Discovery and Genomic Characterization of a Novel Hepadnavirus from Asymptomatic Anadromous Alewife (Alosa pseudoharengus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.1.1. Spawning River Herring
2.1.2. Young-of-the-Year (Age-0) River Herring
2.1.3. Landlocked Alewife
2.2. Viral Screening (Cell Culture)
2.3. DNA Extractions and Template Preparation
2.4. High Throughput Sequencing-Assisted Virus Discovery
2.5. Bioinformatic Discovery of a Novel Hepadnavirus
2.6. Quantitative PCR
3. Results
3.1. Cell Culture Assays, Histology, and Transmission Electron Microscopy
3.2. Virus Prevalence in Wild Caught Herring
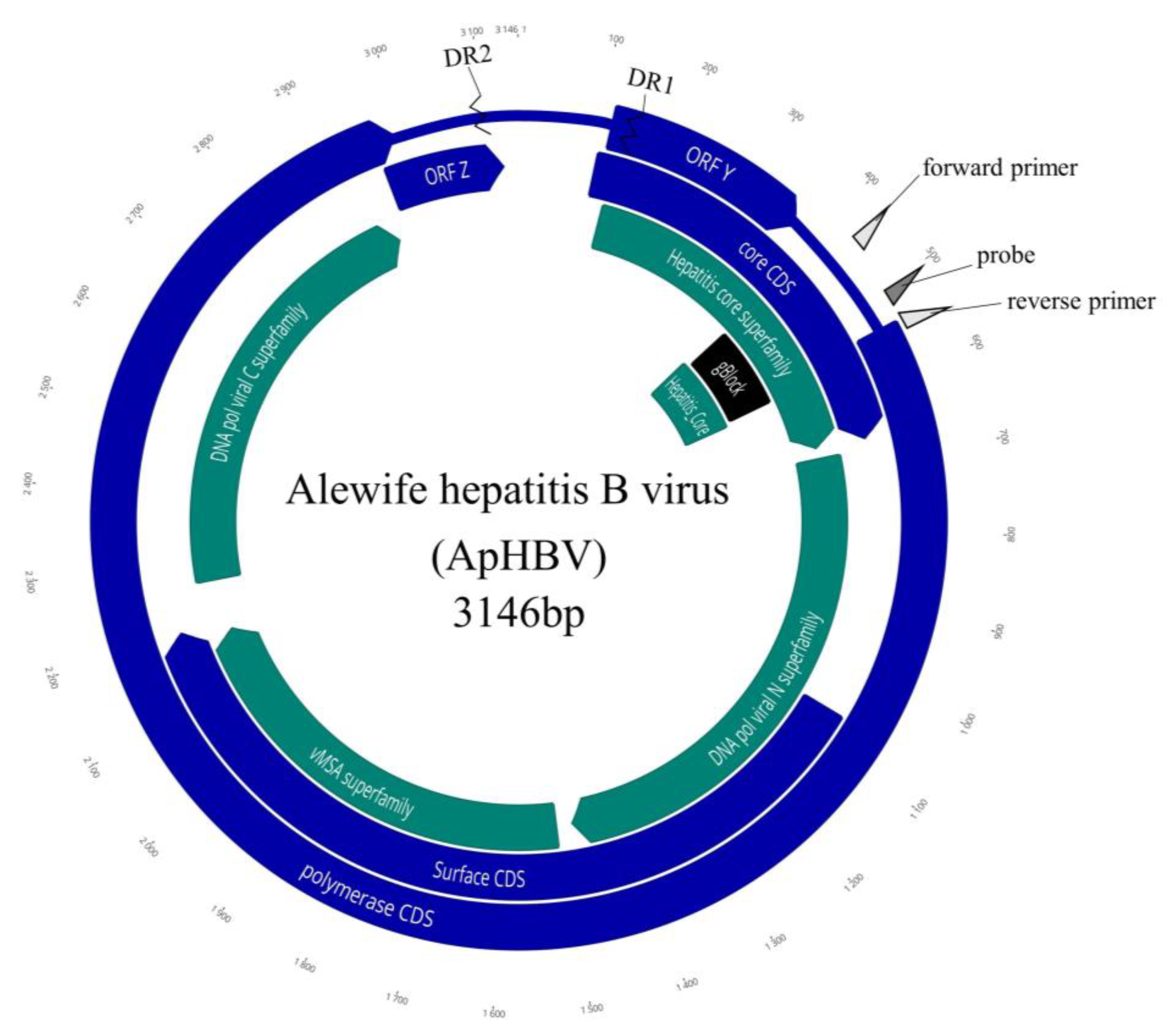
3.3. Sequencing the Viral Genome and ORF Organization
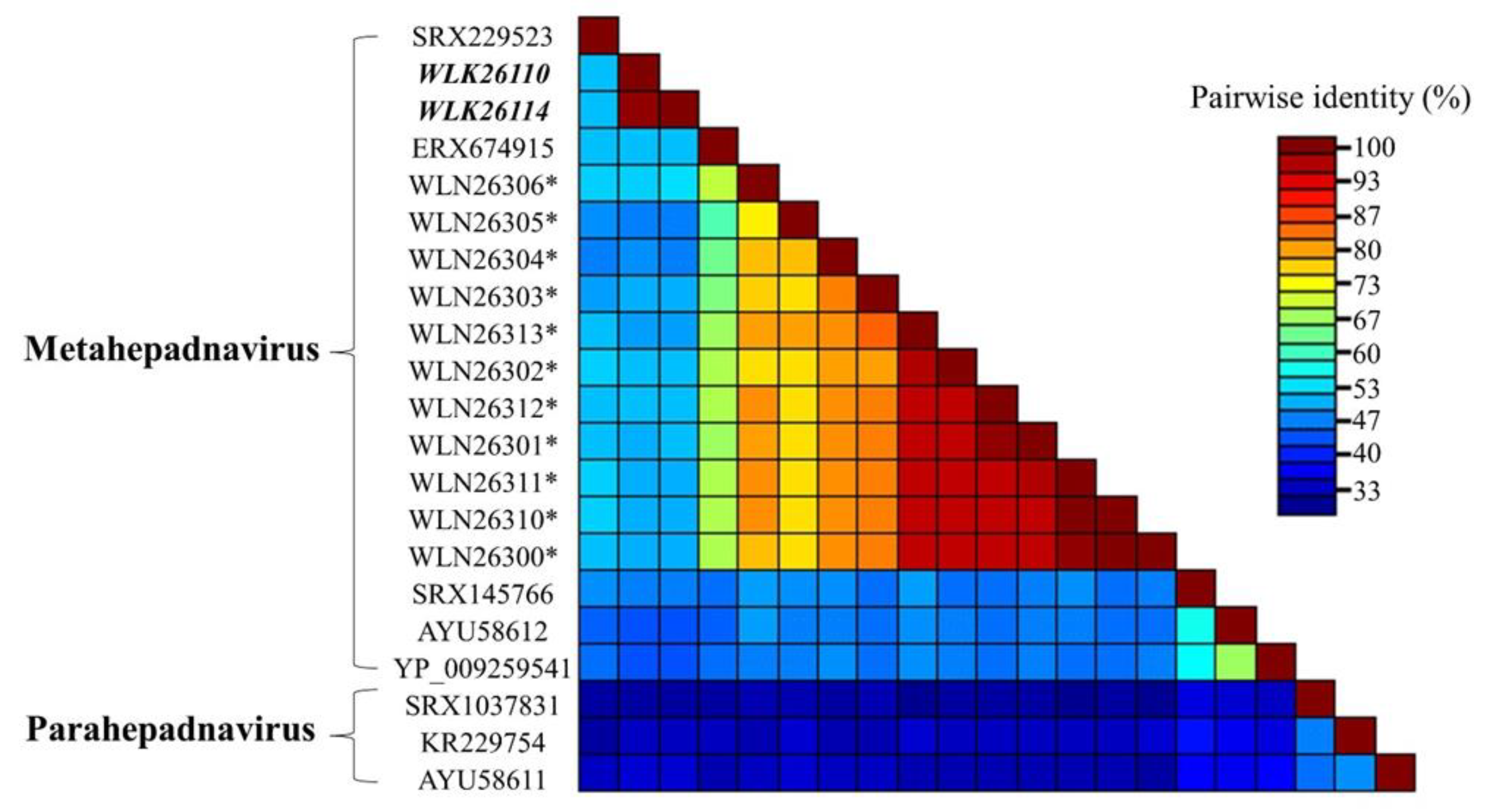
3.4. Genomic Diversity
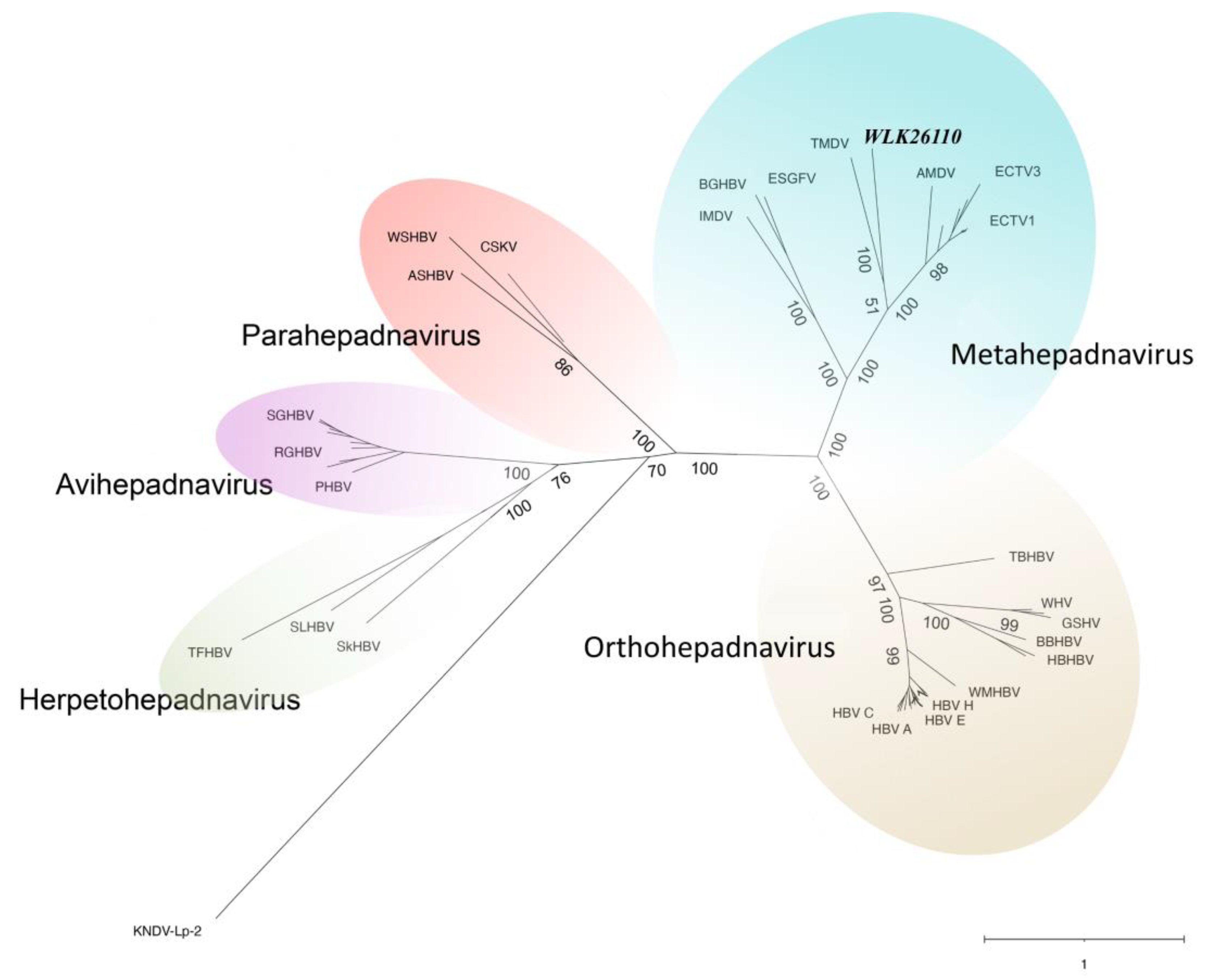
3.5. Pairwise Comparisons and Phylogenetic Analysis of P Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belding, D.L. The Preservation of the Alewife. Trans. Am. Fish. Soc. 1920, 49, 92–104. [Google Scholar] [CrossRef]
- Rounsefell, G.A.; Stringer, L.D. Restoration and Management of the New England Alewife Fisheries with Special Reference to Maine. Trans. Am. Fish. Soc. 1945, 73, 394–424. [Google Scholar] [CrossRef]
- Vogel, V.J. The Blackout of Native American Cultural Achievements. Am. Indian Q. 1987, 11, 11–35. [Google Scholar] [CrossRef]
- Fenichel, E.P.; Horan, R.D.; Bence, J.R. Indirect Management of Invasive Species through Bio-Controls: A Bioeconomic Model of Salmon and Alewife in Lake Michigan. Resour. Energy Econ. 2010, 32, 500–518. [Google Scholar] [CrossRef]
- Havey, K.A. Restoration of Anadromous Alewives at Long Pond, Maine. Trans. Am. Fish. Soc. 1961, 90, 281–286. [Google Scholar] [CrossRef]
- McBride, M.C.; Hasselman, D.J.; Willis, T.V.; Palkovacs, E.P.; Bentzen, P. Influence of Stocking History on the Population Genetic Structure of Anadromous Alewife (Alosa pseudoharengus) in Maine Rivers. Conserv. Genet. 2015, 16, 1209–1223. [Google Scholar] [CrossRef]
- Kissil, G.W. Spawning of the Anadromous Alewife, Alosa Pseudoharengus, in Bride Lake, Connecticut. Trans. Am. Fish. Soc. 1974, 103, 312–317. [Google Scholar] [CrossRef]
- Walsh, H.J.; Settle, L.R.; Peters, D.S. Early Life History of Blueback Herring and Alewife in the Lower Roanoke River, North Carolina. Trans. Am. Fish. Soc. 2005, 134, 910–926. [Google Scholar] [CrossRef]
- Limburg, K.E.; Waldman, J.R. Dramatic Declines in North Atlantic Diadromous Fishes. BioScience 2009, 59, 955–965. [Google Scholar] [CrossRef]
- Hall, C.J.; Jordaan, A.; Frisk, M.G. Centuries of Anadromous Forage Fish Loss: Consequences for Ecosystem Connectivity and Productivity. BioScience 2012, 62, 723–731. [Google Scholar] [CrossRef]
- German, B.; Watson, J.; Best, M. River Herring Habitat Conservation Plan; Greater Atlantic Region Policy Series; Greater Atlantic Regional Fisheries Office: Gloucester, MA, USA, 2023; Volume 23. [Google Scholar]
- Marcogliese, D. The Impact of Climate Change on the Parasites and Infectious Diseases of Aquatic Animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Lovy, J.; Friend, S.E. Intestinal coccidiosis of anadromous and landlocked alewives, Alosa pseudoharengus, caused by Goussia ameliae n. sp. and G. alosii n. sp. (Apicomplexa: Eimeriidae). Int. J. Parasitol. Parasites Wildl. 2015, 4, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H.; Consortium, I.R. ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571. [Google Scholar] [CrossRef] [PubMed]
- Dill, J.A.; Camus, A.C.; Leary, J.H.; Di Giallonardo, F.; Holmes, E.C.; Ng, T.F.F. Distinct Viral Lineages from Fish and Amphibians Reveal the Complex Evolutionary History of Hepadnaviruses. J. Virol. 2016, 90, 7920–7933. [Google Scholar] [CrossRef]
- Lauber, C.; Seitz, S.; Mattei, S.; Suh, A.; Beck, J.; Herstein, J.; Börold, J.; Salzburger, W.; Kaderali, L.; Briggs, J.A.; et al. Deciphering the Origin and Evolution of Hepatitis B Viruses by Means of a Family of Non-Enveloped Fish Viruses. Cell Host Microbe 2017, 22, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.R.; Blazer, V.S.; Sherry, J.; Cornman, R.S.; Iwanowicz, L.R. Phylogeographic Genetic Diversity in the White Sucker Hepatitis B Virus across the Great Lakes Region and Alberta, Canada. Viruses 2021, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.; Hoffman, J.; Walsh, H.; Braham, R.; Hahn, C.; Collins, P.; Jorgenson, Z.; Ledder, T. Health of White Sucker within the St. Louis River Area of Concern Associated with Habitat Usage as Assessed Using Stable Isotopes. Ecotoxicology 2014, 23, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Walsh, H.; Braham, R.; Hahn, C.; Mazik, P.; McIntyre, P. Tumours in White Suckers from Lake Michigan Tributaries: Pathology and Prevalence. J. Fish Dis. 2017, 40, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.M.; Iwanowicz, L.R.; Cornman, R.S.; Conway, C.M.; Winton, J.R.; Blazer, V.S. Characterization of a Novel Hepadnavirus in the White Sucker (Catostomus commersonii) from the Great Lakes Region of the United States. J. Virol. 2015, 89, 11801–11811. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Cousins, K.; Shi, M.; Williamson, J.E.; Holmes, E.C. Hidden Diversity and Evolution of Viruses in Market Fish. Virus Evol. 2018, 4, vey031. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Wille, M.; Ortiz-Baez, A.S.; Costa, V.A.; Ghaly, T.; Mifsud, J.C.; Turnbull, O.M.; Bellwood, D.R.; Williamson, J.E.; et al. Virome Composition in Marine Fish Revealed by Meta-Transcriptomics. Virus Evol. 2021, 7, veab005. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at Its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar]
- Friend, S.E.; Lewis, N.L.; Lovy, J. Myxozoan Parasites Vary in River Herring According to Life History Stage and Habitat. Parasitol. Res. 2021, 120, 3709–3723. [Google Scholar] [CrossRef]
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to Determine Limit of Detection and Limit of Quantification in Quantitative Real-Time PCR (qPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hayer, J.; Jadeau, F.; Deleage, G.; Kay, A.; Zoulim, F.; Combet, C. HBVdb: A Knowledge Database for Hepatitis B Virus. Nucleic Acids Res. 2013, 41, D566–D570. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.N.; Hu, J. Hepatitis B Virus Reverse Transcriptase–Target of Current Antiviral Therapy and Future Drug Development. Antivir. Res. 2015, 123, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus Taxonomy: The Database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Filipa-Silva, A.; Parreira, R.; Martínez-Puchol, S.; Bofill-Mas, S.; Barreto Crespo, M.T.; Nunes, M. The Unexplored Virome of Two Atlantic Coast Fish: Contribution of Next-Generation Sequencing to Fish Virology. Foods 2020, 9, 1634. [Google Scholar] [CrossRef]
- Costa, V.A.; Ronco, F.; Mifsud, J.C.; Harvey, E.; Salzburger, W.; Holmes, E.C. Host Adaptive Radiation Is Associated with Rapid Virus Diversification and Cross-Species Transmission in African Cichlid Fishes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Suh, A.; Brosius, J.; Schmitz, J.; Kriegs, J.O. The Genome of a Mesozoic Paleovirus Reveals the Evolution of Hepatitis B Viruses. Nat. Commun. 2013, 4, 1791. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Meik, J.; Dashevsky, D.; Card, D.; Castoe, T.; Schaack, S. Endogenous Hepadnaviruses, Bornaviruses and Circoviruses in Snakes. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141122. [Google Scholar] [CrossRef] [PubMed]
- Lytras, S.; Arriagada, G.; Gifford, R.J. Ancient Evolution of Hepadnaviral Paleoviruses and Their Impact on Host Genomes. Virus Evol. 2021, 7, veab012. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, M.B.; Spires, J.; Aguilar, R.; Goodison, M.R.; Heggie, K.; Kinnebrew, E.; McBurney, W.; Richie, K.D.; Roberts, P.M.; Hines, A.H. Assessment of River Herring Spawning Runs in a Chesapeake Bay Coastal Plain Stream Using Imaging Sonar. Trans. Am. Fish. Soc. 2017, 146, 22–35. [Google Scholar] [CrossRef]
- Tian, Y.; Kuo, C.; Chen, W.; Ou, J.J. Enhancement of Hepatitis B Virus Replication by Androgen and Its Receptor in Mice. J. Virol. 2012, 86, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Chen, P.-J.; Yeh, S.-H. Gender Disparity in Chronic Hepatitis B: Mechanisms of Sex Hormones. J. Gastroenterol. Hepatol. 2015, 30, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Palkovacs, E.P.; Hasselman, D.J.; Baetscher, D.; Kibele, J.; Gahagan, B.; Bentzen, P.; McBride, M.C.; Garza, J.C. Comprehensive Evaluation of Genetic Population Structure for Anadromous River Herring with Single Nucleotide Polymorphism Data. Fish. Res. 2018, 206, 247–258. [Google Scholar] [CrossRef]
- McBride, M.C.; Willis, T.V.; Bradford, R.G.; Bentzen, P. Genetic Diversity and Structure of Two Hybridizing Anadromous Fishes (Alosa pseudoharengus, Alosa aestivalis) across the Northern Portion of Their Ranges. Conserv. Genet. 2014, 15, 1281–1298. [Google Scholar] [CrossRef]
- Kaján, G.L.; Doszpoly, A.; Tarján, Z.L.; Vidovszky, M.Z.; Papp, T. Virus–Host Coevolution with a Focus on Animal and Human DNA Viruses. J. Mol. Evol. 2020, 88, 41–56. [Google Scholar] [CrossRef]
- Able, K.; Grothues, T.; Shaw, M.; VanMorter, S.; Sullivan, M.; Ambrose, D. Alewife (Alosa Pseudoharengus) Spawning and Nursery Areas in a Sentinel Estuary: Spatial and Temporal Patterns. Environ. Biol. Fishes 2020, 103, 1419–1436. [Google Scholar] [CrossRef]
- Hall, C.J.; Jordaan, A.; Frisk, M.G. The Historic Influence of Dams on Diadromous Fish Habitat with a Focus on River Herring and Hydrologic Longitudinal Connectivity. Landsc. Ecol. 2011, 26, 95–107. [Google Scholar] [CrossRef]
- Song, C.; Omalley, A.; Roy, S.G.; Barber, B.L.; Zydlewski, J.; Mo, W. Managing Dams for Energy and Fish Tradeoffs: What Does a Win-Win Solution Take? Sci. Total Environ. 2019, 669, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Carlos Garza, J.; Gephard, S.R.; Caccone, A.; Post, D.M.; Palkovacs, E.P. Restoration-Mediated Secondary Contact Leads to Introgression of Alewife Ecotypes Separated by a Colonial-Era Dam. Evol. Appl. 2020, 13, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Alcott, D.J.; Goerig, E.; Rillahan, C.; He, P.; Castro-Santos, T. Tide Gates Form Physical and Ecological Obstacles to River Herring (Alosa Spp.) Spawning Migrations. Can. J. Fish. Aquat. Sci. 2021, 78, 869–880. [Google Scholar] [CrossRef]
- Lambert, Y.; Dodson, J.J. Freshwater Migration as a Determinant Factor in the Somatic Cost of Reproduction of Two Anadromous Coregonines of James Bay. Can. J. Fish. Aquat. Sci. 1990, 47, 318–334. [Google Scholar] [CrossRef]
- Wilson, S.M.; Taylor, J.J.; Mackie, T.A.; Patterson, D.A.; Cooke, S.J.; Willmore, W.G. Oxidative Stress in Pacific Salmon (Oncorhynchus Spp.) during Spawning Migration. Physiol. Biochem. Zool. 2014, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B. Stress and Fish Reproduction: The Roles of Allostasis and Hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Thorstad, E.B.; Økland, F.; Aarestrup, K.; Heggberget, T.G. Factors Affecting the Within-River Spawning Migration of Atlantic Salmon, with Emphasis on Human Impacts. Rev. Fish Biol. Fish. 2008, 18, 345–371. [Google Scholar] [CrossRef]
- Hershberger, P.K.; van der Leeuw, B.K.; Gregg, J.L.; Grady, C.A.; Lujan, K.M.; Gutenberger, S.K.; Purcell, M.K.; Woodson, J.C.; Winton, J.R.; Parsley, M.J. Amplification and Transport of an Endemic Fish Disease by an Introduced Species. Biol. Invasions 2010, 12, 3665–3675. [Google Scholar] [CrossRef]
- Altizer, S.; Bartel, R.; Han, B.A. Animal Migration and Infectious Disease Risk. Science 2011, 331, 296–302. [Google Scholar] [CrossRef]
- Rahel, F.J.; McLaughlin, R.L. Selective Fragmentation and the Management of Fish Movement across Anthropogenic Barriers. Ecol. Appl. 2020, 28, 2066–2081. [Google Scholar] [CrossRef] [PubMed]
- Dominy, C. Changes in Blood Lactic Acid Concentrations in Alewives (Alosa pseudoharengus) during Passage through a Pool and Weir Fishway. J. Fish. Board Can. 1971, 28, 1215–1217. [Google Scholar] [CrossRef]
- Dominy, C. Effect of Entrance-Pool Weir Elevation and Fish Density on Passage of Alewives (Alosa pseudoharengus) in a Pool and Weir Fishway. Trans. Am. Fish. Soc. 1973, 102, 398–404. [Google Scholar] [CrossRef]
- Zwollo, P. The Humoral Immune System of Anadromous Fish. Dev. Comp. Immunol. 2018, 80, 24–33. [Google Scholar] [CrossRef]
- Costa, V.A.; Bellwood, D.R.; Mifsud, J.C.; Van Brussel, K.; Geoghegan, J.L.; Holmes, E.C.; Harvey, E. Limited Cross-Species Virus Transmission in a Spatially Restricted Coral Reef Fish Community. Virus Evol. 2023, 9, vead011. [Google Scholar] [CrossRef]
- Lynch, P.D.; Nye, J.A.; Hare, J.A.; Stock, C.A.; Alexander, M.A.; Scott, J.D.; Curti, K.L.; Drew, K. Projected Ocean Warming Creates a Conservation Challenge for River Herring Populations. ICES J. Mar. Sci. 2015, 72, 374–387. [Google Scholar] [CrossRef]
- Marcos-López, M.; Gale, P.; Oidtmann, B.; Peeler, E. Assessing the Impact of Climate Change on Disease Emergence in Freshwater Fish in the United Kingdom. Transbound. Emerg. Dis. 2010, 57, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lovy, J.; Hutcheson, J.M. Myxobolus Mauriensis n. Sp. Infecting Rib Cartilage of Young-of-the-Year River Herring in New Jersey: Notes on Pathology, Prevalence, and Genetics. J. Parasitol. 2016, 102, 419–428. [Google Scholar] [CrossRef]
- Okamoto, H.; Tsuda, F.; Sakugawa, H.; Sastrosoewignjo, R.I.; Imai, M.; Miyakawa, Y.; Mayumi, M. Typing Hepatitis B Virus by Homology in Nucleotide Sequence: Comparison of Surface Antigen Subtypes. J. Gen. Virol. 1988, 69, 2575–2583. [Google Scholar] [CrossRef]



| Site Name | Location | Species | Life Stage | Sample Date | Water Temperature | Collection Method | TL ± SD (mm) | Weight ± SD (g) | Sample Size |
|---|---|---|---|---|---|---|---|---|---|
| Maurice | 39.378760, −75.037418 | Alewife | Sp adult | April 2015 | 6.5–22 °C | 3″ gill net | 301 ± 10.5 | 252 ± 17.9 | n = 12 |
| Maurice | 39.378760, −75.037418 | Blueback | Sp adult | April 2015 | 6.5–22 °C | 3″ gill net | 297 ± 7.8 | 295 ± 21.6 | n = 2 |
| Maurice | 39.378760, −75.037418 | Alewife | Sp adult | March–April 2016 | 9.7–17 °C | 3″ gill net | 282 ± 10 | ND | n = 16 |
| Maurice | 39.378760, −75.037418 | Alewife | Sp adult | April–May 2018 | 5.2–13.8 °C | 3″ gill net | 277 ± 1.1 | 214 ± 26 | n = 15 |
| Maurice | 39.378760, −75.037418 | Blueback | Sp adult | April–May 2018 | 5.2–13.8 °C | 3″ gill net | 277 ± 0.3 | 199 ± 11 | n = 3 |
| GEH | 39.400229, −74.717855 | Alewife | Sp adult | April 2018 | 4.4–15 °C | 3″ gill net | 298 ± 1.3 | 246 ± 27 | n = 4 |
| GEH | 39.400229, −74.717855 | Alewife | YOY | August 2015 | ND | 100′ × 6′ × ¼″ mesh beach seine | 61.5 ± 2.4 | ND | n = 20 |
| GEH | 39.400229, −74.717855 | Blueback | YOY | August 2015 | ND | 100′ × 6′ × ¼″ mesh beach seine | 70.7 ± 4.3 | ND | n = 40 |
| Delaware | 40.153580, −74.721212 | Blueback | YOY | August 2015 | ND | Boat Electrofishing | 65.5 ± 4.2 | ND | n = 60 |
| Hopatcong | 40.934327, −74.643549 | LL Alewife | Juv-Adult | September 2015 | ND | Boatmounted Umbrella net | ND | ND | n = 65 |
| Site Name | Species | Life Stage | Sampling Period | Investigation Method | Tissues Collected |
|---|---|---|---|---|---|
| Maurice | Alewife | Sp adult | April 2015 | histology, viral cell culture, qPCR | AK, PK, spleen, gill |
| Maurice | Blueback | Sp adult | April 2015 | histology, viral cell culture, qPCR | AK, PK, spleen, gill |
| Maurice | Alewife | Sp adult | March–April 2016 | histology | AK, PK, spleen, liver, heart, GI |
| Maurice | Alewife | Sp adult | April–May 2018 | histology, qPCR | AK, PK, spleen, liver, heart, GI |
| Maurice | Blueback | Sp adult | April–May 2018 | histology, qPCR | AK, PK, spleen, liver, heart, GI |
| GEH | Alewife | Sp adult | April 2018 | histology, qPCR | AK, PK, spleen, liver, heart, GI |
| GEH | Alewife | YOY | August 2015 | viral cell culture, qPCR | AK, PK, spleen, gill, brain |
| GEH | Blueback | YOY | August 2015 | viral cell culture, qPCR | AK, PK, spleen, gill, brain |
| Delaware | Blueback | YOY | August 2015 | viral cell culture, qPCR | AK, PK, spleen, gill, brain |
| Lake Hopatcong | LL Alewife | Juv-Adult | September 2015 | viral cell culture, qPCR | AK, PK, spleen, gill, brain |
| Primer ID | Primer Name | Sequence (5′ → 3′) | Purpose | PCR Conditions |
|---|---|---|---|---|
| Resequencing PCR | ||||
| 1 | ApHBV802 F | 5′-TTACAGCTACAGGGCATCAA-3′ | Resequencing reaction 1 F | 30 cycles of 10 s at 98 °C, 60 s at 60 °C, and 5 min at 68 °C. Final hold at 4 °C |
| 2 | ApHBV2914 R | 5′-CAAAACAGCAGATGCGATAC-3′ | Resequencing reaction 1 R | |
| 3 | ApHBVGapF | 5′-CACGCGGTTTAGTGCTAACG-3′ | Resequencing reaction 2 F | |
| 4 | ApHBVGapR | 5′-GCAAGCCCAGTGAAACCAAG-3′ | Resequencing reaction 2 R | |
| Quantitative PCR | ||||
| 5 | ApHBV438_191F | 5′-CTTGGTTTCACTGGGCTTG-3′ | qPCR F Primer | 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 60 s at 60 °C, and 15 s at 95 °C |
| 6 | ApHBV_555R | 5′-AGAATGGGAGCATTCGGTGG-3′ | qPCR R Primer | |
| 7 | ApHBV probe | 5′-/56-FAM/CTGGACGCA/ZEN/GACCCCAGCAG/3IABkFQ/-3′ | qPCR Probe | |
| Accession # | Host | Tissue Source | Virus | % Similarity (M10) |
|---|---|---|---|---|
| WLK26110 | Alosa pseudoharengus | Viscera | Alewife hepatitis B virus | - |
| WLK26114 | Alosa pseudoharengus | Liver | Alewife hepatitis B virus | 99.1 |
| WLN26306 | Julidochromis dickfeldi | Lower pharengeal jaw | Lamprologini metahepadnavirus | 52.6 |
| WLN26302 | Cyathopharynx furcifer | Lower pharengeal jaw | Ectodini metahepadnavirus 1 | 51.1 |
| SRX229523 * | Astyanax mexicanus | Eyes-surface | Mexican tetra hepadnavirus | 51.0 |
| WLN26312 | Ophthalmotilapia ventralis | Lower pharengeal jaw | Ectodini metahepadnavirus 1 | 50.8 |
| ERX674915 * | Astatotilapia sp. | Unknown | Astatotilapia metahepadnavirus | 50.6 |
| WLN26311 | Cyathopharynx furcifer | Lower pharengeal jaw | Ectodini metahepadnavirus 3 | 50.5 |
| WLN26301 | Simochromis diagramma | Lower pharengeal jaw | Simochromis diagramma metahepadnavirus | 50.4 |
| WLN26310 | Xenotilapia sp. | Lower pharengeal jaw | Xenotilapia metahepadnavirus | 50.4 |
| WLN26300 | Lamprologus lemairii | lower pharyngeal jaw | Lamprologini metahepadnavirus | 50.3 |
| WLN26303 | Aulonocranus dewindti | Lower pharengeal jaw | Ectodini metahepadnavirus 1 | 49.8 |
| WLN26313 | Callochromis pleurospilus | Gill | Ectodini metahepadnavirus 2 | 48.8 |
| WLN26304 | Enantiopus melanogenys | Lower pharengeal jaw | Ectodini metahepadnavirus 2 | 47.7 |
| WLN26305 | Ophthalmotilapia ventralis | Gill | Ectodini metahepadnavirus 3 | 47.0 |
| SRX145766 * | Boulengerochromis microlepis | Lower pharengeal jaw | Boulengerochromis microlepis metahepadnavirus | 47.0 |
| AYU58612 | Hyporhamphus australis | Gills | Eastern sea garfish hepatitis B virus | 43.7 |
| YP_009259541 | Lepomis macrochirus | Lip | Bluegill hepatitis B virus | 43.1 |
| AYU58611 | Pagrus auratus | Liver | Australasian snapper hepatitis B virus | 34.4 |
| KR229754 | Catostomus commersonii | Liver | White Sucker hepatitis B virus | 33.3 |
| SRX1037831 * | Oncorhynchus kisutch | Kidney | Coho Salmon parahepadnavirus | 31.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raines, C.; Lovy, J.; Phelps, N.; Mor, S.; Ng, T.F.F.; Iwanowicz, L. Discovery and Genomic Characterization of a Novel Hepadnavirus from Asymptomatic Anadromous Alewife (Alosa pseudoharengus). Viruses 2024, 16, 824. https://doi.org/10.3390/v16060824
Raines C, Lovy J, Phelps N, Mor S, Ng TFF, Iwanowicz L. Discovery and Genomic Characterization of a Novel Hepadnavirus from Asymptomatic Anadromous Alewife (Alosa pseudoharengus). Viruses. 2024; 16(6):824. https://doi.org/10.3390/v16060824
Chicago/Turabian StyleRaines, Clayton, Jan Lovy, Nicolas Phelps, Sunil Mor, Terry Fei Fan Ng, and Luke Iwanowicz. 2024. "Discovery and Genomic Characterization of a Novel Hepadnavirus from Asymptomatic Anadromous Alewife (Alosa pseudoharengus)" Viruses 16, no. 6: 824. https://doi.org/10.3390/v16060824
APA StyleRaines, C., Lovy, J., Phelps, N., Mor, S., Ng, T. F. F., & Iwanowicz, L. (2024). Discovery and Genomic Characterization of a Novel Hepadnavirus from Asymptomatic Anadromous Alewife (Alosa pseudoharengus). Viruses, 16(6), 824. https://doi.org/10.3390/v16060824









