Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection
Abstract
1. Introduction
2. Materials and Methods
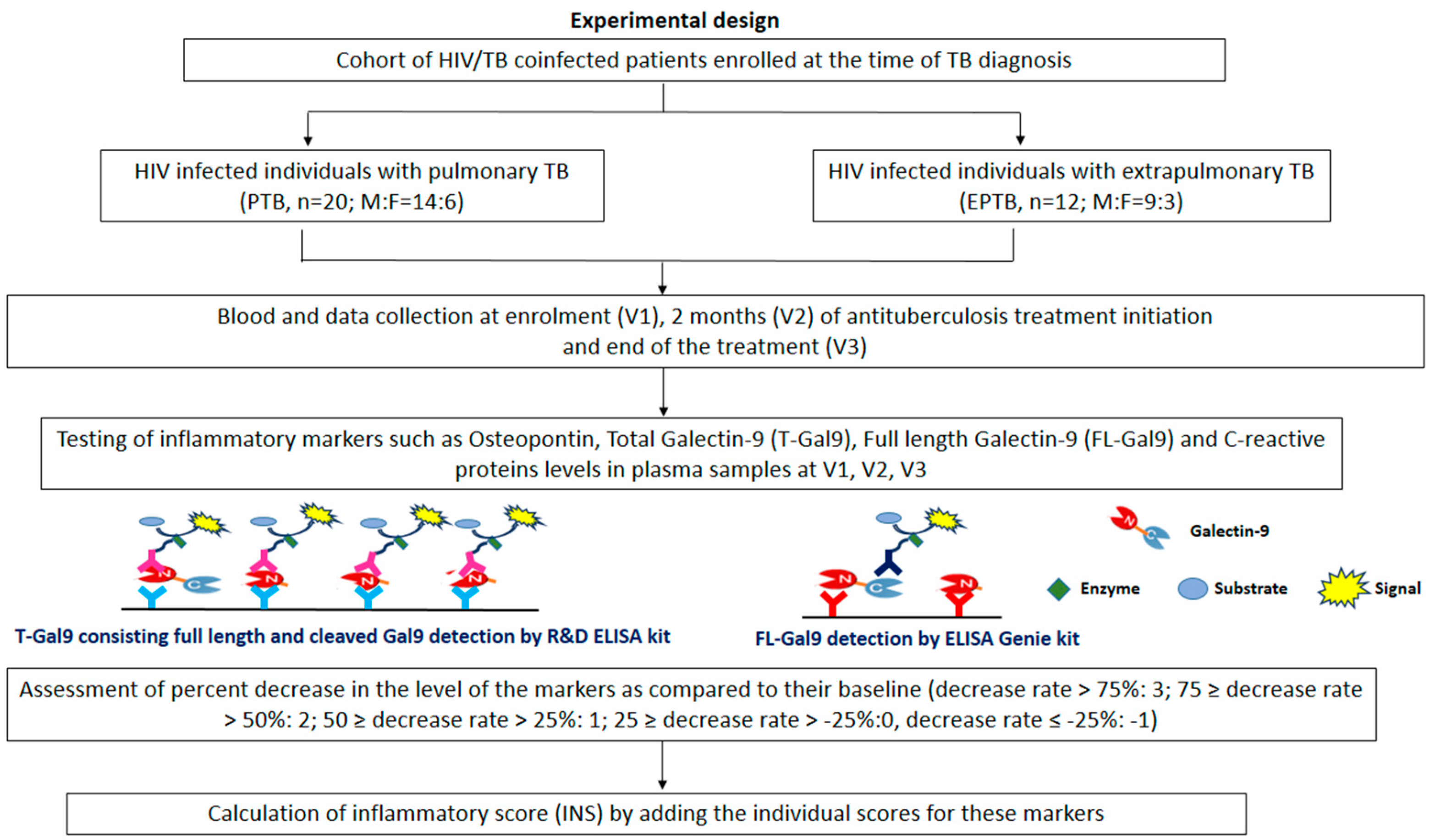
2.1. Study Settings and Participants
2.2. Sample Collection and Data Collection
2.3. Estimation of Matricellular Proteins and Other Inflammatory Markers
2.3.1. OPN ELISA Assay
2.3.2. T-Gal9 ELISA Assay
2.3.3. Full-Length Galectin-9 ELISA Assay
2.3.4. CRP ELISA Assay
2.4. Classification of the Severity of the Chest X-ray Findings
- Moderate involvement is defined as lesions present in one or both lungs, with a total extent which does not exceed the following:
- Scattered lesions of slight to moderate density that may extend throughout the total volume of one lung or may partially involve both lungs.
- Dense, confluent lesions that extend to up to 1/3 of the volume of one lung. Cavitation with a diameter of <4 cm.
2.5. Scoring for Inflammatory Markers
- For OPN, T-Gal9, FL-Gal9, and CRP levels, each decrease rate was calculated as the percentage of concentration at the first visit minus concentration at the third visit. Based on each decrease rate, the score for each marker was defined as follows: decrease rate > 75%: 3; 75 ≥ decrease rate > 50%: 2; 50 ≥ decrease rate > 25%: 1; 25 ≥ decrease rate > −25%:0; decrease rate ≤ −25%: −1. Finally, the total score, calculated by adding the individual scores for these markers, was designated as INS.
- INS classified each patient into immunologic successor (above 6 for all TB patients and 8 for only PTB) and failure (below 5 for all TB patients and 7 for only PTB). The median of the total score was used to classify each patient as a successor or failure and obtain the cut-off value of each marker to discriminate successors from failures using ROC curve analysis.
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Patients
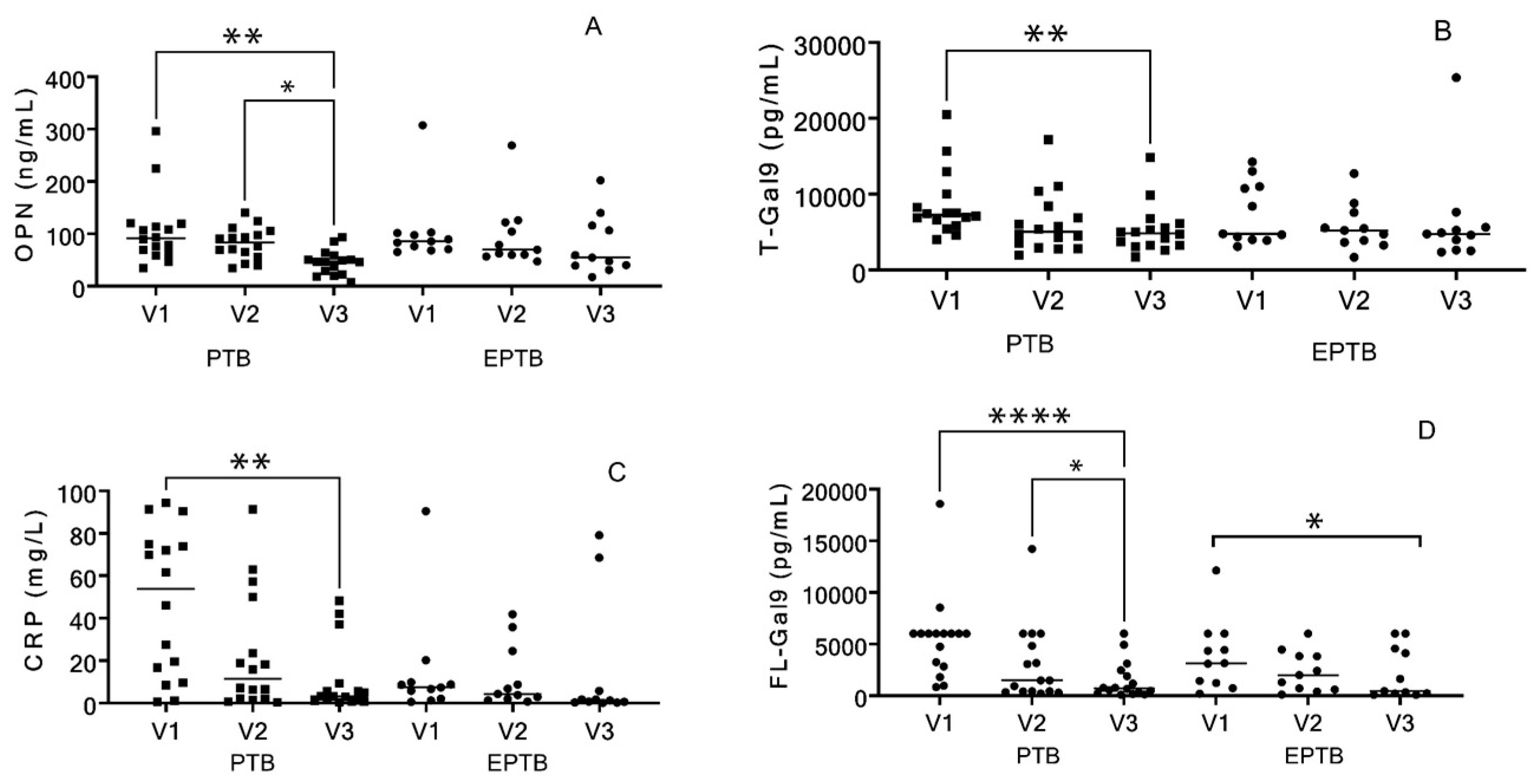
3.2. Analysis of Markers of the Patients
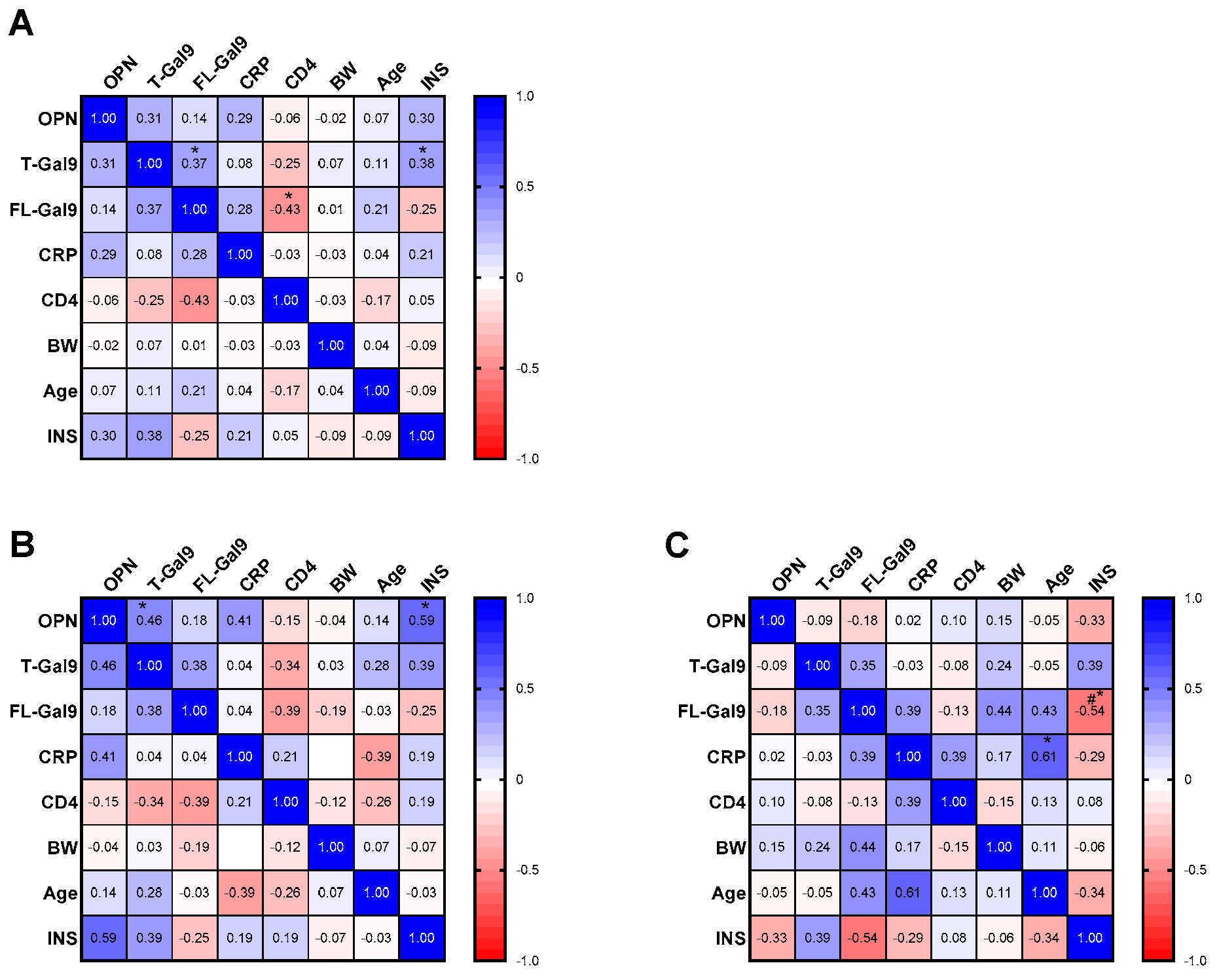
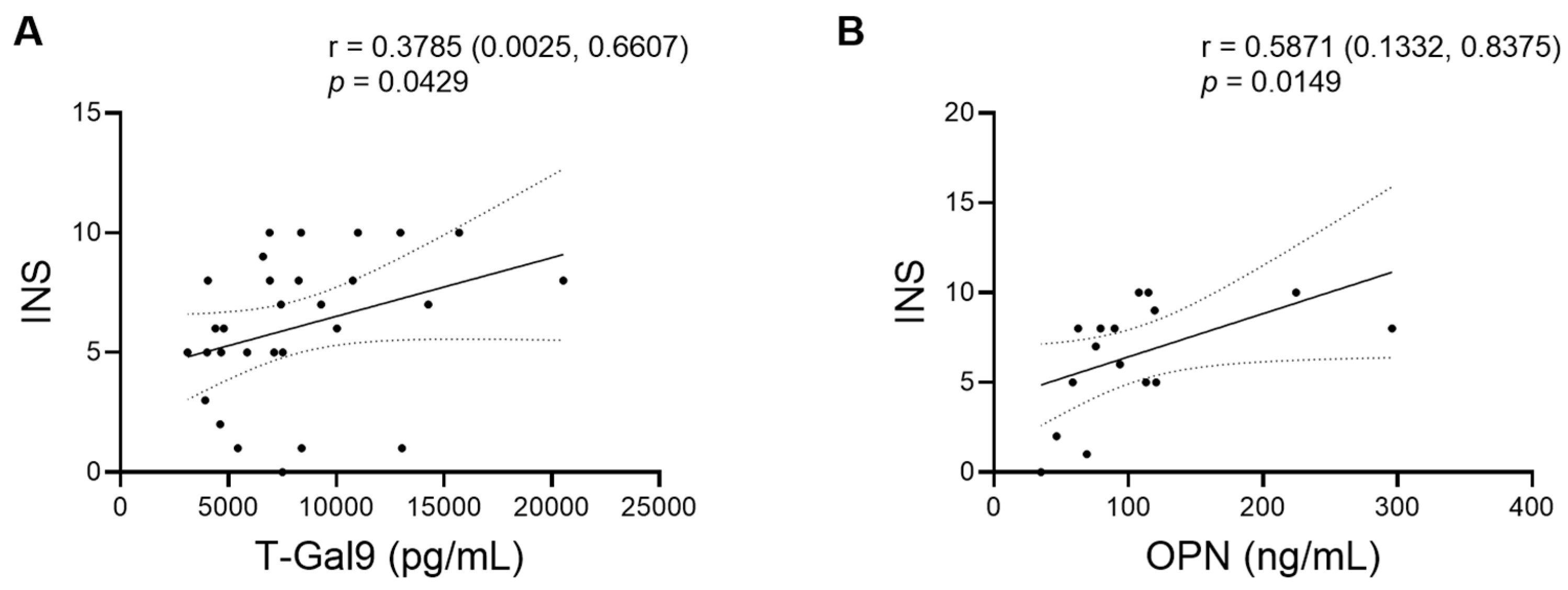
3.3. Correlation of Baseline Levels of Inflammatory Markers with Each Other and with INS
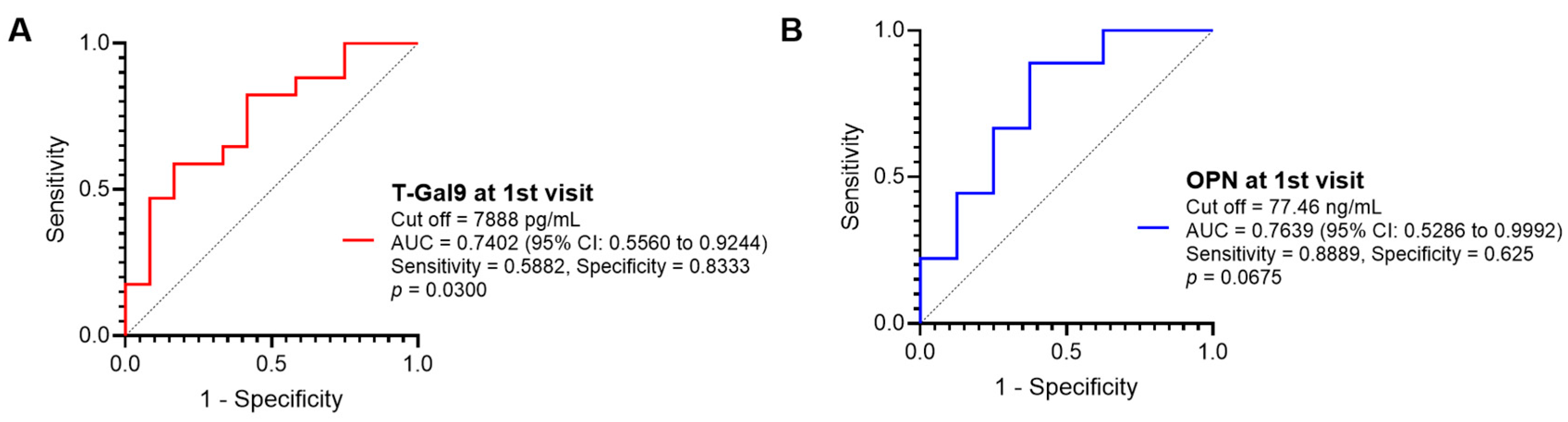
3.4. Role of Baseline Inflammatory Markers in Predicting Immunological Recovery in Response to Antituberculosis Treatment
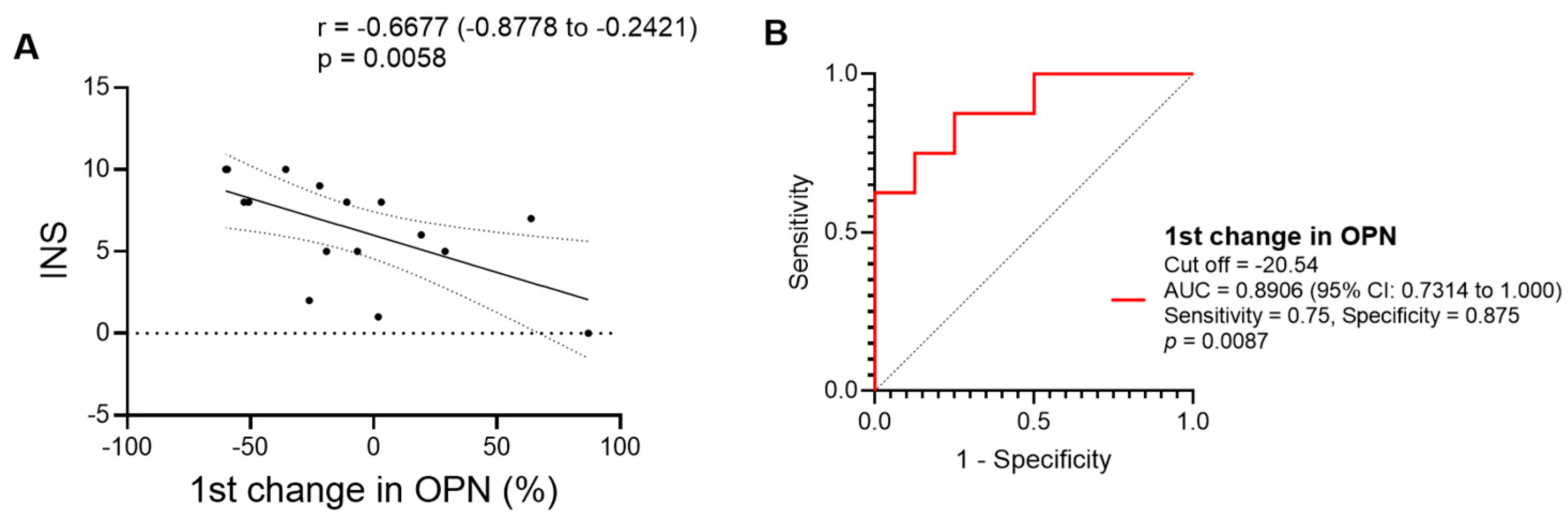
3.5. Role of Early Changes in the Levels of the Inflammatory Markers in Predicting Immunological Recovery in Response to Antituberculosis Treatment
3.6. Chest X-ray (CXR) Severity and Role of Different Markers
3.7. Effect of Gender on the Levels of the Different Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiratori, B.; Saitoh, H.; Siddiqi, U.R.; Zhao, J.; Chagan-Yasutan, H.; Usuzawa, M.; Nakajima, C.; Suzuki, Y.; Hattori, T. Immunological Diagnosis of Active and Latent TB. In Understanding Tuberculosis-Global Experiences and Innovative Approaches to the Diagnosis; IntechOpen: London, UK, 2012. [Google Scholar]
- Suthar, A.B.; Lawn, S.D.; del Amo, J.; Getahun, H.; Dye, C.; Sculier, D.; Sterling, T.R.; Chaisson, R.E.; Williams, B.G.; Harries, A.D.; et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001270. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wood, R.; Kaplan, R.; Bekker, L.G.; Lawn, S.D. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: Comparison with rates in the community. PLoS ONE 2012, 7, e34156. [Google Scholar] [CrossRef] [PubMed]
- Dravid, A.; Natarajan, K.; Medisetty, M.; Gawali, R.; Mahajan, U.; Kulkarni, M.; Saraf, C.; Ghanekar, C.; Kore, S.; Rathod, N.; et al. Incidence of tuberculosis among HIV infected individuals on long term antiretroviral therapy in private healthcare sector in Pune, Western India. BMC Infect. Dis. 2019, 19, 714. [Google Scholar] [CrossRef] [PubMed]
- Gatechompol, S.; Sophonphan, J.; Ubolyam, S.; Avihingsanon, A.; van Leth, F.; Cobelens, F.; Kerr, S.J. Incidence and factors associated with active tuberculosis among people living with HIV after long-term antiretroviral therapy in Thailand: A competing risk model. BMC Infect. Dis. 2022, 22, 346. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Evans, A.J.; Sedgwick, P.M.; Acheampong, J.W. Pulmonary tuberculosis: Radiological features in west Africans coinfected with HIV. Br. J. Radiol. 1999, 72, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.; Saadoun, D.; Calatroni, I.; Launay, O.; Mémain, N.; Vincent, V.; Marchal, G.; Dupont, B.; Bouchaud, O.; Valeyre, D.; et al. Tuberculosis in HIV-infected patients: A comprehensive review. Clin. Microbiol. Infect. 2004, 10, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Yew, K.; Berkowitz, K.A.; Aranda, C.P. Factors affecting the yield of acid-fast sputum smears in patients with HIV and tuberculosis. Chest 1994, 106, 684–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaisson, R.E.; Clermont, H.C.; Holt, E.A.; Cantave, M.; Johnson, M.P.; Atkinson, J.; Davis, H.; Boulos, R.; Quinn, T.C.; Halsey, N.A. Six-month supervised intermittent tuberculosis therapy in Haitian patients with and without HIV infection. Am. J. Respir. Crit. Care Med. 1996, 154, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Perriëns, J.H.; St Louis, M.E.; Mukadi, Y.B.; Brown, C.; Prignot, J.; Pouthier, F.; Portaels, F.; Willame, J.C.; Mandala, J.K.; Kaboto, M.; et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N. Engl. J. Med. 1995, 332, 779–784. [Google Scholar] [CrossRef]
- Darboe, F.; Mbandi, S.K.; Naidoo, K.; Yende-Zuma, N.; Lewis, L.; Thompson, E.G.; Duffy, F.J.; Fisher, M.; Filander, E.; van Rooyen, M.; et al. Detection of Tuberculosis Recurrence, Diagnosis and Treatment Response by a Blood Transcriptomic Risk Signature in HIV-Infected Persons on Antiretroviral Therapy. Front. Microbiol. 2019, 10, 1441. [Google Scholar] [CrossRef]
- Douek, D.C.; Roederer, M.; Koup, R.A. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 2009, 60, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases. Biomolecules 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Iwasaki-Hozumi, H.; Bai, G.; Chagan-Yasutan, H.; Shete, A.; Telan, E.F.; Takahashi, A.; Ashino, Y.; Matsuba, T. Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases. Biomedicines 2021, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Dhayarkar, S.; Dhamanage, A.; Kulkarni, S.; Ghate, M.; Sangle, S.; Medhe, U.; Verma, V.; Rajan, S.; Hattori, T.; et al. Possible role of plasma Galectin-9 levels as a surrogate marker of viremia in HIV infected patients on antiretroviral therapy in resource-limited settings. AIDS Res. Ther. 2020, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Premeaux, T.A.; Moser, C.B.; McKhann, A.; Hoenigl, M.; Laws, E.I.; Aquino, D.L.; Lederman, M.M.; Landay, A.L.; Gianella, S.; Ndhlovu, L.C.; et al. Plasma galectin-9 as a predictor of adverse non-AIDS events in persons with chronic HIV during suppressive antiretroviral therapy. AIDS 2021, 35, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, B.; Leano, S.; Nakajima, C.; Chagan-Yasutan, H.; Niki, T.; Ashino, Y.; Suzuki, Y.; Telan, E.; Hattori, T. Elevated OPN, IP-10, and neutrophilia in loop-mediated isothermal amplification confirmed tuberculosis patients. Mediators Inflamm. 2014, 2014, 513263. [Google Scholar] [CrossRef]
- Wang, D.; Tong, X.; Wang, L.; Zhang, S.; Huang, J.; Zhang, L.; Fan, H. The association between osteopontin and tuberculosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0242702. [Google Scholar] [CrossRef]
- Nau, G.J.; Chupp, G.L.; Emile, J.F.; Jouanguy, E.; Berman, J.S.; Casanova, J.L.; Young, R.A. Osteopontin expression correlates with clinical outcome in patients with mycobacterial infection. Am. J. Pathol. 2000, 157, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bazán, S.; Mata-Espinosa, D.; Lozano-Ordaz, V.; Ramos-Espinosa, O.; Barrios-Payán, J.; López-Casillas, F.; Hernández Pando, R. Immune Regulatory Effect of Osteopontin Gene Therapy in a Murine Model of Multidrug Resistant Pulmonary Tuberculosis. Hum. Gene Ther. 2022, 33, 1037–1051. [Google Scholar] [CrossRef]
- Niki, T.; Fujita, K.; Rosen, H.; Hirashima, M.; Masaki, T.; Hattori, T.; Hoshino, K. Plasma Galectin-9 Concentrations in Normal and Diseased Condition. Cell Physiol. Biochem. 2018, 50, 1856–1868. [Google Scholar] [CrossRef]
- Padilla, S.T.; Niki, T.; Furushima, D.; Bai, G.; Chagan-Yasutan, H.; Telan, E.F.; Tactacan-Abrenica, R.J.; Maeda, Y.; Solante, R.; Hattori, T. Plasma Levels of a Cleaved Form of Galectin-9 Are the Most Sensitive Biomarkers of Acquired Immune Deficiency Syndrome and Tuberculosis Coinfection. Biomolecules 2020, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Bichare, S.; Pujari, V.; Virkar, R.; Thakar, M.; Ghate, M.; Patil, S.; Vyakarnam, A.; Gangakhedkar, R.; Bai, G.; et al. Elevated Levels of Galectin-9 but Not Osteopontin in HIV and Tuberculosis Infections Indicate Their Roles in Detecting MTB Infection in HIV Infected Individuals. Front. Microbiol. 2020, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Motoda, H.; Ozuru, R.; Chagan-Yasutan, H.; Hattori, T.; Matsuba, T. Synthesis of a Cleaved Form of Osteopontin by THP-1 Cells and Its Alteration by Phorbol 12-Myristate 13-Acetate and BCG Infection. Int. J. Mol. Sci. 2018, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Adult pulmonary tuberculosis as a pathological manifestation of hyperactive antimycobacterial immune response. Clin. Transl. Med. 2016, 5, 38. [Google Scholar] [CrossRef]
- Saripalli, A.; Ramapuram, J. C-Reactive Protein as a Screening Test for Tuberculosis in People Living with HIV in Southern India: A Cross-Sectional, Observational Study. J. Clin. Med. 2022, 11, 3566. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.F.; Clark, S.O.; Oni, T.; Andreu, N.; Tezera, L.; Singh, S.; Saraiva, L.; Pedersen, B.; Kelly, D.L.; Tree, J.A.; et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2012, 185, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Oomizu, S.; Arikawa, T.; Niki, T.; Kadowaki, T.; Ueno, M.; Nishi, N.; Yamauchi, A.; Hattori, T.; Masaki, T.; Hirashima, M. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS ONE 2012, 7, e48574. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Bhat, M.; Sawant, J.; Deshpande, S. Both N- and C-terminal domains of galectin-9 are capable of inducing HIV reactivation despite mediating differential immunomodulatory functionalities. Front. Immunol. 2022, 13, 994830. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, K.; Arikawa, T.; Oomizu, S.; Kontani, K.; Nobumoto, A.; Tateno, H.; Watanabe, K.; Niki, T.; Katoh, S.; Miyake, M.; et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J. Immunol. 2008, 181, 7660–7669. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, T.; Li, H.; Xiong, Y.; Tao, Y. Gal-9/Tim-3 signaling pathway activation suppresses the generation of Th17 cells and promotes the induction of Foxp3+ regulatory T cells in renal ischemia-reperfusion injury. Mol. Immunol. 2023, 156, 136–147. [Google Scholar] [CrossRef]
- Rahmati, A.; Bigam, S.; Elahi, S. Galectin-9 promotes natural killer cells activity via interaction with CD44. Front. Immunol. 2023, 14, 1131379. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sui, Y.; Wang, Y.; Song, L.; Li, D.; Li, G.; Liu, J.; Shu, Q. Galectin-9 expression correlates with therapeutic effect in rheumatoid arthritis. Sci. Rep. 2021, 11, 5562. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.; Wehrmann, M.; Meister, S.; Kolben, T.M.; Trillsch, F.; Burges, A.; Czogalla, B.; Schmoeckel, E.; Mahner, S.; Jeschke, U.; et al. Galectin-8 and -9 as prognostic factors for cervical cancer. Arch. Gynecol. Obstet. 2022, 306, 1211–1220. [Google Scholar] [CrossRef]
- Hirashima, M.; Kashio, Y.; Nishi, N.; Yamauchi, A.; Imaizumi, T.A.; Kageshita, T.; Saita, N.; Nakamura, T. Galectin-9 in physiological and pathological conditions. Glycoconj. J. 2002, 19, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shiratori, B.; Chagan-Yasutan, H.; Matsumoto, M.; Niki, T.; Tanaka, M.; Takahashi, Y.; Usami, O.; Ashino, Y.; Hattori, T. Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. Int. J. Mol. Sci. 2017, 18, 1382. [Google Scholar] [CrossRef] [PubMed]
- Howlett, P.; Du Bruyn, E.; Morrison, H.; Godsent, I.C.; Wilkinson, K.A.; Ntsekhe, M.; Wilkinson, R.J. The immunopathogenesis of tuberculous pericarditis. Microbes Infect. 2020, 22, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, N.; Mashhouri, S.; Perez Rosero, E.; Xu, L.; Shahbaz, S.; Sligl, W.; Osman, M.; Kutsogiannis, D.J.; MacIntyre, E.; O’Neil, C.R.; et al. Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection. mBio 2021, 12, e00384-21. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Takeuchi, S.Y.; Renjifo, M.E.; Medina, F.J. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics 2019, 39, 2023–2037. [Google Scholar] [CrossRef]
- Krautter, F.; Hussain, M.T.; Zhi, Z.; Lezama, D.R.; Manning, J.E.; Brown, E.; Marigliano, N.; Raucci, F.; Recio, C.; Chimen, M.; et al. Galectin-9: A novel promoter of atherosclerosis progression. Atherosclerosis 2022, 363, 57–68. [Google Scholar] [CrossRef]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. J. Respir. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Nau, G.J.; Guilfoile, P.; Chupp, G.L.; Berman, J.S.; Kim, S.J.; Kornfeld, H.; Young, R.A. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6414–6419. [Google Scholar] [CrossRef]
- Kon, S.; Nakayama, Y.; Matsumoto, N.; Ito, K.; Kanayama, M.; Kimura, C.; Kouro, H.; Ashitomi, D.; Matsuda, T.; Uede, T. A novel cryptic binding motif, LRSKSRSFQVSDEQY, in the C-terminal fragment of MMP-3/7-cleaved osteopontin as a novel ligand for alpha9beta1 integrin is involved in the anti-type II collagen antibody-induced arthritis. PLoS ONE 2014, 9, e116210. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Zawaideh, S.; Hikita, S.; Kumar, V.A.; Cantor, H.; Ashkar, S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 2002, 72, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.L.; Myles, T.; Morser, J. Thrombin Cleavage of Osteopontin and the Host Anti-Tumor Immune Response. Cancers 2023, 15, 3480. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Xu, Y.; Aguilar, M.S.; Jing, L.; Layton, E.D.; Gilleron, M.; Minnaard, A.J.; Scriba, T.J.; Day, C.L.; Warren, E.H.; et al. CD4 and CD8 co-receptors modulate functional avidity of CD1b-restricted T cells. Nat. Commun. 2022, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Chamie, G.; Luetkemeyer, A.; Walusimbi-Nanteza, M.; Okwera, A.; Whalen, C.C.; Mugerwa, R.D.; Havlir, D.V.; Charlebois, E.D. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int. J. Tuberc. Lung Dis. 2010, 14, 1295–1302. [Google Scholar]
- Meintjes, G.; Lawn, S.D.; Scano, F.; Maartens, G.; French, M.A.; Worodria, W.; Elliott, J.H.; Murdoch, D.; Wilkinson, R.J.; Seyler, C.; et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008, 8, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed]
- Perlman, D.C.; El-Sadr, W.M.; Nelson, E.T.; Matts, J.P.; Telzak, E.E.; Salomon, N.; Chirgwin, K.; Hafner, R. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG). Clin. Infect. Dis. 1997, 25, 242–246. [Google Scholar] [CrossRef]
- Bourgarit, A.; Carcelain, G.; Martinez, V.; Lascoux, C.; Delcey, V.; Gicquel, B.; Vicaut, E.; Lagrange, P.H.; Sereni, D.; Autran, B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006, 20, F1–F7. [Google Scholar] [CrossRef]
- Shaik, R.A.; Holyachi, S.K.; Ahmad, M.S.; Miraj, M.; Alzahrani, M.; Ahmad, R.K.; Almehmadi, B.A.; Aljulifi, M.Z.; Alzahrani, M.A.; Alharbi, M.B.; et al. Clinico-demographic and survival profile of people living with HIV on antiretroviral treatment. Front. Public. Health 2023, 11, 1084210. [Google Scholar] [CrossRef] [PubMed]
- Roshanaei, G.; Sabouri Ghannad, M.; Poorolajal, J.; Mohraz, M.; Molaeipoor, L. Survival Rates among Co-infected Patients with Human Immunodeficiency Virus/Tuberculosis in Tehran, Iran. Iran. J. Public. Health 2017, 46, 1123–1131. [Google Scholar] [PubMed]
- Murata, H.; Tanaka, S.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; Okada, H. Transcriptional regulation of LGALS9 by HAND2 and FOXO1 in human endometrial stromal cells in women with regular cycles. Mol. Hum. Reprod. 2021, 27, gaab063. [Google Scholar] [CrossRef] [PubMed]


| Parameters, Median (IQR) | PTB | EPTB | All TB | p Value |
|---|---|---|---|---|
| n = 20 | n = 12 | n = 32 | ||
| Age (Years) | 46 (40–52) | 40.5 (33–48) | 45.5 (38–50) | 0.1431 |
| Body weight (kg) | 46.7 (41–56) | 49.5 (38–64) | 48.5 (40–56) | 0.7228 |
| Duration of therapy (months) | 6 (6–12) | 6 (6–12) | 6 (6–12) | 0.8956 |
| Gender:Male:Female | 14:06 | 09:03 | 23:09 | >0.9999 |
| CD4 count (cells/μL) | 181.5 (128–360.5) | 383 (325–528.8) | 143 (304–399.8) | 0.0055 |
| ≤200 no. (%) | 11 (55) | 1 (8.3) | 12 (37.5) | - |
| 201–500 no. (%) | 8 (40) | 7 (58.3) | 15 (46.9) | - |
| >500 no. (%) | 1 (5) | 4 (33.3) | 5 (15.6) | - |
| Viral Load (copies/mL), | 155.5 (0–65,547) | 0 (0–31,204) | 0(0–54,508) | 0.2014 |
| INS | 8 (5–9.75) | 5 (3–7) | 6 (5–8) | 0.1024 |
| OPN (ng/mL) | 91.7 (64.1–114.4) | 87.5 (71.8–100.5) | 89.6 (69.4–107.6) | 0.833 |
| T-Gal9 (pg/mL) | 7473 (6056–9095) | 6593 (4099–10,952) | 7473 (4689–9862) | 0.7445 |
| FL-Gal9 (pg/mL) | 6000 (3409–6000) | 3502 (1274–5604) | 5371 (2895–6000) | 0.0305 (one tailed) |
| CRP (mg/L) | 53.9 (9.6–74.7) | 8.0(2.9–20.0) | 19.9 (6.8–71.5) | 0.0081 |
| Parameters Median (IQR) | Male (n = 23) | Female (n = 9) | p Value |
|---|---|---|---|
| PTB:EPTB | 14:09 | 06:03 | NS |
| ART naïve (%) | 30.4 | 44.4 | NS |
| Weight (Kg) | 52.4 (43.9–80) | 38.25 (33–70.9) | 0.0042 |
| CD4 (cells/μL) | 291 (141–569) | 317 (180.3–794) | NS |
| Viral load (copies/mL) | 0 (0–376,409) | 150 (0–331,792) | NS |
| OPN (ng/mL) | 90.3 (69.0–307.7) | 86.3 (73.4–224.5) | NS |
| T-Gal9 (pg/mL) | 7439 (4657–20,539) | 9512 (6281–15,706) | NS |
| CRP (mg/L) | 20.18 (6.664–94.44) | 16.72 (4.936–90.48) | NS |
| FL-Gal9 (pg/mL) | 6000 (3855–18,573) | 3197 (906–6000) | 0.0353 |
| INS | 5 (3–10) | 9 (8–10) | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shete, A.; Ghate, M.; Iwasaki-Hozumi, H.; Patil, S.; Shidhaye, P.; Bai, G.; Matsuba, T.; Pharande, P.; Mahajan, B.; Randive, A.; et al. Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection. Viruses 2024, 16, 664. https://doi.org/10.3390/v16050664
Shete A, Ghate M, Iwasaki-Hozumi H, Patil S, Shidhaye P, Bai G, Matsuba T, Pharande P, Mahajan B, Randive A, et al. Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection. Viruses. 2024; 16(5):664. https://doi.org/10.3390/v16050664
Chicago/Turabian StyleShete, Ashwini, Manisha Ghate, Hiroko Iwasaki-Hozumi, Sandip Patil, Pallavi Shidhaye, Gaowa Bai, Takashi Matsuba, Pratiksha Pharande, Bharati Mahajan, Aarti Randive, and et al. 2024. "Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection" Viruses 16, no. 5: 664. https://doi.org/10.3390/v16050664
APA StyleShete, A., Ghate, M., Iwasaki-Hozumi, H., Patil, S., Shidhaye, P., Bai, G., Matsuba, T., Pharande, P., Mahajan, B., Randive, A., Mukherjee, A., & Hattori, T. (2024). Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection. Viruses, 16(5), 664. https://doi.org/10.3390/v16050664







