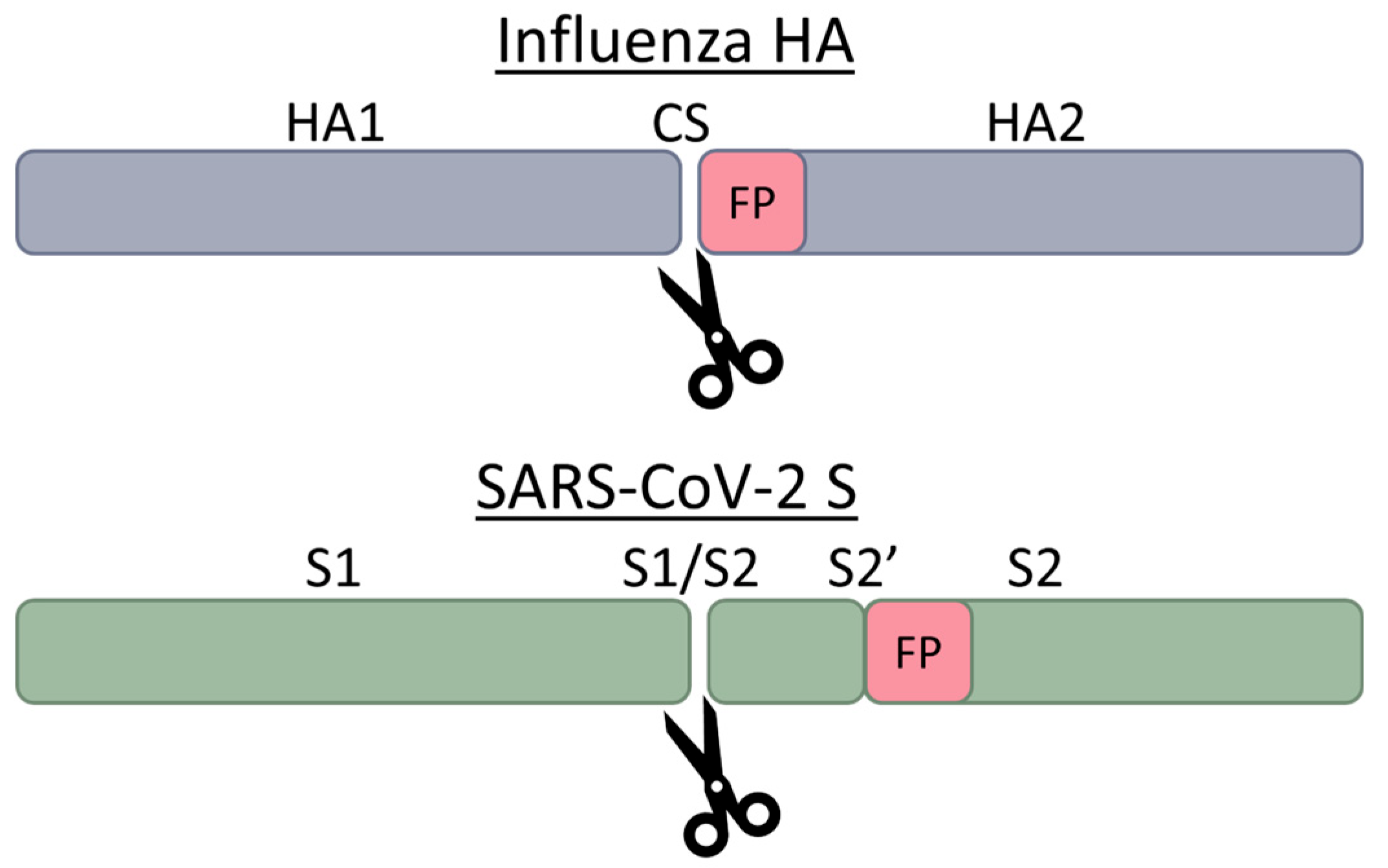


| Serine Proteases | Cleavage Preferences | Exploiting Respiratory Viruses |
|---|---|---|
| Chymase | Aromatic amino acids at P1, aliphatic amino acids from P2 to P4, S at P1′, E/D at P2′, A/V/G at P3′ [17] | Mammalian Orthoreovirus [18] |
| Factor Xa | Preference for PXG/AR↓XXD [19] | SARS-CoV-1 [20], SARS-CoV-2 [21] |
| Furin | R-X-X-R↓S [22] | Influenza virus [23], SARS-CoV-2 [24], MERS-CoV [25], RSV [26], HPIV [27], measles virus (MeV) [28], mumps virus [29] |
| Kallikrein-related peptidase 13 (KLK13] | V/Y-R/L/F/M-R↓ [30] | HKU-1 [31] |
| KLK1 | R/Y↓S/R [32] | Influenza virus [33] |
| KLK5 | X(aliphatic/aromatic)-R/K -X(polar/aliphatic)- R↓ [30] | Influenza virus [33] |
| Neutrophil elastase | A/V/I/T↓ [34] | Mammalian Orthoreovirus [35], SARS-CoV-1 [36,37] |
| PC5/6 | R-X-R/K-R↓ [38] | Influenza virus [39], RSV [39] |
| Plasmin | R/L↓ [40], preference for aromatic hydrophobic residue at P2 [41] | Influenza virus [42], RSV [43], SARS-CoV-1 [44], SARS-CoV-2 [45] |
| Thrombin | L-X-P-R↓S/A/G/T-X(aromatic)-R [46] | RSV [43], SARS-CoV-2 [21] |
| Trypsin | K/R↓ [34] | SARS-CoV-1 [47,48], HMPV [49], RSV [43], HPIV [50] |
| Cathepsin G | Preference for F, Y, W, or L at P1 [51], S at P6, negatively charged amino acid in P2′ position | Mammalian Orthoreovirus [18], RSV [52] |
| Type II Transmembrane Serine Proteases (TTSPs) | Cleavage Preferences | Exploiting Respiratory Viruses |
|---|---|---|
| DESC1 | R-R/A/L-L-A↓ [87] | Influenza virus [88], MERS-CoV [88], SARS-CoV-1 [88] |
| Human Airway Trypsin-like Protease | R/K↓ [89,90] | Influenza virus [91], HCoV-229E [92], SARS-CoV-1 [93], Mammalian Orthoreovirus [18] |
| Matriptase | Minimum: R/K [34]↓ Preferred: R-X(non-basic)-S-R↓ [87] | Influenza A virus [94,95] |
| TMPRSS11a | Unconfirmed, putative R/K↓ | SARS-CoV-1 [44], influenza virus [96], MERS-CoV [96] |
| TMPRSS13/MSPL | R/K↓, preference for dibasic P2-P1 [97] | Influenza virus [88], SARS-CoV-1 [88], MERS-CoV [88], SARS-CoV-2 [98] |
| TMPRSS2 | R/K↓ [99] | Influenza A + B virus [91], SARS-CoV-2 [100], HMPV [101], HCoV-229E [92], MERS-CoV [102], SARS-CoV [102], HPIV [103], Mammalian Orthoreovirus [18] |
| TMPRSS4 | Unconfirmed, putative R/K↓ [104] | Influenza A virus [105], SARS-CoV-2 [106] |
| Cysteine Proteases | Cleavage Determinants | Exploiting Respiratory Viruses |
|---|---|---|
| Cathepsin L | Prefers aromatic or aliphatic residues in P2 [127] | HCoV-229E [92], SARS-CoV-1 [128,129,130], SARS-CoV-2 [131], Mammalian Orthoreovirus [132], MERS-CoV [133], Nipah [134], RSV [126], Hendra [134] |
| Cathepsin B | Prefers an aromatic or aliphatic residue and tolerates a basic P2, an aromatic residue in P1′ and a P3′ G [127] | Influenza A virus [135], Mammalian Orthoreovirus [132], Nipah [124], SARS-CoV-2 [136] |
| Cathepsin S | Prefers aliphatic residues in P2, G/E in P1 [127] | Mammalian Orthoreovirus [137], SARS-CoV-2, SARS-CoV-1, RSV [52] |
| Cathepsin W | W/F–L/V–G/A/R↓V–D/N/E/Q (suggested [138]) | Influenza A virus [139], RSV [52] |
| Cathepsin K | Prefers non-aromatic hydrophobic residues in P2 [140] | SARS-CoV-2 [136] |
| Cathepsin V | Prefers hydrophobic residues in P2, P in P3 [141] | SARS-CoV-2 [136] |
| Metalloprotease | Exploiting Respiratory Viruses | Role |
|---|---|---|
| MT-MMP | SARS-CoV-2 [149] | Proteolytic cleavage |
| ADAM | SARS-CoV-2 [149] | Proteolytic cleavage |
| ACE2 | SARS-CoV-2 [160], SARS-CoV [161], HcoV NL63 [162]|Influenza A virus [159], MERS-CoV [159] | Receptor|Regulation of TMPRSS2 |
| APN | HCoV-229E [163] | Receptor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubinski, B.; Whittaker, G.R. Host Cell Proteases Involved in Human Respiratory Viral Infections and Their Inhibitors: A Review. Viruses 2024, 16, 984. https://doi.org/10.3390/v16060984
Lubinski B, Whittaker GR. Host Cell Proteases Involved in Human Respiratory Viral Infections and Their Inhibitors: A Review. Viruses. 2024; 16(6):984. https://doi.org/10.3390/v16060984
Chicago/Turabian StyleLubinski, Bailey, and Gary R. Whittaker. 2024. "Host Cell Proteases Involved in Human Respiratory Viral Infections and Their Inhibitors: A Review" Viruses 16, no. 6: 984. https://doi.org/10.3390/v16060984
APA StyleLubinski, B., & Whittaker, G. R. (2024). Host Cell Proteases Involved in Human Respiratory Viral Infections and Their Inhibitors: A Review. Viruses, 16(6), 984. https://doi.org/10.3390/v16060984






