The NDV-MLS as an Immunotherapeutic Strategy for Breast Cancer: Proof of Concept in Female Companion Dogs with Spontaneous Mammary Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Newcastle Disease Virus
2.3. Dogs
2.4. Viral Administration and Follow-Up
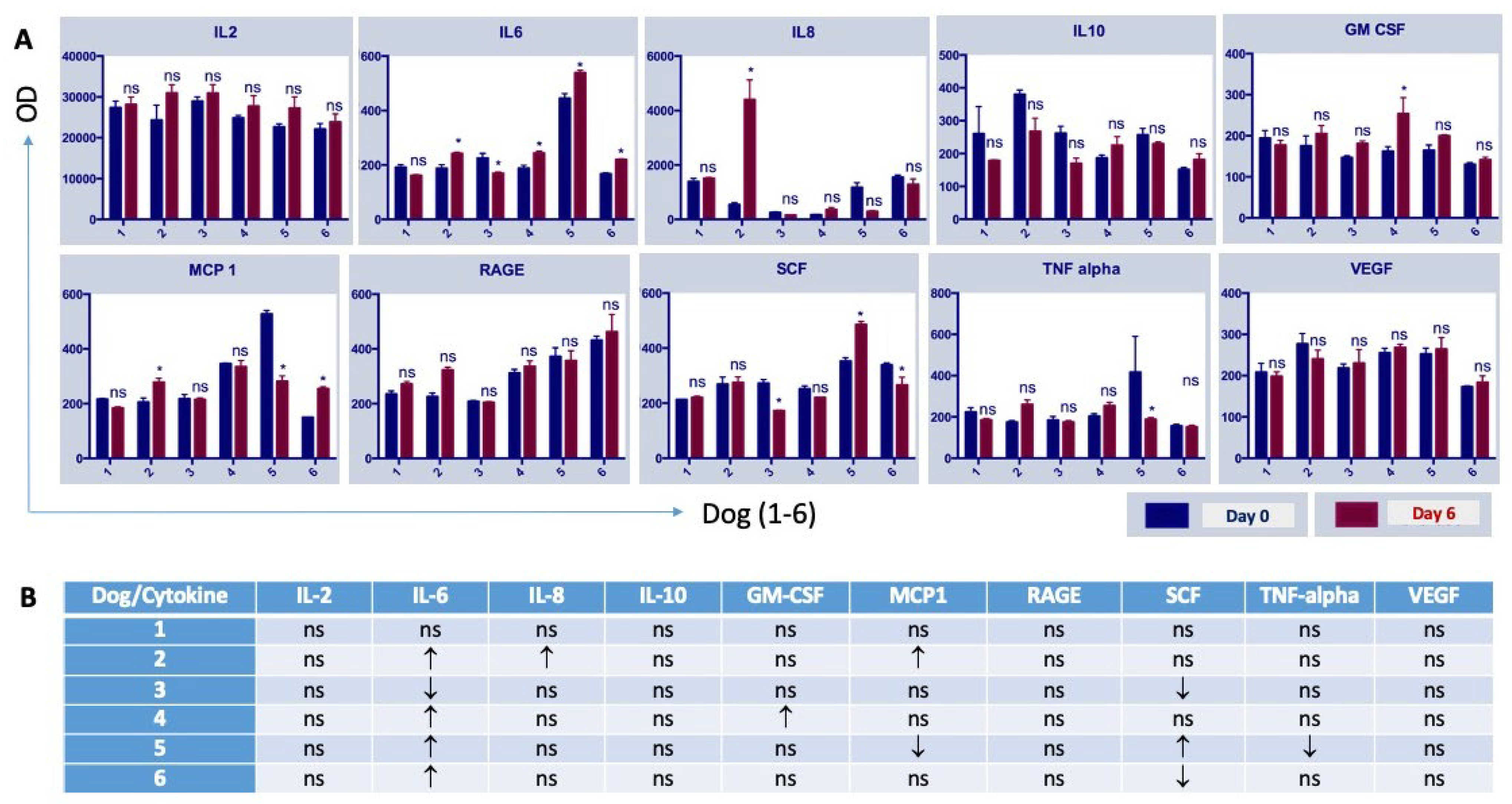
2.5. Cytokine Measurement
2.6. Virological Studies
2.7. Histopathology and Immunohistochemistry
2.8. Histopathologic Evaluation
2.9. Statistical Analysis
3. Results
3.1. Simultaneous Intravenous and Intratumoral Injection of NDV-MLS to Female Companion Dogs with Spontaneous Mammary Cancer Was Well Tolerated and Did Not Result in Severe Adverse Events over the 6-Day Period Post-Administration
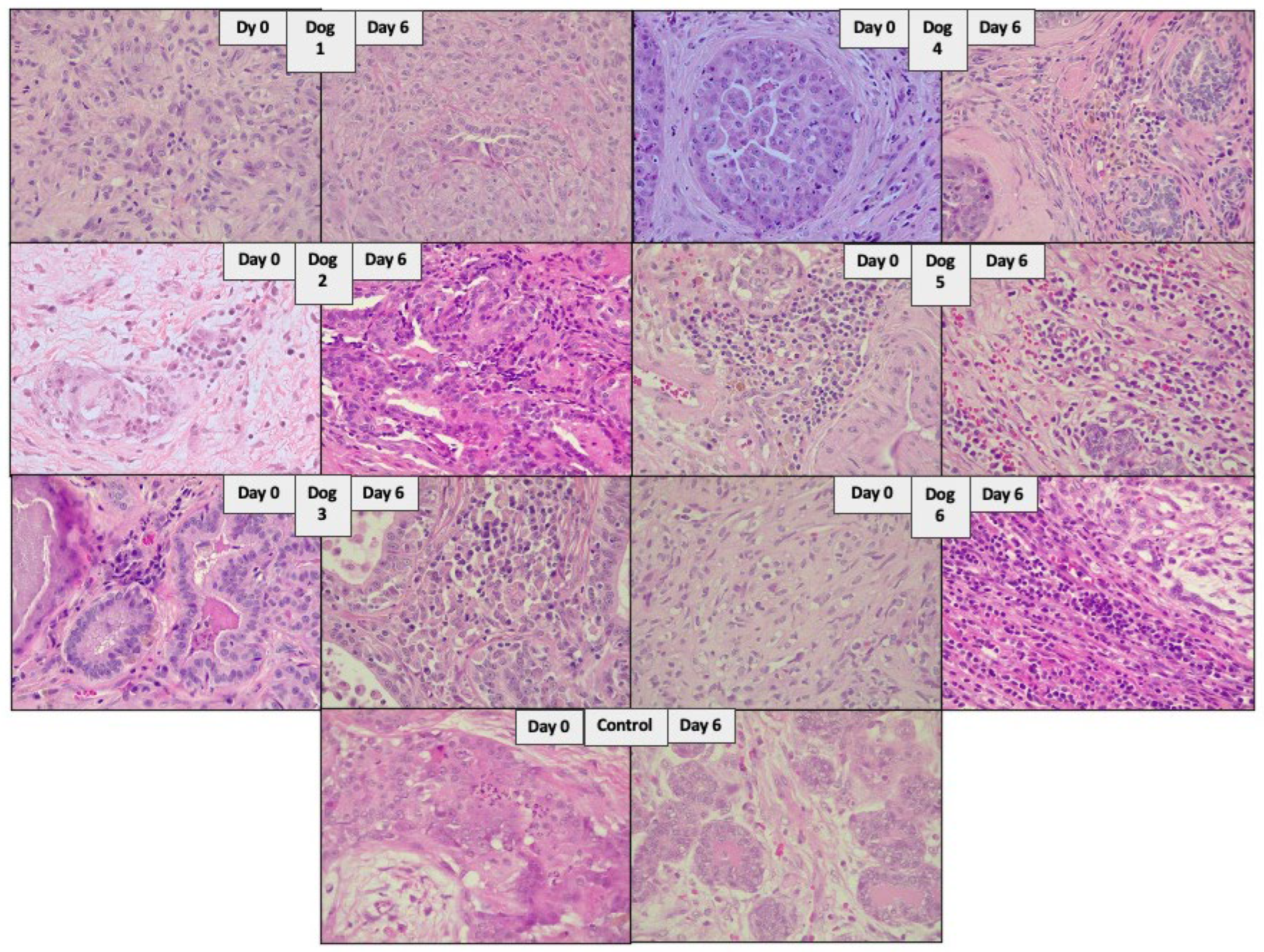
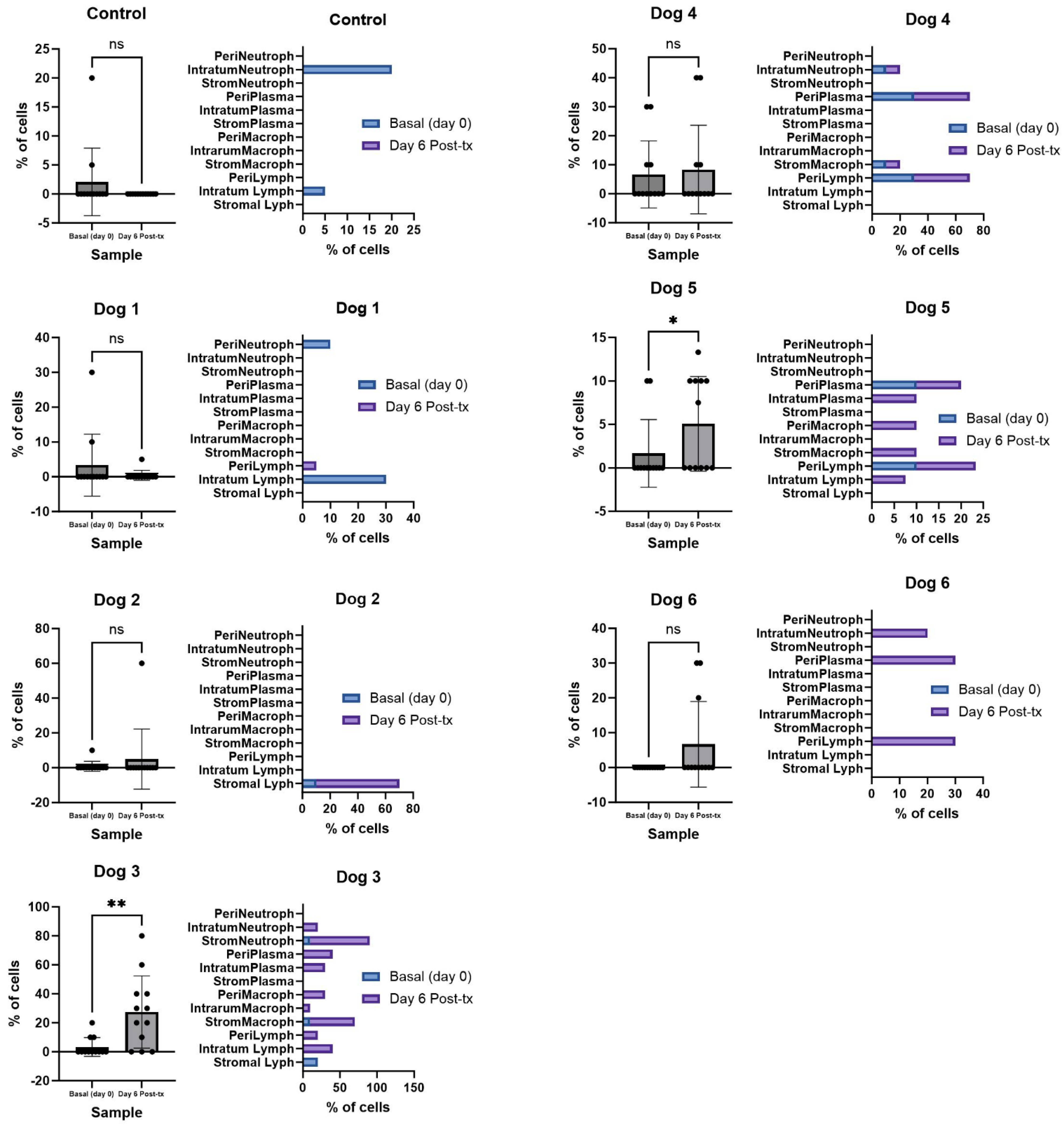
3.2. The Administration of NDV-MLS to Companion Dogs with Spontaneous Mammary Cancer Resulted in an Increase in Tumor-Infiltrating Immune Cells and Changes in Plasma Pro-Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in Patients with Chemotherapy-Refractory Metastatic Merkel Cell Carcinoma: A Multicentre, Single-Group, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Olszanski, A.J. Current and Future Roles of Targeted Therapy and Immunotherapy in Advanced Melanoma. J. Manag. Care Pharm. 2014, 20, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chasalow, S.D.; Wang, L.; Hamid, O.; Schmidt, H.; Cogswell, J.; Alaparthy, S.; Berman, D.; Jure-Kunkel, M.; Siemers, N.O.; et al. An Immune-Active Tumor Microenvironment Favors Clinical Response to Ipilimumab. Cancer Immunol. Immunother. 2012, 61, 1019–1031. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of Immune Evasion in Breast Cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers from Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Gao, Y.; Welte, T.; Wang, H.; Liu, J.; Janghorban, M.; Sheng, K.; Niu, Y.; Goldstein, A.; Zhao, N.; et al. Immuno-Subtyping of Breast Cancer Reveals Distinct Myeloid Cell Profiles and Immunotherapy Resistance Mechanisms. Nat. Cell Biol. 2019, 21, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.M.; Oikari, S.; Auvinen, P. High Numbers of Macrophages, Especially M2-like (CD163-Positive), Correlate with Hyaluronan Accumulation and Poor Outcome in Breast Cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, C.; Bendahl, P.O.; Ekholm, M.; Fernö, M.; Forsare, C.; Krüger, U.; Nordenskjöld, B.; Stål, O.; Rydén, L. Tumour-Infiltrating Lymphocytes as a Prognostic and Tamoxifen Predictive Marker in Premenopausal Breast Cancer: Data from a Randomised Trial with Long-Term Follow-Up. Breast Cancer Res. 2020, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, O.; Bacha, D.; Rahoui, N.; Zran, M.D.; Lahmar, A.; Slama, S. Ben Prognostic Value of Tumor-Infiltrating Lymphocytes (TILS) and Their Association with Clinicopathological Features in Breast Cancer: A Retrospective Study Involving 53 Cases. Rev. Senol. Patol. Mamar. 2022, 35, 160–166. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic Virus Immunotherapy: Future Prospects for Oncology 11 Medical and Health Sciences 1107 Immunology 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef]
- Raman, S.S.; Hecht, J.R.; Chan, E. Talimogene Laherparepvec: Review of Its Mechanism of Action and Clinical Efficacy and Safety. Immunotherapy 2019, 11, 705–723. [Google Scholar] [CrossRef]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef]
- Sánchez, D.; Cesarman-Maus, G.; Amador-Molina, A.; Lizano, M. Oncolytic Viruses for Canine Cancer Treatment. Cancers 2018, 10, 404. [Google Scholar] [CrossRef]
- Sánchez, D.; Pelayo, R.; Medina, L.A.; Vadillo, E.; Sánchez, R.; Núñez, L.; Cesarman-Maus, G.; Sarmiento-Silva, R.E. Newcastle Disease Virus: Potential Therapeutic Application for Human and Canine Lymphoma. Viruses 2015, 8, 3. [Google Scholar] [CrossRef]
- Schirrmacher, V. Oncolytic Newcastle Disease Virus as a Prospective Anti-Cancer Therapy. A Biologic Agent with Potential to Break Therapy Resistance. Expert Opin. Biol. Ther. 2015, 15, 1757–1771. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmed, I.; Keong, Y.Y.; Abd Manan, N.; Othman, F. Inhibitory and Apoptosis-Inducing Effects of Newcastle Disease Virus Strain AF2240 on Mammary Carcinoma Cell Line. Biomed. Res. Int. 2015, 2015, 127828. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef]
- Zamarin, D.; Ricca, J.M.; Sadekova, S.; Oseledchyk, A.; Yu, Y.; Blumenschein, W.M.; Wong, J.; Gigoux, M.; Merghoub, T.; Wolchok, J.D. PD-L1 in Tumor Microenvironment Mediates Resistance to Oncolytic Immunotherapy. J. Clin. Investig. 2018, 128, 1413–1428. [Google Scholar] [CrossRef]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Gool, S.W. Newcastle Disease Virotherapy Induces Long-Term Survival and Tumor-Specific Immune Memory in Orthotopic Glioma through the Induction of Immunogenic Cell Death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef] [PubMed]
- Buijs, P.R.A.; Van Amerongen, G.; Van Nieuwkoop, S.; Bestebroer, T.M.; Van Run, P.R.W.A.; Kuiken, T.; Fouchier, R.A.M.; Van Eijck, C.H.J.; Van Den Hoogen, B.G. Intravenously Injected Newcastle Disease Virus in Non-Human Primates Is Safe to Use for Oncolytic Virotherapy. Cancer Gene Ther. 2014, 21, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle Disease Virus-Vectored Rabies Vaccine Is Safe, Highly Immunogenic, and Provides Long-Lasting Protection in Dogs and Cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to Celebrate! Biomedicines 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, N.; King, D.J.; Seal, B.S.; Samal, S.K.; Brown, C.C. The Pathogenesis of Newcastle Disease: A Comparison of Selected Newcastle Disease Virus Wild-Type Strains and Their Infectious Clones. Virology 2006, 353, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Burman, B.; Pesci, G.; Zamarin, D. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers 2020, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.; Kim, K.; Kim, S.H.; Chung, Y.J.; Lee, C. Studying Cancer Immunotherapy Using Patient-Derived Xenografts (PDXs) in Humanized Mice. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buqué, A.; Galluzzi, L. Modeling Tumor Immunology and Immunotherapy in Mice. Trends Cancer 2018, 4, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, P.; Zhang, A.; Mao, X. Advances in Rodent Models for Breast Cancer Formation, Progression, and Therapeutic Testing. Front. Oncol. 2021, 11, 593337. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S. Canine Mammary Tumors as a Model for Human Disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response Evaluation Criteria for Solid Tumours in Dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) Consensus Document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILS) in Breast Cancer: Recommendations by an International TILS Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N. TNM Classification of Tumours in Domestic Animals, 1st ed.; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, W.; He, D.; Wang, S.; Lou, J.; Jiang, Y.; Wang, S. Recent Progress on Immunotherapy for Breast Cancer: Tumor Microenvironment, Nanotechnology and More. Front. Bioeng. Biotechnol. 2021, 9, 680315. [Google Scholar] [CrossRef]
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast Cancer Immunotherapy: An Update. Breast Cancer 2018, 12, 1178223418774802. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Al-Ziaydi, A.G.; Al-Shammari, A.M.; Hamzah, M.I.; Kadhim, H.S.; Jabir, M.S. Newcastle Disease Virus Suppress Glycolysis Pathway and Induce Breast Cancer Cells Death. Virusdisease 2020, 31, 341–348. [Google Scholar] [CrossRef]
- Raihan, J.; Ahmad, U.; Yong, Y.K.; Eshak, Z.; Othman, F.; Ideris, A. Regression of Solid Breast Tumours in Mice by Newcastle Disease Virus Is Associated with Production of Apoptosis Related-Cytokines. BMC Cancer 2019, 19, 315. [Google Scholar] [CrossRef]
- Pecora, A.L.; Rizvi, N.; Cohen, G.I.; Meropol, N.J.; Sterman, D.; Marshall, J.L.; Goldberg, S.; Gross, P.; O’Neil, J.D.; Groene, W.S.; et al. Phase I Trial of Intravenous Administration of PV701, an Oncolytic Virus, in Patients with Advanced Solid Cancers. J. Clin. Oncol. 2002, 20, 2251–2266. [Google Scholar] [CrossRef]
- Hotte, S.J.; Lorence, R.M.; Hirte, H.W.; Polawski, S.R.; Bamat, M.K.; O’Neil, J.D.; Roberts, M.S.; Groene, W.S.; Major, P.P. An Optimized Clinical Regimen for the Oncolytic Virus PV701. Clin. Cancer Res. 2007, 13, 977–985. [Google Scholar] [CrossRef]
- Lorence, R.M.; Roberts, M.S.; O’neil, J.D.; Groene, W.S.; Miller, J.A.; Mueller, S.N.; Bamat, M.K. Phase 1 Clinical Experience Using Intravenous Administration of PV701, an Oncolytic Newcastle Disease Virus. Curr. Cancer Drug Targets 2007, 7, 157–167. [Google Scholar] [CrossRef]
- Laurie, S.A.; Bell, J.C.; Atkins, H.L.; Roach, J.; Bamat, M.K.; O’Neil, J.D.; Roberts, M.S.; Groene, W.S.; Lorence, R.M. A Phase 1 Clinical Study of Intravenous Administration of PV701, an Oncolytic Virus, Using Two-Step Desensitization. Clin. Cancer Res. 2006, 12, 2555–2562. [Google Scholar] [CrossRef]
- Hotte, S.J.; Major, P.P.; Hirte, H.W.; Polawski, S.; Bamat, M.K.; Rheaume, N.; Groene, W.S.; Roberts, M.S.; Miller, J.A.; Lorence, R.M. Slow Intravenous Infusion of PV701, an Oncolytic Virus: Final Results of a Phase I Study. J. Clin. Oncol. 2004, 22, 3037. [Google Scholar] [CrossRef]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II Trial of Intravenous NDV-HUJ Oncolytic Virus in Recurrent Glioblastoma Multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, K.S.; Zhou, P.; Kovner, A.V.; Zavjalov, E.L.; Shestopalova, L.V.; Shestopalov, A.M. Oncolytic Effect of Wild-Type Newcastle Disease Virus Isolates in Cancer Cell Lines In Vitro and In Vivo on Xenograft Model. PLoS ONE 2018, 13, e0195425. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, K.S.; Glushchenko, A.V.; Gulyaeva, M.A.; Bi, Y.; Chen, J.; Shi, W.; Adamenko, L.S.; Shestopalov, A.M. Intratumoral Virotherapy with Wild-Type Newcastle Disease Virus in Carcinoma Krebs-2 Cancer Model. Viruses 2021, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, A.K.; Munusamy, P.; Kumar, D.; Latheef, S.K.; Singh, S.D.; Singh, R.; Dhama, K. Pathological and Molecular Investigation of Velogenic Viscerotropic Newcastle Disease Outbreak in a Vaccinated Chicken Flocks. Virusdisease 2018, 29, 180–191. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Ferreira, H.L.; Pantin-Jackwood, M.J.; Taylor, T.L.; Goraichuk, I.V.; Crossley, B.M.; Killian, M.L.; Bergeson, N.H.; Torchetti, M.K.; Afonso, C.L.; et al. Pathogenicity and Transmission of Virulent Newcastle Disease Virus from the 2018–2019 California Outbreak and Related Viruses in Young and Adult Chickens. Virology 2019, 531, 203–218. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Koch, G.; Rottier, P.J.; Peeters, B.P. Virulence of Newcastle Disease Virus: What Is Known so Far? Vet. Res. 2011, 42, 122. [Google Scholar] [CrossRef]
- Jin, J.H.; Cheng, J.L.; He, Z.R.; Ren, Y.C.; Yu, X.H.; Song, Y.; Yang, H.M.; Yang, Y.L.; Liu, T.; Zhang, G.Z. Different Origins of Newcastle Disease Virus Hemagglutinin-Neuraminidase Protein Modulate the Replication Efficiency and Pathogenicity of the Virus. Front. Microbiol. 2017, 8, 1607. [Google Scholar] [CrossRef] [PubMed]
- Taebipour, M.J.; Dadras, H.; Nazifi, S.; Afsar, M.; Ansari-Lari, M. Evaluation of Blood Monocyte and Lymphocyte Population in Broiler Chicken after Vaccination and Experimental Challenge with Newcastle Disease Virus. Vet. Immunol. Immunopathol. 2017, 190, 31–38. [Google Scholar] [CrossRef]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399. [Google Scholar] [CrossRef]
- Long, K.B.; Gladney, W.L.; Tooker, G.M.; Graham, K.; Fraietta, J.A.; Beatty, G.L. IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016, 6, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Washburn, B.; Weigand, M.A.; Grosse-Wilde, A.; Janke, M.; Stahl, H.; Rieser, E.; Sprick, M.R.; Schirrmacher, V.; Walczak, H. TNF-Related Apoptosis-Inducing Ligand Mediates Tumoricidal Activity of Human Monocytes Stimulated by Newcastle Disease Virus. J. Immunol. 2003, 170, 1814–1821. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 903800. [Google Scholar] [CrossRef]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. STEM CELL FACTOR RECEPTOR/c-KIT: FROM BASIC SCIENCE TO CLINICAL IMPLICATIONS. Clin. Implic. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- You, K.; Gu, H.; Yuan, Z.; Xu, X. Tumor Necrosis Factor Alpha Signaling and Organogenesis. Front. Cell Dev. Biol. 2021, 9, 727075. [Google Scholar] [CrossRef]
- Yao, J.; Li, S.; Wang, X. Identification of Breast Cancer Immune Subtypes by Analyzing Bulk Tumor and Single Cell Transcriptomes. Front. Cell Dev. Biol. 2022, 9, 781848. [Google Scholar] [CrossRef]
- Sfacteria, A.; Napoli, E.; Rifici, C.; Commisso, D.; Giambrone, G.; Mazzullo, G.; Marino, G. Immune Cells and Immunoglobulin Expression in the Mammary Gland Tumors of Dog. Animals 2021, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in Cancer: Prognostic Role and Therapeutic Strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Yeong, J.; Lim, J.C.T.; Lee, B.; Li, H.; Chia, N.; Ong, C.C.H.; Lye, W.K.; Putti, T.C.; Dent, R.; Lim, E.; et al. High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer. Front. Immunol. 2018, 9, 1209. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Horimoto, Y.; Onagi, H.; Ikarashi, D.; Nakayama, T.; Nakatsura, T.; Shimizu, H.; Kojima, K.; Yao, T.; Matsumoto, T.; et al. Plasma Cell Infiltration and Treatment Effect in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Breast Cancer Res. 2021, 23, 99. [Google Scholar] [CrossRef]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Enkhbat, B.; Masunaga, A. Prognostic Value of Tumor-Infiltrating B Lymphocytes and Plasma Cells in Triple-Negative Breast Cancer. Breast Cancer 2021, 28, 904–914. [Google Scholar] [CrossRef]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical Relevance of Tumour-Associated Macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Oncotarget 30576 Www.Impactjournals.Com/Oncotarget Prognostic Significance of Tumor-Associated Macrophages in Breast Cancer: A Meta-Analysis of the Literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef]
- Monteiro, L.N.; Rodrigues, M.A.; Gomes, D.A.; Salgado, B.S.; Cassali, G.D. Tumour-Associated Macrophages: Relation with Progression and Invasiveness, and Assessment of M1/M2 Macrophages in Canine Mammary Tumours. Vet. J. 2018, 234, 119–125. [Google Scholar] [CrossRef]
- Petty, A.J.; Owen, D.H.; Yang, Y.; Huang, X. Targeting Tumor-associated Macrophages in Cancer Immunotherapy. Cancers 2021, 13, 5318. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Bai, L.; Umansky, V.; Yu, L.; Xing, Y.; Qian, Z. Newcastle Disease Virus Activates Macrophages for Anti-Tumor Activity. Int. J. Oncol. 2000, 16, 363–436. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Shatrov, V.A.; Lehmann, V.; Schirrmacher, V. Induction of NO Synthesis in Macrophages by Newcastle Disease Virus Is Associated with Activation of Nuclear Factor-KB. Int. Immunol. 1996, 8, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.H.; Li, C.X.; Liu, M.; Jiang, J.Y. Predictive and Prognostic Role of Tumour-Infiltrating Lymphocytes in Breast Cancer Patients with Different Molecular Subtypes: A Meta-Analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The Tale of TILs in Breast Cancer: A Report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- Termeer, C.C.; Schirrmacher, V.; Bröcker, E.-B.; Becker, J.C. Newcastle Disease Virus Infection Induces B7-1/B7-2-Independent T-Cell Costimulatory Activity in Human Melanoma Cells. Cancer Gene Ther. 2000, 7, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Jarahian, M.; Watzl, C.; Fournier, P.; Arnold, A.; Djandji, D.; Zahedi, S.; Cerwenka, A.; Paschen, A.; Schirrmacher, V.; Momburg, F. Activation of Natural Killer Cells by Newcastle Disease Virus Hemagglutinin-Neuraminidase. J. Virol. 2009, 83, 8108–8121. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, E.; Lipovka, Y.; Cervantes-Arias, A.; Garibay-Escobar, A.; Haby, M.M.; Queiroga, F.L.; Velazquez, C. Canine Mammary Cancer: State of the Art and Future Perspectives. Animals 2023, 13, 3147. [Google Scholar] [CrossRef]
- Ghali, S.A.; Al-Hmudi, H.A.; AI-Ali, A.A. The Anti-Cancer Effect of LaSota Strain of Newcastle Disease Virus (NDV) against Iraqi Breast Cancer Cell Line AMJ13. In Proceedings of the American Institute of Physics Conference Series, Washington, DC, USA, 6–8 October 2022; Volume 2398, p. 40006. [Google Scholar]
- Hassan, S.A.H.; Al-Shammari, A.M.; Allawe, A.B. Oncolytic Effect of Non-Virulent Newcastle Disease Virus Lasota Strain in Human Breast Cancer Cell Lines in Vitro. Biochem. Cell Arch. 2020, 20, 1013–1022. [Google Scholar]
- Kalantari, A.; Farashi Bonab, S.; Keyvanfar, H.; Mortazavi, P. Evaluation of Apoptosis Induction by Newcastle Disease Virus Lasota Strain in Human Breast Carcinoma Cells. Arch. Razi Inst. 2020, 75, 367–376. [Google Scholar] [CrossRef] [PubMed]
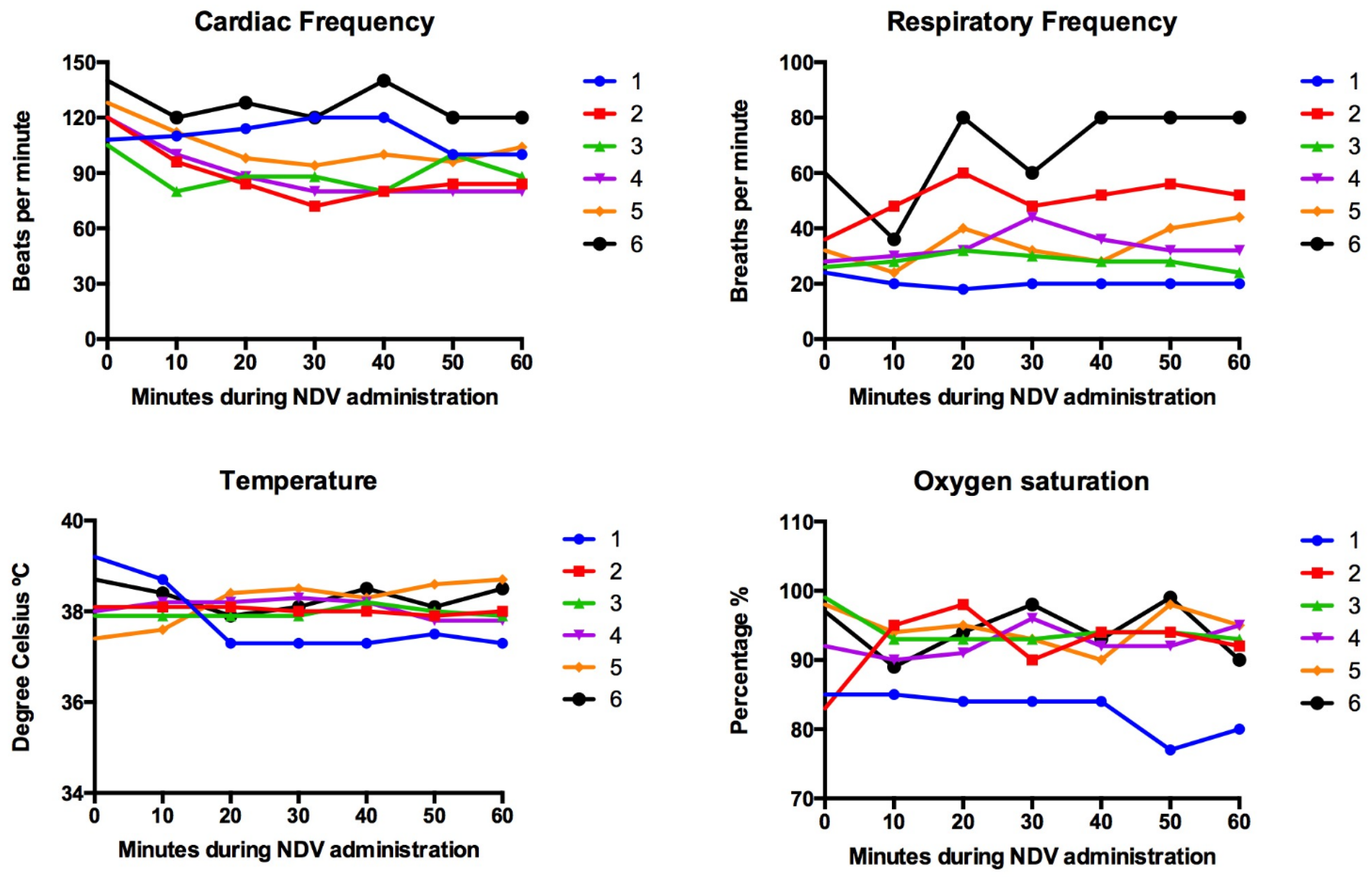
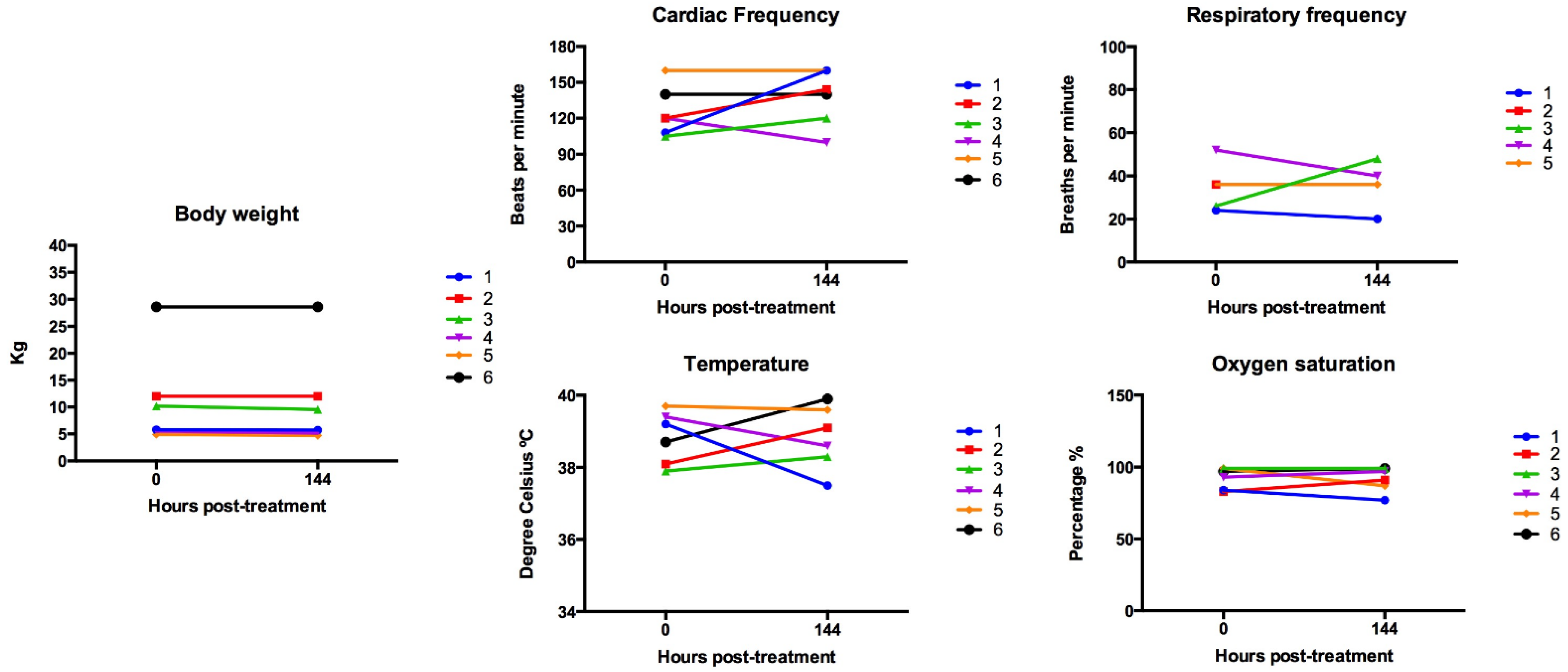
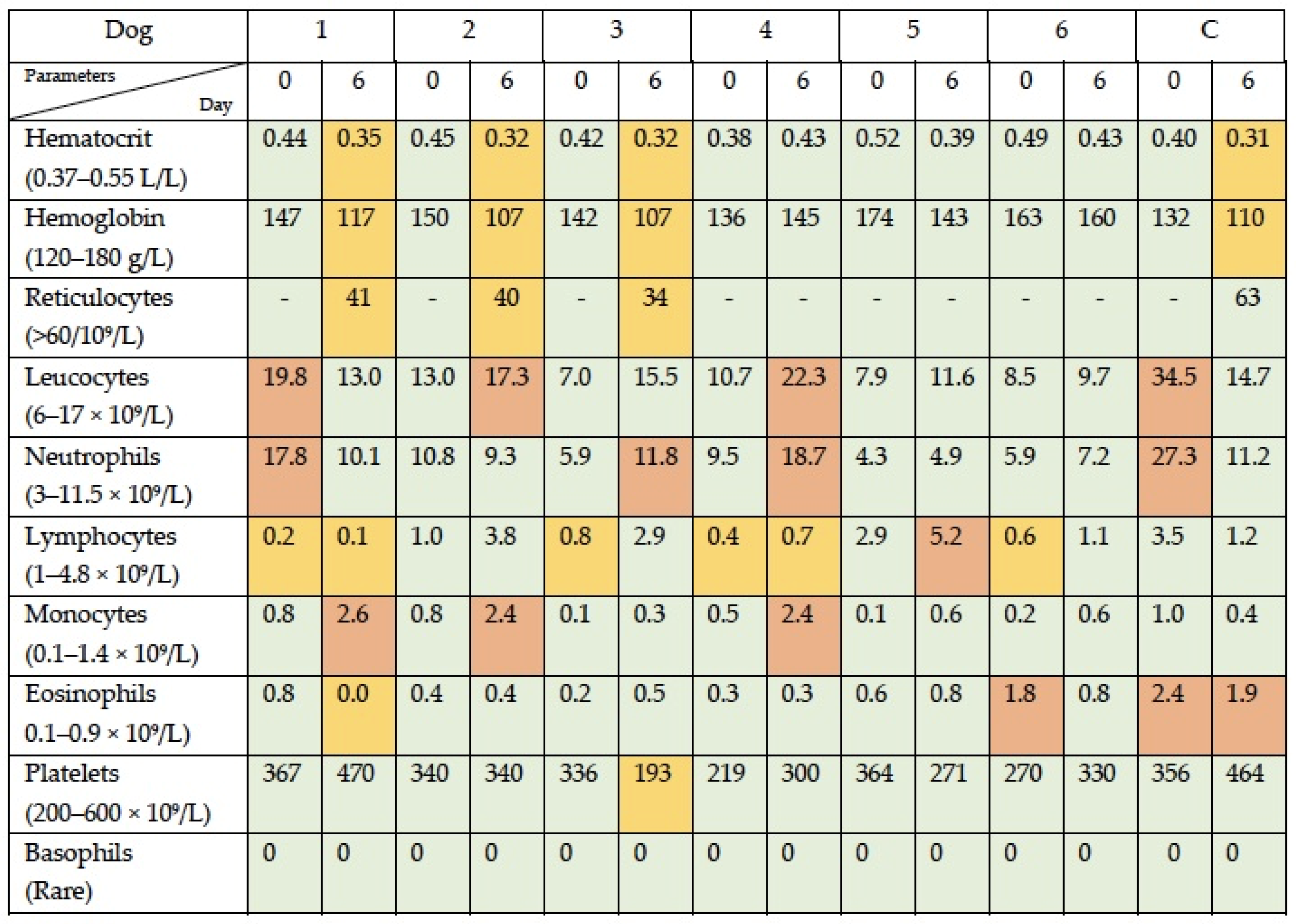
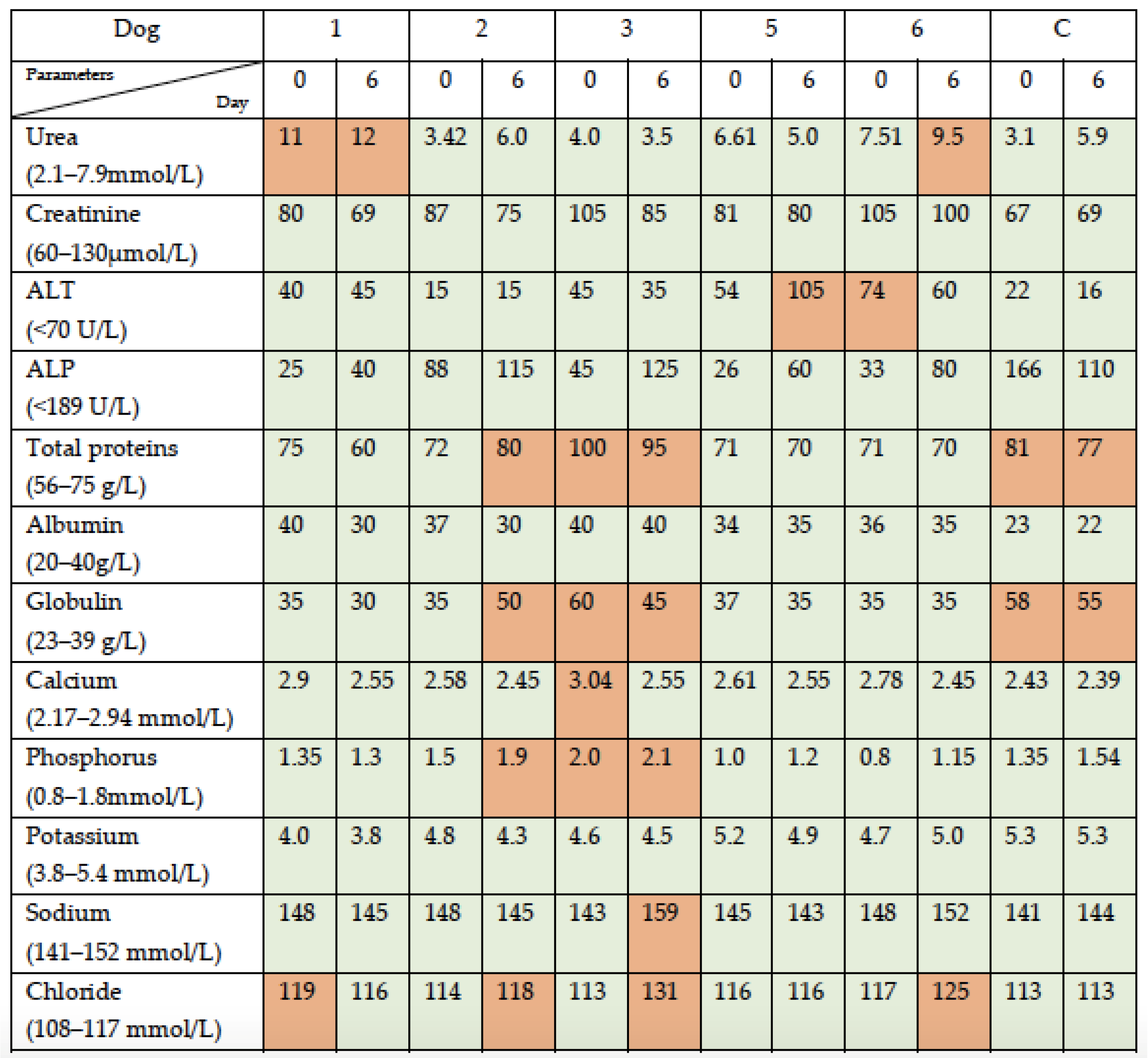
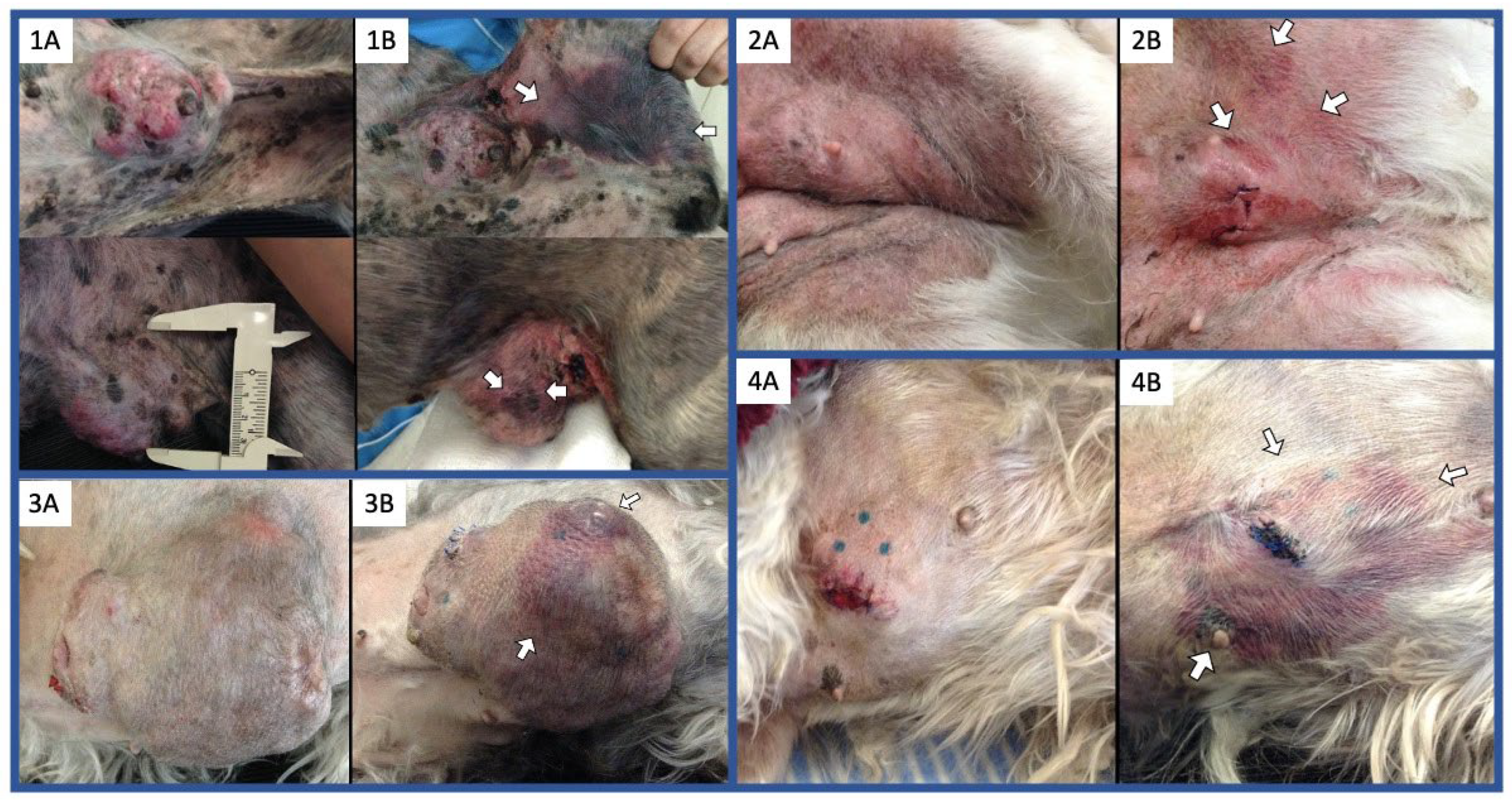
| Dog | Breed | Castration Status | Age (Years) | Performance Status 1 | Tumor Histologic Subtype/Histologic Grade | WHO Clinical Staging 2 |
|---|---|---|---|---|---|---|
| 1 | Miniature Schnauzer | Spayed | 10 | 2 | Simple carcinoma/Intermediate | V (lung) |
| 2 | Mix breed | Spayed | 11 | 2 | Simple carcinoma/Intermediate | V (lung) |
| 3 | Miniature Schnauzer | Spayed | 7 | 1 | Tubular carcinoma/Intermediate | III |
| 4 | Miniature Schnauzer | Intact | 12 | 1 | Comedocarcinoma/High | V (lung) |
| 5 | Mix breed | Intact | 11 | 1 | Tubular carcinoma/Low | II |
| 6 | Labrador | Intact | 10 | 1 | Simple carcinoma/Intermediate | II |
| Control | Giant schnauzer | Intact | 11 | 2 | Simple carcinoma/Intermediate | At least III |
| Dog | 1 | 2 | 3 | 4 | 5 | 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (Day) | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 6 |
| Prothrombin time (PT) Reference interval: 7.0–9.4 s | 7.4 | 6.6 | 6.8 | 6.5 | 6.9 | 7 | 7.4 | 8.1 | 7.5 | 7.9 | 8.1 | 7.9 |
| Partial thromboplastin time (PTT) Reference interval: 8.5–13.8 s | 20 | 17.6 | 13.7 | 13.9 | 12.3 | 12.2 | 12.8 | 17.4 | 16.2 | 15.9 | 14.6 | 15.2 |
| Dog | HAU 1, Baseline (Day 0) | HAU 1 Day 6 Post-NDV-MLS |
|---|---|---|
| 1 | 0 | 64 |
| 2 | 0 | 0 |
| 3 | 0 | 32 |
| 4 | 0 | 0 |
| 5 | 0 | 0 |
| 6 | 0 | 0 |
| D A Y 0 | Necrosis | Apoptosis | Fibrosis | Cellular Confluence | ||||||||||||||||||||||||
| C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | |
| 20 | 40 | 10 | 30 | 60 | 0 | 0 | 0 | 5 | 0 | 10 | 20 | 0 | 10 | 30 | 0 | 20 | 40 | 10 | 10 | 70 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| D A Y 6 | Necrosis | Apoptosis | Fibrosis | Cellular Confluence | ||||||||||||||||||||||||
| C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | |
| 90 | 0 | NE | 60 | 60 | 5 | 30 | 20 | 0 | NE | 20 | 30 | 5 | 10 | 70 | 0 | NE | 40 | 10 | 10 | 70 | 100 | 80 | NE | 70 | 90 | 80 | 100 | |
D A Y 0 | Tumor compartment | Stromal | Intratumoral | Peritumoral | |||||||||||||||||||
| Dog | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | ||
| MNs | Lymphocytes | 10 | 20 | 5 | 30 | 30 | 10 | ||||||||||||||||
| CD79a (+) | 20 | 10 | 10 | 20 | 50 | ||||||||||||||||||
| CD3 (+) | 80 | 90 | 90 | 80 | 50 | ||||||||||||||||||
| Both negative | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Macrophages | 10 | 10 | |||||||||||||||||||||
| Plasma cells | 30 | 10 | |||||||||||||||||||||
| PMNs | Neutrophils | 10 | 20 | 10 | 10 | ||||||||||||||||||
| Eosinophils | |||||||||||||||||||||||
| Basophils | |||||||||||||||||||||||
D A Y 6 | Tumor compartment | Stromal | Intratumoral | Peritumoral | |||||||||||||||||||
| Dog | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | C | 1 | 2 | 3 | 4 | 5 | 6 | ||
| MNs | Lymphocytes | 60 | 40 | 5/10 | 5 | 10/30 | 40 | 10/10/20 | 30 | ||||||||||||||
| CD79a (+) | 40 | 60 | 0/0 | 0 | 0/0 | 10 | 0/10/0 | 10 | |||||||||||||||
| CD3 (+) | 60 | 40 | 50/80 | 100 | 50/10 | 90 | 20/90/80 | 90 | |||||||||||||||
| Both negative | 0 | 0 | 50/20 | 0 | 50/90 | 0 | 80/0/20 | 0 | |||||||||||||||
| Macrophages | 60 | 10 | 10 | 10 | 30 | 10 | |||||||||||||||||
| Plasma cells | 30 | 10 | 40 | 40 | 10 | 30 | |||||||||||||||||
| PMNs | Neutrophils | 80 | 20 | 10 | 20 | ||||||||||||||||||
| Eosinophils | |||||||||||||||||||||||
| Basophils | |||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, D.; Cesarman-Maus, G.; Romero, L.; Sánchez-Verin, R.; Vail, D.; Guadarrama, M.; Pelayo, R.; Sarmiento-Silva, R.E.; Lizano, M. The NDV-MLS as an Immunotherapeutic Strategy for Breast Cancer: Proof of Concept in Female Companion Dogs with Spontaneous Mammary Cancer. Viruses 2024, 16, 372. https://doi.org/10.3390/v16030372
Sánchez D, Cesarman-Maus G, Romero L, Sánchez-Verin R, Vail D, Guadarrama M, Pelayo R, Sarmiento-Silva RE, Lizano M. The NDV-MLS as an Immunotherapeutic Strategy for Breast Cancer: Proof of Concept in Female Companion Dogs with Spontaneous Mammary Cancer. Viruses. 2024; 16(3):372. https://doi.org/10.3390/v16030372
Chicago/Turabian StyleSánchez, Diana, Gabriela Cesarman-Maus, Laura Romero, Rogelio Sánchez-Verin, David Vail, Marina Guadarrama, Rosana Pelayo, Rosa Elena Sarmiento-Silva, and Marcela Lizano. 2024. "The NDV-MLS as an Immunotherapeutic Strategy for Breast Cancer: Proof of Concept in Female Companion Dogs with Spontaneous Mammary Cancer" Viruses 16, no. 3: 372. https://doi.org/10.3390/v16030372
APA StyleSánchez, D., Cesarman-Maus, G., Romero, L., Sánchez-Verin, R., Vail, D., Guadarrama, M., Pelayo, R., Sarmiento-Silva, R. E., & Lizano, M. (2024). The NDV-MLS as an Immunotherapeutic Strategy for Breast Cancer: Proof of Concept in Female Companion Dogs with Spontaneous Mammary Cancer. Viruses, 16(3), 372. https://doi.org/10.3390/v16030372







