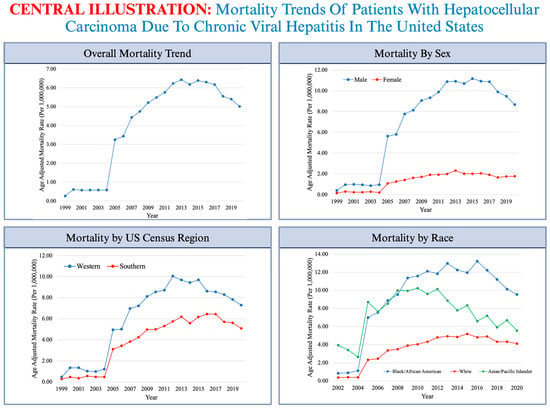
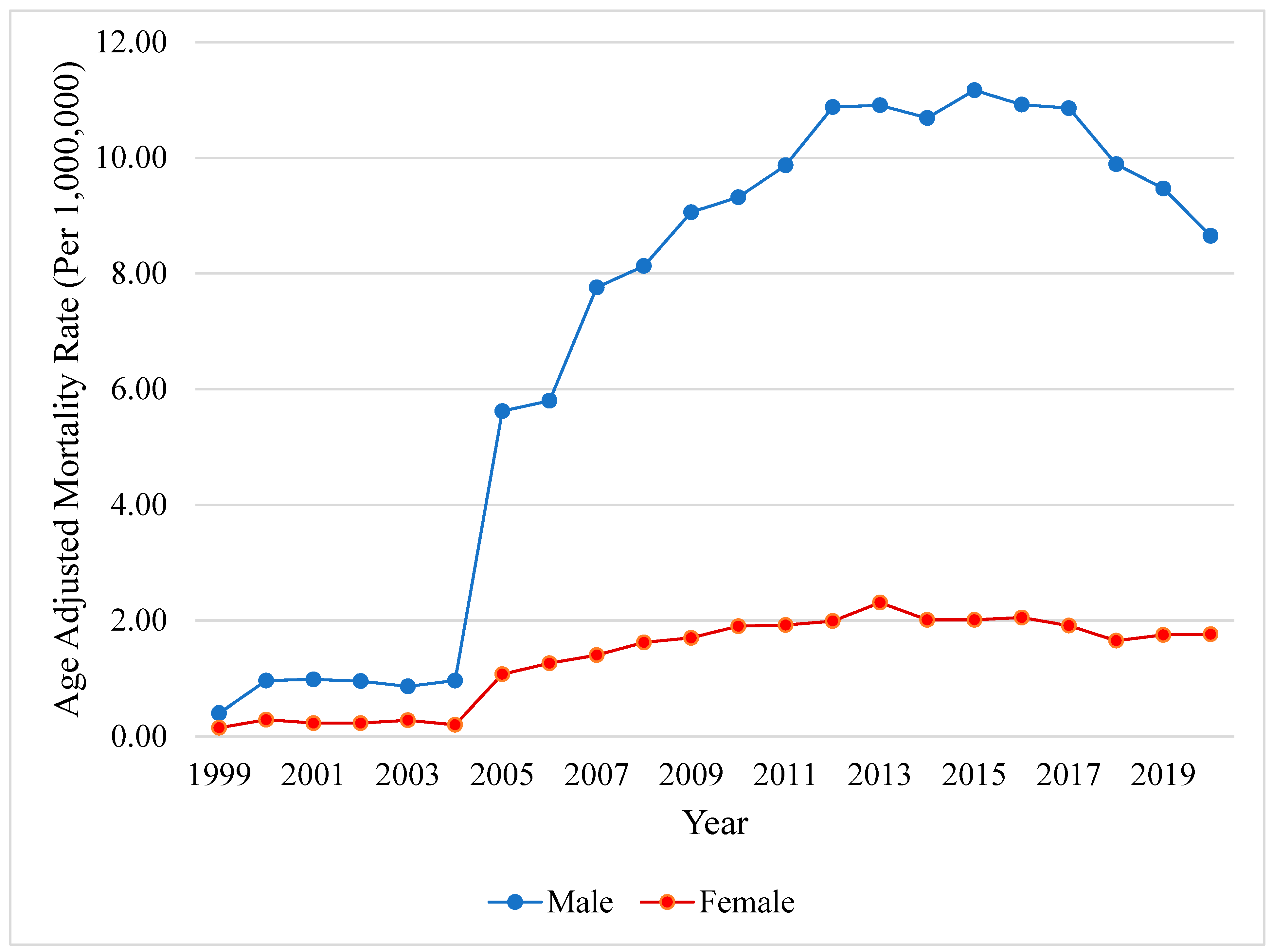
A Longitudinal Analysis of Mortality Related to Chronic Viral Hepatitis and Hepatocellular Carcinoma in the United States
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Cancer IARC Globocan. 2022. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_groupearth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D (accessed on 20 March 2024).
- Zhuo, Y.; Chen, Q.; Chhatwal, J. Changing Epidemiology of Hepatocellular Carcinoma and Role of Surveillance. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches; Hoshida, Y., Ed.; Humana Press: Cham, Germany, 2019; ISBN 978-3-030-21539-2. [Google Scholar]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Cruz-Ramón, V.; Chinchilla-López, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 262–279. [Google Scholar] [CrossRef]
- Russo, F.P.; Zanetto, A.; Pinto, E.; Battistella, S.; Penzo, B.; Burra, P.; Farinati, F. Hepatocellular Carcinoma in Chronic Viral Hepatitis: Where Do We Stand? Int. J. Mol. Sci. 2022, 23, 500. [Google Scholar] [CrossRef]
- Rizzo, G.E.M.; Cabibbo, G.; Craxì, A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses 2022, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, B.; Thimme, R.; Blum, H.E.; Zoulim, F. Hepatitis C virus-induced hepatocarcinogenesis. J. Hepatol. 2009, 51, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Gupta, P.; Irshad, K. Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection. World J. Hepatol. 2017, 9, 1305–1314. [Google Scholar] [CrossRef]
- Yang, M.; Parikh, N.D.; Liu, H.; Wu, E.; Rao, H.; Feng, B.; Lin, A.; Wei, L.; Lok, A.S. Incidence and risk factors of hepatocellular carcinoma in patients with hepatitis C in China and the United States. Sci. Rep. 2020, 10, 20922. [Google Scholar] [CrossRef]
- Melaram, R. Environmental Risk Factors Implicated in Liver Disease: A Mini-Review. Front. Public Health 2021, 9, 683719. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.C.; Yuan, J.-M. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology 2004, 127, S72–S78. [Google Scholar] [CrossRef] [PubMed]
- Edlin, B.R.; Eckhardt, B.J.; Shu, M.A.; Holmberg, S.D.; Swan, T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatol. Baltim. Md 2015, 62, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Nguyen, M.H.; Kim, W.R.; Gish, R.; Perumalswami, P.; Jacobson, I.M. Prevalence of Chronic Hepatitis B Virus Infection in the United States. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 1429. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention National Center for Health Statistics. CDC Wonder: Multiple Cause of Death 1999–2018; Center for Disease Control and Prevention: Washington, DC, USA, 2020. [Google Scholar]
- Registry, Agency for Toxic Substances and Disease Registry. DC/ATSDR Social Vulnerability Index. 2023. Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed on 4 April 2024).
- Sciences, National Cancer Institute Division of Cancer Control and Population Sciences. Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 4 April 2024).
- Backus, L.I.; Belperio, P.S.; Shahoumian, T.A.; Mole, L.A. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatol. Baltim. Md 2019, 69, 487–497. [Google Scholar] [CrossRef]
- Kim, H.-S.; El-Serag, H.B. Tenofovir vs. entecavir in reducing hepatocellular carcinoma risk in patients with chronic HBV infection?—Still an unsolved question. Hepatobiliary Surg. Nutr. 2021, 10, 119. [Google Scholar] [CrossRef]
- Dietz, C.; Maasoumy, B. Direct-Acting Antiviral Agents for Hepatitis C Virus Infection—From Drug Discovery to Successful Implementation in Clinical Practice. Viruses 2022, 14, 1325. [Google Scholar] [CrossRef]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef]
- Ramani, A.; Tapper, E.B.; Griffin, C.; Shankar, N.; Parikh, N.D.; Asrani, S.K. Hepatocellular Carcinoma-Related Mortality in the USA, 1999–2018. Dig. Dis. Sci. 2022, 67, 4100–4111. [Google Scholar] [CrossRef]
- Shiels, M.S.; Engels, E.A.; Yanik, E.L.; McGlynn, K.A.; Pfeiffer, R.M.; O’Brien, T.R. Incidence of Hepatocellular Carcinoma among Older Americans Attributable to Hepatitis C and Hepatitis B, 2001–2013. Cancer 2019, 125, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, G.; Guan, L.; Liu, Z.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Xu, H.; Ouyang, G. The burden of primary liver cancer caused by specific etiologies from 1990 to 2019 at the global, regional, and national levels. Cancer Med. 2022, 11, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, S.J.; Bird, S.M.; Goldberg, D.J. Influence of alcohol on the progression of hepatitis C virus infection: A meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2005, 3, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef]
- Picchio, C.A.; Lens, S.; Hernandez-Guerra, M.; Arenas, J.; Andrade, R.J.; Crespo, J.; García-Samaniego, J.; Romero-Gómez, M.; Turnes, J.; Calleja, J.L.; et al. Late presentation of chronic HBV and HCV patients seeking first time specialist care in Spain: A 2-year registry review. Sci. Rep. 2021, 11, 24133. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.S.; Mejia, M.C.; Salemi, J.L.; Gonzalez, S.J.; Aliyu, M.H.; Husaini, B.A.; Zoorob, R.J.; Hennekens, C.H. A descriptive study of racial inequalities in mortality from hepatocellular cancer before and after licensure of lifesaving drugs for hepatitis C virus in the United States. eClinicalMedicine 2020, 22, 100350. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S., Jr.; Dang, J. Hepatitis B among Asian Americans: Prevalence, progress, and prospects for control. World J. Gastroenterol. 2015, 21, 11924–11930. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; O’Brien, T.R. Recent Decline in Hepatocellular Carcinoma Rates in the United States. Gastroenterology 2020, 158, 1503–1505.e2. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatol. Baltim. Md 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Razavi-Shearer, D.; Gamkrelidze, I.; Pan, C.Q.; Razavi-Shearer, K.; Blach, S.; Estes, C.; Mooneyhan, E.; Razavi, H. The impact of immigration on hepatitis B burden in the United States: A modelling study. Lancet Reg. Health Am. 2023, 22, 100516. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.F.; McMahon, B.J.; Brown, R.S.; Wong, J.B.; Ahmed, A.T.; Farah, W.; Almasri, J.; Alahdab, F.; Benkhadra, K.; Mouchli, M.A.; et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatol. Baltim. Md 2016, 63, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Y.; Ismail, M.; Khan, H.; Medina-Morales, E.; Alsakarneh, S.; Jaber, F.; Pyrsopoulos, N.T. Hepatocellular Carcinoma Incidence and Mortality in the USA by Sex, Age, and Race: A Nationwide Analysis of Two Decades. J. Clin. Transl. Hepatol. 2024, 12, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.S.; Petrick, J.L.; Parisi, D.; McMahon, B.J.; Graubard, B.I.; McGlynn, K.A. Racial/ethnic disparities in hepatocellular carcinoma incidence and mortality rates in the United States, 1992–2018. Hepatol. Baltim. Md 2022, 76, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Cravero, A.; VoPham, T.; Vutien, P.; Carr, R.; Issaka, R.B.; Johnston, J.; McMahon, B.; Mera, J.; Ioannou, G.N. Addressing racial and ethnic disparities in US liver cancer care. Hepatol. Commun. 2023, 7, e00190. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Hoang, T.; Spiegel, B.M.R.; Eisen, S.; Dominitz, J.A.; Gifford, A.; Goetz, M.; Asch, S.M. Predictors of treatment in patients with chronic hepatitis C infection—Role of patient versus nonpatient factors. Hepatol. Baltim. Md 2007, 46, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Sloane, D.; Chen, H.; Howell, C. Racial disparity in primary hepatocellular carcinoma: Tumor stage at presentation, surgical treatment and survival. J. Natl. Med. Assoc. 2006, 98, 1934–1939. [Google Scholar] [PubMed]
- Saberi, B.; Gurakar, A.; Tamim, H.; Schneider, C.V.; Sims, O.T.; Bonder, A.; Fricker, Z.; Alqahtani, S.A. Racial Disparities in Candidates for Hepatocellular Carcinoma Liver Transplant After 6-Month Wait Policy Change. JAMA Netw. Open 2023, 6, e2341096. [Google Scholar] [CrossRef]
- Sobotka, L.A.; Hinton, A.; Conteh, L.F. African Americans are less likely to receive curative treatment for hepatocellular carcinoma. World J. Hepatol. 2018, 10, 849–855. [Google Scholar] [CrossRef]
- Zhou, K.; Pickering, T.A.; Gainey, C.S.; Cockburn, M.; Stern, M.C.; Liu, L.; Unger, J.B.; El-Khoueiry, A.B.; Terrault, N.A. Presentation, Management, and Outcomes Across the Rural-Urban Continuum for Hepatocellular Carcinoma. JNCI Cancer Spectr. 2021, 5, pkaa100. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Lee, Y.-T.; Agopian, V.G.; Zhu, Y.; You, S.; Tseng, H.-R.; Yang, J.D. Hepatocellular carcinoma surveillance: Current practice and future directions. Hepatoma Res. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Kim, D.; Ahmed, A.; Singal, A.K. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer 2021, 127, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Alawadi, Z.M.; Phatak, U.R.; Kao, L.S.; Ko, T.C.; Wray, C.J. Race not rural residency is predictive of surgical treatment for hepatocellular carcinoma: Analysis of the Texas Cancer Registry. J. Surg. Oncol. 2016, 113, 84–88. [Google Scholar] [CrossRef]
- Vo Quang, E.; Shimakawa, Y.; Nahon, P. Epidemiological projections of viral-induced hepatocellular carcinoma in the perspective of WHO global hepatitis elimination. Liver Int. 2021, 41, 915–927. [Google Scholar] [CrossRef]



| SVI-Q1 [95% CI] | SVI-Q2 [95% CI] | SVI-Q3 [95% CI] | SVI-Q4 [95% CI] | |
|---|---|---|---|---|
| All | 2.53 [2.43–2.62] | 3.82 [3.74–3.91] | 4.99 [4.90–5.08] | 5.20 [5.11–5.29] |
| Sex | ||||
| Female | 0.80 [0.73–0.88] | 1.24 [1.17–1.30] | 1.64 [1.56–1.71] | 1.72 [1.65–1.79] |
| Male | 4.36 [4.18–4.53] | 6.62 [6.45–6.78] | 8.67 [8.49–8.85] | 9.15 [8.98–9.33] |
| Ethnicity | ||||
| Hispanic | 4.66 [3.73–5.59] | 6.77 [6.17–7.37] | 8.66 [8.17–9.14] | 6.22 [5.98–6.45] |
| NH | 2.49 [2.40–2.58] | 3.70 [3.61–3.79] | 4.70 [4.61–4.80] | 4.95 [4.85–5.04] |
| Race | ||||
| NH Black | 8.38 [7.36–9.40] | 9.99 [9.40–10.59] | 11.11 [10.65–11.58] | 7.99 [7.73–8.25] |
| NH White | 2.25 [2.15–2.34] | 2.99 [2.91–3.08] | 3.81 [3.71–3.90] | 3.93 [3.83–4.04] |
| NH Asian | 5.53 [4.43–6.62] | 8.46 [7.81–9.11] | 7.92 [7.35–8.50] | 6.20 [5.74–6.66] |
| NH Native | NA | 9.49 [7.60–11.71] | 9.87 [8.16–11.58] | 6.21 [5.26–7.16] |
| Census Region | ||||
| Northeast | 2.65 [2.45–2.84] | 3.01 [2.86–3.17] | 3.22 [3.05–3.40] | 4.56 [4.33–4.80] |
| Midwest | 2.17 [2.04–2.29] | 3.07 [2.91–3.23] | 4.60 [4.39–4.81] | 4.02 [3.78–4.26] |
| South | 2.57 [2.33–2.82] | 3.15 [3.01–3.29] | 4.43 [4.29–4.57] | 4.90 [4.78–5.02] |
| West | 4.12 [3.72–4.51] | 6.99 [6.72–7.27] | 7.37 [7.15–7.59] | 6.51 [6.32–6.71] |
| Urbanization | ||||
| Urban | 2.59 [2.48–2.70] | 4.01 [3.92–4.11] | 5.26 [5.15–5.36] | 5.36 [5.26–5.46] |
| Rural | 2.34 [2.14–2.53] | 2.96 [2.77–3.15] | 3.61 [3.41–3.81] | 4.36 [4.15–4.57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.B.; Pham, H.N.; Mouhaffel, R.; Ibrahim, R.; Alsaqa, M.; Gurakar, A.; Saberi, B. A Longitudinal Analysis of Mortality Related to Chronic Viral Hepatitis and Hepatocellular Carcinoma in the United States. Viruses 2024, 16, 694. https://doi.org/10.3390/v16050694
Ozturk NB, Pham HN, Mouhaffel R, Ibrahim R, Alsaqa M, Gurakar A, Saberi B. A Longitudinal Analysis of Mortality Related to Chronic Viral Hepatitis and Hepatocellular Carcinoma in the United States. Viruses. 2024; 16(5):694. https://doi.org/10.3390/v16050694
Chicago/Turabian StyleOzturk, N. Begum, Hoang Nhat Pham, Rama Mouhaffel, Ramzi Ibrahim, Marwan Alsaqa, Ahmet Gurakar, and Behnam Saberi. 2024. "A Longitudinal Analysis of Mortality Related to Chronic Viral Hepatitis and Hepatocellular Carcinoma in the United States" Viruses 16, no. 5: 694. https://doi.org/10.3390/v16050694
APA StyleOzturk, N. B., Pham, H. N., Mouhaffel, R., Ibrahim, R., Alsaqa, M., Gurakar, A., & Saberi, B. (2024). A Longitudinal Analysis of Mortality Related to Chronic Viral Hepatitis and Hepatocellular Carcinoma in the United States. Viruses, 16(5), 694. https://doi.org/10.3390/v16050694