Genomic and Proteomic Analysis of Six Vi01-like Phages Reveals Wide Host Range and Multiple Tail Spike Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Phage Isolation and Host Range
2.2. Genome Analysis
2.3. Electron Microscopy
2.4. Mass Spectrometry
3. Results
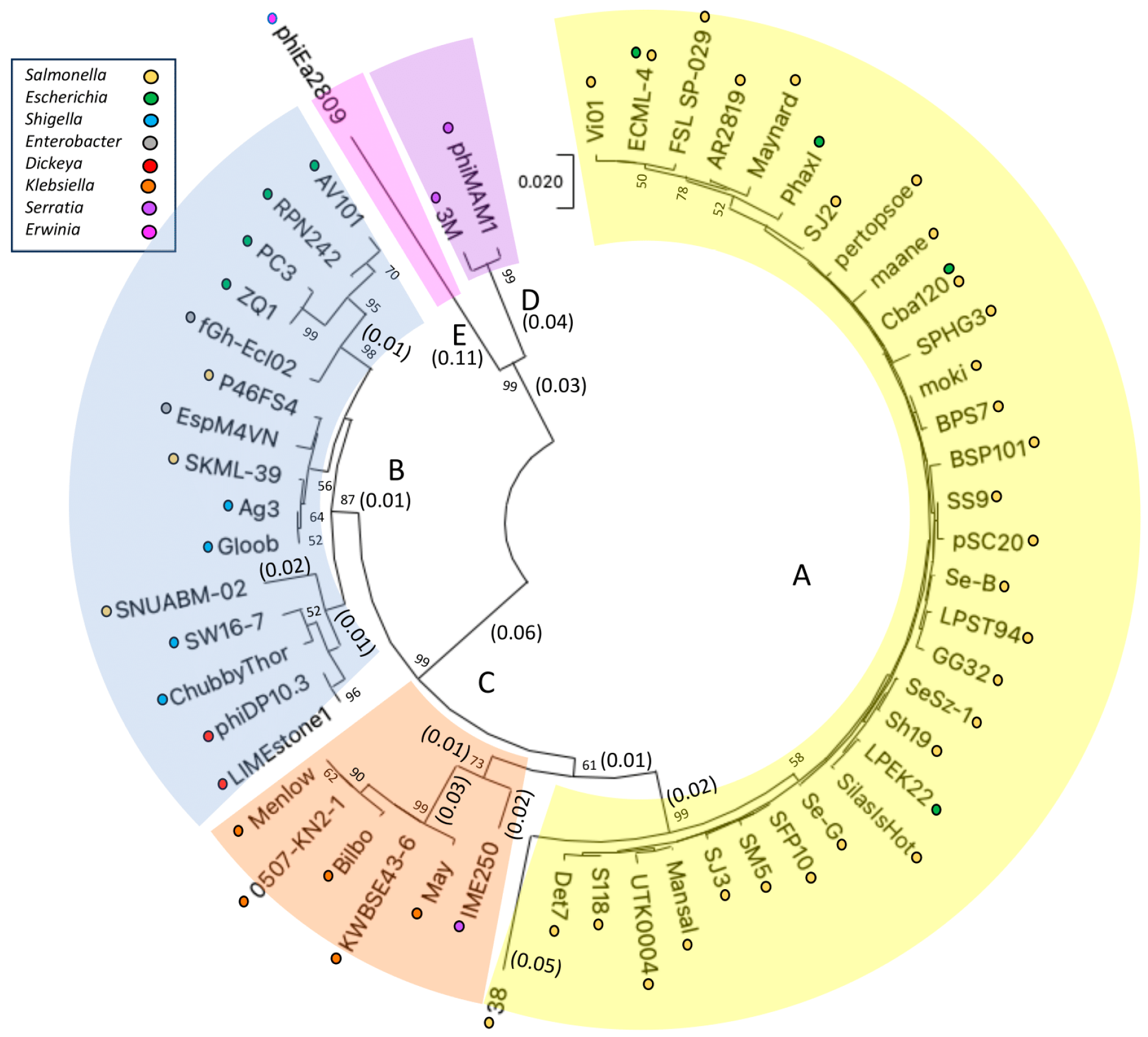
3.1. Analysis of Six Vi01-like Phages and Their Replationship to 144 Vi01-like Enterobacteriacae of the Ackermannviridae
3.1.1. FrontPhageNews, Guerrero, Sajous1, SilasIsHot, AR2819, and ChubbyThor Lie in Two of Five Enterobacteriaceae Ackermannviridae Subclusters
3.1.2. Characteristics of Representative Phages of Vi01-like Subclusters
3.1.3. Analysis of the Conserved Proteins among the Subclusters of the Vi01-like Enterobacteriaceae
3.2. Proteome Characterization of Vi01-like Phages through Structural and Operon Analysis, as Well as Mass Spectrometry
3.2.1. Structural and Operon Analysis of a Salmonella phage (FrontPhageNews) of Cluster A and a Shigella Phage (ChubbyThor) of Cluster B Provides Putative Functions for 45 Hypothetical Proteins
3.2.2. Mass Spectrometry Analysis of Sajous1 Identifies Putative Virion Proteins
3.3. Host Range Analysis of Five Enterobacteriaceae Vi01-like Phages
3.3.1. Host Range of Iron-Uptake Mutant Strains and Common Laboratory Enterobacteriaceae
3.3.2. Host Range of Clinical Enterobacteriaceae Isolates
3.3.3. Tail Spike Protein Analysis Reveals Four Tail Spike Proteins in Five Enterobacteriaceae Vi01-like Phages That May Explain Their Broad Host Range
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergh, O.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in Aquatic Ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Hambly, E.; Suttle, A.C. The viriosphere, diversity, and genetic exchange within phage communities. Curr. Opin. Microbiol. 2005, 8, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Grose, J.H.; Casjens, S.R. Understanding the enormous diversity of bacteriophages: The tailed phages that infect the bacterial family Enterobacteriaceae. Virology 2014, 468–470, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Novick, R.P. Phage-Mediated Intergeneric Transfer of Toxin Genes. Science 2009, 323, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Schicklmaier, P.; Schmieger, H. Frequency of generalized transducing phages in natural isolates of the Salmonella typhimurium complex. Appl. Environ. Microbiol. 1995, 61, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.F. Bacteriophage-encoded bacterial virulence factors and phage-pathogenicity island interactions. Adv. Virus Res. 2012, 82, 91–118. [Google Scholar]
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Carascal, M.B.; Cruz-Papa, D.M.D.; Remenyi, R.; Cruz, M.C.B.; Destura, R.V. Phage Revolution against Multidrug-Resistant Clinical Pathogens in Southeast Asia. Front. Microbiol. 2022, 13, 820572. [Google Scholar] [CrossRef]
- Rahn, O. New Principles for the Classification of Bacteria. Zentralblatt Bakteriol. Parasitenkd. Infekt. Hyg. 1937, 96, 273–286. [Google Scholar]
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospital urinary tract infections: Incidence and antibiotic susceptibility profile over 9 years. World J. Urol. 2016, 34, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am. J. Med. 2006, 119 (Suppl. 1), S20–S28; discussion S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ye, G.; Olesky, M.; Lawrence, K.; Murray, J.; Yu, K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC Infect. Dis. 2019, 19, 742. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef] [PubMed]
- Hooton, S.P.; Timms, A.R.; Rowsell, J.; Connerton, I.J. Salmonella Typhimurium-specific bacteriophage PhiSH19 and the origins of species specificity in the Vi01-like phage family. Virol. J. 2011, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- ICTV. Virus Taxonomy: 2020 Release. Available online: https://ictv.global/taxonomy (accessed on 28 December 2023).
- Harris, E.B.; Anthony, L.B.; Ali, S.; Atkin, H.; Bowden, L.C.; Brugger, S.W.; Carr, E.L.; Eberhard, N.; Flor, S.; Gaertner, R.K.; et al. Complete genome sequences of five Ackermannviridae that infect Enterobacteriaceae hosts. Microbiol. Resour. Announc. 2024, e0095023. [Google Scholar] [CrossRef]
- Ugarriza, L.E.A.; Michalik-Provasek, J.; Newkirk, H.; Liu, M.; Gill, J.J.; Ramsey, J. Complete Genome Sequence of Klebsiella pneumoniae Myophage Magnus. Microbiol. Resour. Announc. 2019, 8, e01049-19. [Google Scholar]
- Adriaenssens, E.M.; Van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; De Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-related bacteriophage LIME stone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef]
- Akter, M.; Brown, N.; Clokie, M.; Yeasmin, M.; Tareq, T.M.; Baddam, R.; Azad, M.A.K.; Ghosh, A.N.; Ahmed, N.; Talukder, K.A. Prevalence of Shigella boydii in Bangladesh: Isolation and Characterization of a Rare Phage MK-13 That Can Robustly Identify Shigellosis Caused by Shigella boydii Type 1. Front. Microbiol. 2019, 10, 2461. [Google Scholar] [CrossRef]
- Bai, J.; Jeon, B.; Ryu, S. Effective inhibition of Salmonella Typhimurium in fresh produce by a phage cocktail targeting multiple host receptors. Food Microbiol. 2019, 77, 52–60. [Google Scholar] [CrossRef]
- Casjens, S.R.; Jacobs-Sera, D.; Hatfull, G.F.; Hendrix, R.W. Genome Sequence of Salmonella enterica Phage Det7. Genome Announc. 2015, 3, e00279-15. [Google Scholar] [CrossRef] [PubMed]
- Chamblee, J.; Zeng, C.; O’Leary, C.J.; Gill, J.J.; Liu, M. Complete Genome Sequence of Salmonella enterica Serovar Enteritidis Myophage Mooltan. Microbiol. Resour. Announc. 2019, 8, e00187-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guan, G.; Liu, Q.; Yuan, S.; Yan, T.; Tian, L.; Zhou, Y.; Zhao, Y.; Ma, Y.; Wei, T.; et al. Characterization and complete genomic analysis of two Salmonella phages, SenALZ1 and SenASZ3, new members of the genus Cba120virus. Arch. Virol. 2019, 164, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Azab, E.; Gobouri, A.A.; Nasr-Eldin, M.A.; Moustafa, M.M.A.; Mohamed, S.A.; Badr, O.A.M.; Abdelatty, A.M. Isolation and Characterization of Two Lytic Bacteriophages Infecting a Multi-Drug Resistant Salmonella Typhimurium and Their Efficacy to Combat Salmonellosis in Ready-to-Use Foods. Microorganisms 2021, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Tie, D.; Sun, Y.; Jiang, J.; Huang, H.; Gong, Y.; Zhao, C. Characterization and Genomic Analysis of Escherichia coli O157:H7 Bacteriophage FEC14, a New Member of Genus Kuttervirus. Curr. Microbiol. 2021, 78, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.; Shang, X.; Luo, H.; Zhou, Y.; Linden, S.B.; Heselpoth, R.D.; Leiman, P.G.; Nelson, D.C.; Herzberg, O. Structure and function of bacteriophage CBA120 ORF211 (TSP2), the determinant of phage specificity towards E. coli O157:H7. Sci. Rep. 2020, 10, 15402. [Google Scholar] [CrossRef]
- Gutierrez, J.; Xie, Y.; Gill, J.J.; Liu, M. Complete Genome Sequence of Salmonella enterica Serovar Typhimurium Myophage Mutine. Microbiol. Resour. Announc. 2019, 8, e00401-19. [Google Scholar] [CrossRef]
- Hsu, C.-R.; Lin, T.-L.; Pan, Y.-J.; Hsieh, P.-F.; Wang, J.-T. Isolation of a Bacteriophage Specific for a New Capsular Type of Klebsiella pneumoniae and Characterization of Its Polysaccharide Depolymerase. PLoS ONE 2013, 8, e70092. [Google Scholar] [CrossRef]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Yan, T.; Willias, S.P.; Mia, M.Z.; Bei, W.; Connerton, I.F.; Fischetti, V.A.; et al. Application of a Broad Range Lytic Phage LPST94 for Biological Control of Salmonella in Foods. Microorganisms 2020, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Juliette, J.; Xie, Y.; Newkirk, H.; Liu, M.; Gill, J.J.; Ramsey, J. Complete Genome Sequence of Salmonella enterica Myophage Matapan. Microbiol. Resour. Announc. 2019, 8, e01017-19. [Google Scholar] [CrossRef] [PubMed]
- Kabanova, A.P.; Shneider, M.M.; Korzhenkov, A.A.; Bugaeva, E.N.; Miroshnikov, K.K.; Zdorovenko, E.L.; Kulikov, E.E.; Toschakov, S.V.; Ignatov, A.N.; Knirel, Y.A.; et al. Host Specificity of the Dickeya Bacteriophage PP35 Is Directed by a Tail Spike Interaction with Bacterial O-Antigen, Enabling the Infection of Alternative Non-pathogenic Bacterial Host. Front. Microbiol. 2019, 9, 3288. [Google Scholar] [CrossRef] [PubMed]
- Knecht, L.E.; Born, Y.; Pothier, J.F.; Loessner, M.J.; Fieseler, L. Complete Genome Sequences of Erwinia amylovora Phages vB_EamP-S2 and vB_EamM-Bue1. Microbiol. Resour. Announc. 2018, 7, e00891-18. [Google Scholar] [CrossRef]
- Korf, I.H.E.; Meier-Kolthoff, J.P.; Adriaenssens, E.M.; Kropinski, A.M.; Nimtz, M.; Rohde, M.; van Raaij, M.J.; Wittmann, J. Still Something to Discover: Novel Insights into Escherichia coli Phage Diversity and Taxonomy. Viruses 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Ciemińska, K.; Grabski, M.; Grabowski, Ł.; Górniak, M.; Jurczak-Kurek, A.; Węgrzyn, G.; Węgrzyn, A. Characteristics of a Series of Three Bacteriophages Infecting Salmonella enterica Strains. Int. J. Mol. Sci. 2020, 21, 6152. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, S.G.; Kim, H.J.; Giri, S.S.; Bin Lee, S.; Park, S.C. Bacteriophage as an alternative to prevent reptile-associated Salmonella transmission. Zoonoses Public Health 2021, 68, 131–143. [Google Scholar] [CrossRef]
- Matsushita, K.; Uchiyama, J.; Kato, S.I.; Ujihara, T.; Hoshiba, H.; Sugihara, S.; Muraoka, A.; Wakiguchi, H.; Matsuzaki, S. Morphological and genetic analysis of three bacteriophages of Serratia marcescens isolated from environmental water. FEMS Microbiol. Lett. 2009, 291, 201–208. [Google Scholar] [CrossRef]
- Modi, R.; Hirvi, Y.; Hill, A.; Griffiths, M.W.; Heyse, S.; Hanna, L.F.; Woolston, J.; Sulakvelidze, A.; Charbonneau, D. Effect of Phage on Survival of Salmonella Enteritidis during Manufacture and Storage of Cheddar Cheese Made from Raw and Pasteurized Milk. J. Food Prot. 2001, 64, 927–933. [Google Scholar] [CrossRef]
- Mutusamy, P.; Jothi, S.J.; Lee, S.Y.; Petersen, B.; Sicheritz-Ponten, T.; Clokie, M.R.J.; Loke, S.; Millard, A.; Parimannan, S.; Rajandas, H. Complete Genome Sequence of Salmonella enterica Bacteriophage PRF-SP1. Microbiol. Resour. Announc. 2021, 10, e0096521. [Google Scholar] [CrossRef]
- Newase, S.; Kapadnis, B.P.; Shashidhar, R. Isolation and Genome Sequence Characterization of Bacteriophage vB_SalM_PM10, a Cba120virus, Concurrently Infecting Salmonella enterica Serovars Typhimurium, Typhi, and Enteritidis. Curr. Microbiol. 2019, 76, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, H.N.; Lessor, L.; Gill, J.J.; Liu, M. Complete Genome Sequence of Klebsiella pneumoniae Myophage Menlow. Microbiol. Resour. Announc. 2019, 8, e00192-19. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, J.-H.; Shin, H.; Kim, M.; Choi, J.; Kang, D.-H.; Heu, S.; Ryu, S. Characterization and Comparative Genomic Analysis of a Novel Bacteriophage, SFP10, Simultaneously Inhibiting both Salmonella enterica and Escherichia coli O157:H7. Appl. Environ. Microbiol. 2012, 78, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Petrzik, K.; Vacek, J.; Brázdová, S.; Ševčík, R.; Koloniuk, I. Diversity of limestone bacteriophages infecting Dickeya solani isolated in the Czech Republic. Arch. Virol. 2021, 166, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Phothaworn, P.; Supokaivanich, R.; Lim, J.; Klumpp, J.; Imam, M.; Kutter, E.; Galyov, E.E.; Dunne, M.; Korbsrisate, S. Development of a broad-spectrum Salmonella phage cocktail containing Viunalike and Jerseylike viruses isolated from Thailand. Food Microbiol. 2020, 92, 103586. [Google Scholar] [CrossRef] [PubMed]
- Santiviago, C.A.; Blondel, C.J.; Quezada, C.P.; Silva, C.A.; Tobar, P.M.; Porwollik, S.; McClelland, M.; Andrews-Polymenis, H.L.; Toro, C.S.; Zaldívar, M.; et al. Spontaneous excision of the Salmonella enterica serovar Enteritidis-specific defective prophage-like element phiSE14. J. Bacteriol. 2010, 192, 2246–2254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahrbabak, S.S.; Khodabandehlou, Z.; Shahverdi, A.R.; Skurnik, M.; Ackermann, H.-W.; Varjosalo, M.; Yazdi, M.T.; Sepehrizadeh, Z. Isolation, characterization and complete genome sequence of PhaxI: A phage of Escherichia coli O157:H7. Microbiology 2013, 159 Pt 8, 1629–1638. [Google Scholar] [CrossRef]
- Sørensen, A.N.; Woudstra, C.; Sørensen, M.C.H.; Brøndsted, L. Subtypes of tail spike proteins predicts the host range of Ackermannviridae phages. Comput. Struct. Biotechnol. J. 2021, 19, 4854–4867. [Google Scholar] [CrossRef]
- Tatsch, C.O.; Wood, T.L.; Chamakura, K.R.; Everett, G.F.K. Complete Genome of Salmonella enterica Serovar Typhimurium Myophage Maynard. Genome Announc. 2013, 1, e00866-13. [Google Scholar] [CrossRef]
- Thanh, N.C.; Nagayoshi, Y.; Fujino, Y.; Iiyama, K.; Furuya, N.; Hiromasa, Y.; Iwamoto, T.; Doi, K. Characterization and Genome Structure of Virulent Phage EspM4VN to Control Enterobacter sp. M4 Isolated From Plant Soft Rot. Front. Microbiol. 2020, 11, 885. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, J.; Zhang, Z.; Chen, X.; Wei, X.; Li, H.; Lin, W.; Ke, Y.; Hu, L.; Jiang, A.; et al. Identification and molecular characterization of Serratia marcescens phages vB_SmaA_2050H1 and vB_SmaM_2050HW. Arch. Virol. 2019, 164, 1085–1094. [Google Scholar] [CrossRef]
- Xing, S.; Ma, T.; Zhang, X.; Huang, Y.; Mi, Z.; Sun, Q.; An, X.; Fan, H.; Wu, S.; Wei, L.; et al. First complete genome sequence of a virulent bacteriophage infecting the opportunistic pathogen Serratia rubidaea. Arch. Virol. 2017, 162, 2021–2028. [Google Scholar] [CrossRef]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Jacobs-Sera, D.; Hatfull, G.F.; Hansen, L.H. Unlocking the Potential of 46 New Bacteriophages for Biocontrol of Dickeya Solani. Viruses 2018, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-J.; Kwon, T.; Lee, S.; Kang, Y.H.; Chung, G.T.; Kim, D.-W.; Lee, D.-Y. Genome Sequence of Bacteriophage GG32, Which Can Infect both Salmonella enterica Serovar Typhimurium and Escherichia coli O157:H7. Genome Announc. 2016, 4, e00802-16. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; Zwirowski, S.; Lojkowska, E. Complete genome sequence of a broad-host-range lytic Dickeya spp. bacteriophage ϕD5. Arch. Virol. 2014, 159, 3153–3155. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; Siwinska, J. The complete genome, structural proteome, comparative genomics and phylogenetic analysis of a broad host lytic bacteriophage varphiD3 infecting pectinolytic Dickeya spp. Stand. Genomic Sci. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Day, A.; Ahn, J.; Fang, X.; Salmond, G.P.C. Environmental Bacteriophages of the Emerging Enterobacterial Phytopathogen, Dickeya solani, Show Genomic Conservation and Capacity for Horizontal Gene Transfer between Their Bacterial Hosts. Front. Microbiol. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- Gencay, Y.E.; Gambino, M.; Prüssing, T.F.; Brøndsted, L. The genera of bacteriophages and their receptors are the major determinants of host range. Environ. Microbiol. 2019, 21, 2095–2111. [Google Scholar] [CrossRef] [PubMed]
- Gendre, J.; Ansaldi, M.; Olivenza, D.R.; Denis, Y.; Casadesús, J.; Ginet, N. Genetic Mining of Newly Isolated Salmophages for Phage Therapy. Int. J. Mol. Sci. 2022, 23, 8917. [Google Scholar] [CrossRef]
- Hooton, S.P.; Atterbury, R.J.; Connerton, I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011, 151, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Imklin, N.; Sriprasong, P.; Thanantong, N.; Lekcharoensuk, P.; Nasanit, R. Characterization and complete genome analysis of a novel Escherichia phage, vB_EcoM-RPN242. Arch. Virol. 2022, 167, 1675–1679. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Yan, Y.; Han, P.; Tong, Y.; Li, X. SW16-7, a Novel Ackermannviridae Bacteriophage with Highly Effective Lytic Activity Targets Salmonella enterica Serovar Weltevreden. Microorganisms 2023, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-T.; Zhang, Y.; Salvador, A.; Ho, K.-J.; Cooley, M.B.; Wu, V.C.H. Characterization of polyvalent Escherichia phage Sa157lw for the biocontrol potential of Salmonella Typhimurium and Escherichia coli O157:H7 on contaminated mung bean seeds. Front. Microbiol. 2022, 13, 1053583. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.M.; Skutt-Kakaria, K.; Blasdel, B.; El-Shibiny, A.; Castano, A.; Bryan, D.; Kropinski, A.M.; Villegas, A.; Ackermann, H.W.; Toribio, A.L.; et al. Characterization of a ViI-like phage specific to Escherichia coli O157:H7. Virol. J. 2011, 8, 430. [Google Scholar] [CrossRef]
- Lagonenko, A.L.; Sadovskaya, O.; Valentovich, L.N.; Evtushenkov, A.N. Characterization of a new ViI-like Erwinia amylovora bacteriophage phiEa2809. FEMS Microbiol. Lett. 2015, 362, fnv031. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.J.; Wood, T.L.; Chamakura, K.R.; Everett, G.F.K. Complete Genome of Salmonella enterica Serovar Enteritidis Myophage Marshall. Genome Announc. 2013, 1, e00867-13. [Google Scholar] [CrossRef]
- Matilla, M.A.; Salmond, G.P.C. Bacteriophage ϕMAM1, a Viunalikevirus, Is a Broad-Host-Range, High-Efficiency Generalized Transducer That Infects Environmental and Clinical Isolates of the Enterobacterial Genera Serratia and Kluyvera. Appl. Environ. Microbiol. 2014, 80, 6446–6457. [Google Scholar] [CrossRef]
- Switt, A.I.M.; Bakker, H.C.D.; Vongkamjan, K.; Hoelzer, K.; Warnick, L.D.; Cummings, K.J.; Wiedmann, M. Salmonella bacteriophage diversity reflects host diversity on dairy farms. Food Microbiol. 2013, 36, 275–285. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Gil, J.; Brown, M.; Tondo, E.C.; de Aquino, N.S.M.; Eisenberg, M.; Erickson, S. Accurate and sensitive detection of Salmonella in foods by engineered bacteriophages. Sci. Rep. 2020, 10, 1746. [Google Scholar] [CrossRef]
- Parmar, K.M.; Dafale, N.A.; Tikariha, H.; Purohit, H.J. Genomic characterization of key bacteriophages to formulate the potential biocontrol agent to combat enteric pathogenic bacteria. Arch. Microbiol. 2018, 200, 611–622. [Google Scholar] [CrossRef]
- Pickard, D.; Toribio, A.L.; Petty, N.K.; van Tonder, A.; Yu, L.; Goulding, D.; Barrell, B.; Rance, R.; Harris, D.; Wetter, M.; et al. A Conserved Acetyl Esterase Domain Targets Diverse Bacteriophages to the Vi Capsular Receptor of Salmonella enterica Serovar Typhi. J. Bacteriol. 2010, 192, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Bonasera, R.; Benson, G.; Hernandez-Morales, A.C.; Gill, J.J.; Liu, M. Complete Genome Sequence of Klebsiella pneumoniae Myophage May. Microbiol. Resour. Announc. 2019, 8, e00252-19. [Google Scholar] [CrossRef] [PubMed]
- Soffer, N.; Abuladze, T.; Woolston, J.; Li, M.; Hanna, L.F.; Heyse, S.; Charbonneau, D.; Sulakvelidze, A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage 2016, 6, e1220347. [Google Scholar] [CrossRef] [PubMed]
- van Mierlo, J.; Hagens, S.; Witte, S.; Klamert, S.; van de Straat, L.; Fieseler, L. Complete Genome Sequences of Escherichia coli Phages vB_EcoM-EP75 and vB_EcoP-EP335. Microbiol. Resour. Announc. 2019, 8, e00078-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-W.; Wang, J.-T.; Lin, T.-L.; Liu, Y.-Z.; Wu, L.-T.; Pan, Y.-J. Identification of three capsule depolymerases in a bacteriophage infecting Klebsiella pneumoniae capsular types K7, K20, and K27 and therapeutic application. J. Biomed. Sci. 2023, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hong, Y.; Fealey, M.; Singh, A.; Walton, K.; Martin, C.; Harman, N.; Mahlie, J.; Ebner, P. Physiological and Molecular Characterization of Salmonella Bacteriophages Previously Used in Phage Therapy. J. Food Prot. 2015, 78, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.; Xie, Y.; Newkirk, H.; Liu, M.; Gill, J.J.; Ramsey, J. Complete Genome Sequence of Salmonella enterica Siphophage Shelanagig. Microbiol. Resour. Announc. 2019, 8, e01033-19. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.J.; Ooi, M.L.; Paramasivan, S.; Finnie, J.W.; Morales, S.; Psaltis, A.J.; Vreugde, S.; Wormald, P.-J. Safety and efficacy of a bacteriophage cocktail in an in vivo model of Pseudomonas aeruginosa sinusitis. Transl. Res. 2019, 206, 41–56. [Google Scholar] [CrossRef]
- Karpe, Y.A.; Kanade, G.D.; Pingale, K.D.; Arankalle, V.A.; Banerjee, K. Genomic characterization of Salmonella bacteriophages isolated from India. Virus Genes 2016, 52, 117–126. [Google Scholar] [CrossRef]
- Liu, F.; Liao, Y.-T.; Li, R.W.; Wu, V.C.H. Complete Genome Sequence of Escherichia coli Phage vB_EcoM Sa157lw, Isolated from Surface Water Collected in Salinas, California. Microbiol. Resour. Announc. 2019, 8, e00718-19. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- DNA Master. 2007. Available online: http://cobamide2.bio.pitt.edu/computer.htm (accessed on 28 December 2023).
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Krumsiek, J.; Arnold, R.; Rattei, T. Gepard: A rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics 2007, 23, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Goldman, N. A General Empirical Model of Protein Evolution Derived from Multiple Protein Families Using a Maximum-Likelihood Approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Reynolds, D.; Seto, D.; Mahadevan, P. CoreGenes3.5: A webserver for the determination of core genes from sets of viral and small bacterial genomes. BMC Res. Notes 2013, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Chooi, Y.-H. clinker & clustermap.js: Automatic generation of gene cluster comparison figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Assafiri, O.; Song, A.A.-L.; Tan, G.H.; Hanish, I.; Hashim, A.M.; Yusoff, K. Klebsiella virus UPM2146 lyses multiple drug-resistant Klebsiella pneumoniae in vitro and in vivo. PLoS ONE 2021, 16, e0245354. [Google Scholar] [CrossRef] [PubMed]
- Regue, M.; Fabregat, C.; Vinas, M. A generalized transducing bacteriophage forSerratia marcescens. Res. Microbiol. 1991, 142, 23–27. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Mourosi, J.T.; Awe, A.; Guo, W.; Batra, H.; Ganesh, H.; Wu, X.; Zhu, J. Understanding Bacteriophage Tail Fiber Interaction with Host Surface Receptor: The Key “Blueprint” for Reprogramming Phage Host Range. Int. J. Mol. Sci. 2022, 23, 12146. [Google Scholar] [CrossRef]
- Hatfull, G.F. Dark Matter of the Biosphere: The Amazing World of Bacteriophage Diversity. J. Virol. 2015, 89, 8107–8110. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Alcorlo, M.; Straume, D.; Lutkenhaus, J.; Håvarstein, L.S.; Hermoso, J.A. Structural Characterization of the Essential Cell Division Protein FtsE and Its Interaction with FtsX in Streptococcus pneumoniae. mBio 2020, 11. [Google Scholar] [CrossRef]
- Yan, Y.; Moult, J. Detection of operons. Proteins 2006, 64, 615–628. [Google Scholar] [CrossRef]
- Zulkower, V.; Rosser, S. DNA Features Viewer: A sequence annotation formatting and plotting library for Python. Bioinformatics 2020, 36, 4350–4352. [Google Scholar] [CrossRef]
- Taboada, B.; Estrada, K.; Ciria, R.; Merino, E. Operon-mapper: A web server for precise operon identification in bacterial and archaeal genomes. Bioinformatics 2018, 34, 4118–4120. [Google Scholar] [CrossRef] [PubMed]
- Bonnain, C.; Breitbart, M.; Buck, K.N. The Ferrojan Horse Hypothesis: Iron-Virus Interactions in the Ocean. Front. Mar. Sci. 2016, 3, 82. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J.; Heffron, F.; Stojiljkovic, I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect. Immun. 1996, 64, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.F.; Mol, J.P.; Silva, A.P.C.; Macêdo, A.A.; Silva, T.M.; Alves, G.E.; Winter, S.; Winter, M.G.; Velazquez, E.M.; Byndloss, M.X.; et al. Iron acquisition pathways and colonization of the inflamed intestine by Salmonella enterica serovar Typhimurium. Int. J. Med. Microbiol. 2016, 306, 604–610. [Google Scholar] [CrossRef]
- Gil, J.; Paulson, J.; Brown, M.; Zahn, H.; Nguyen, M.M.; Eisenberg, M.; Erickson, S. Tailoring the Host Range of Ackermannviridae Bacteriophages through Chimeric Tailspike Proteins. Viruses 2023, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Plattner, M.; Shneider, M.M.; Arbatsky, N.P.; Shashkov, A.S.; Chizhov, A.O.; Nazarov, S.; Prokhorov, N.S.; Taylor, N.M.; Buth, S.A.; Gambino, M.; et al. Structure and Function of the Branched Receptor-Binding Complex of Bacteriophage CBA120. J. Mol. Biol. 2019, 431, 3718–3739. [Google Scholar] [CrossRef] [PubMed]
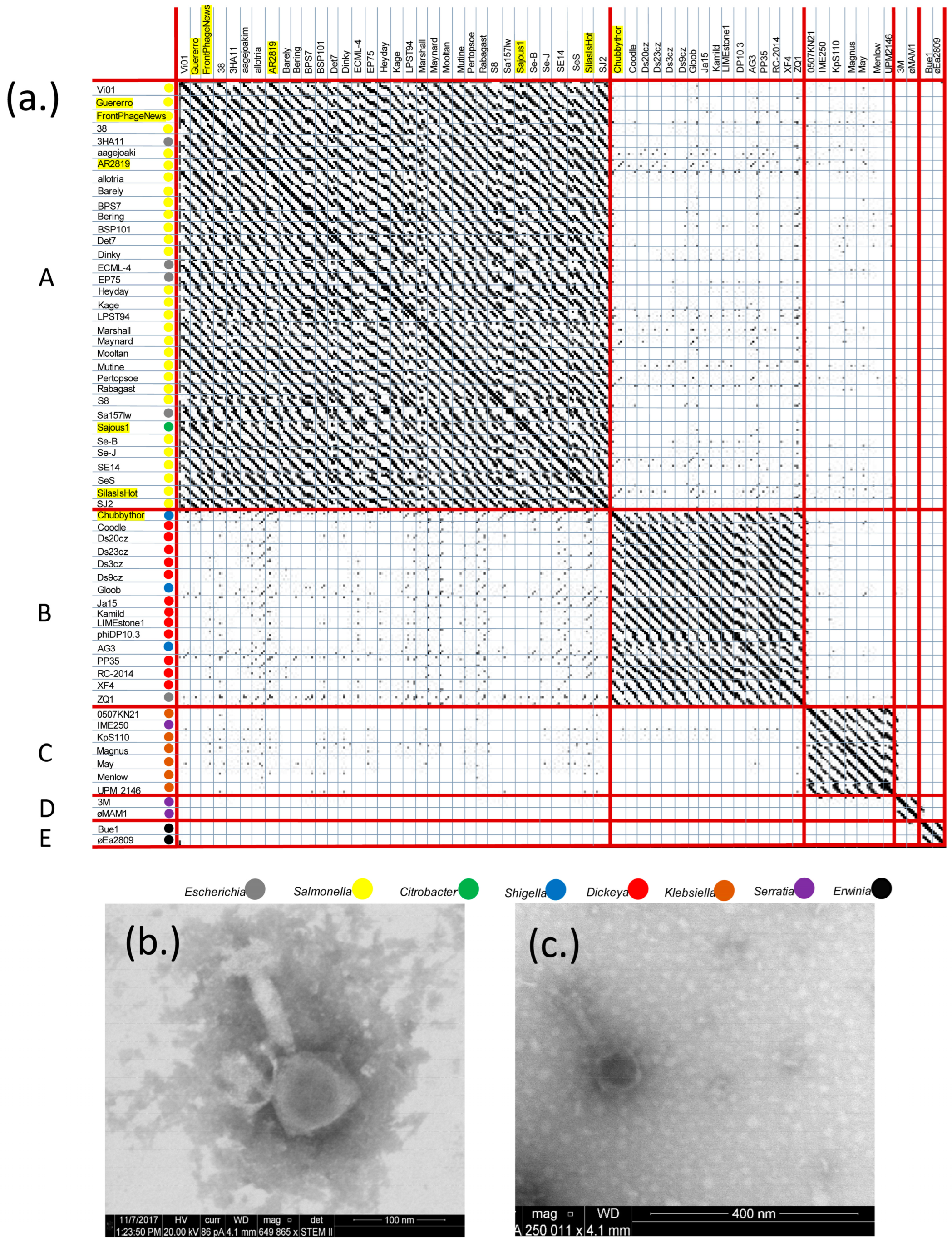


| Phage Name | Bacterial Host | GenBank Accession # | Genome Length (bp) | GC % | Gene Products |
|---|---|---|---|---|---|
| AR2819 | Salmonella typhimurium | MW021753 | 156,899 | 44.97 | 223 |
| SilasIsHot | Salmonella typhimurium | MW021760 | 160,559 | 45.13 | 227 |
| Sajous1 | Salmonella typhimurium | MW021757 | 157,255 | 44.86 | 219 |
| FrontPhageNews | Salmonella typhimurium | MW021754 | 157,832 | 44.61 | 220 |
| ChubbyThor | Shigella boydii | OL615013 | 159,319 | 50.1 | 208 |
| Guerrero | Salmonella typhimurium | OP610151 | 157,565 | 44.9 | 215 |
| Phage | Cluster | Capsid Diameter | Tail Length | Tail Width | Neck | Reference |
|---|---|---|---|---|---|---|
| Vi01 | A | 89 nm | 115 nm | 18 nm | * | [73] |
| Guerrero | A | 103 nm | 118 nm | 23 nm | 10 nm | This study |
| AR2819 | A | 90 nm | 113 nm | 28 nm | ** | This study |
| LIMEstone 1 | B | 91 nm | 114 nm | 17 nm | 20 nm | [20] |
| UPM2146 | C | 51 nm | 173 nm | 10 nm | * | [93] |
| ϕMAM1 | D | 90 nm | 120 nm | 21 nm | 11 nm | [69] |
| 3M | D | 82 nm | 123 nm | 18 nm | * | [94] |
| Bue1 | E | 79 nm | 126 nm | * | * | [35] |
| Phage | Cluster | Length (bp) | # of Genes | %GC | GenBank Accession | Reference |
|---|---|---|---|---|---|---|
| Vi01 | A | 157,061 | 208 | 45.22 | NC_015296 | [73] |
| ChubbyThor | B | 159,319 | 208 | 50.37 | OL615013 | [18] |
| Magnus | C | 157,741 | 217 | 46.26 | MN045230 | [19] |
| 3M | D | 159,398 | 203 | 51.41 | NC_048736 | (Day, Monson and Salmond, unpublished) |
| Bue1 | E | 164,037 | 176 | 50.2 | NC_048702 | [35] |
| Representative | Cluster | Vi01 | ChubbyThor | Magnus | 3M | Bue1 |
|---|---|---|---|---|---|---|
| Vi01 | A | 1 | 0.7287 | 0.7165 | 0.617 | 0.6086 |
| ChubbyThor | B | 0.7287 | 1 | 0.7146 | 0.6353 | 0.6233 |
| Magnus | C | 0.7165 | 0.7146 | 1 | 0.621 | 0.6168 |
| 3M | D | 0.617 | 0.6353 | 0.621 | 1 | 0.633 |
| Bue1 | E | 0.6086 | 0.6233 | 0.6168 | 0.633 | 1 |
| Function | Type | Vi01 Gene Product # |
|---|---|---|
| RIIA lysis inhibitor | Lysis | 1 |
| RIIB lysis inhibitor | Lysis | 2 |
| tail fiber | Assembly | 3 |
| putative histone like protein | DNA/RNA | 4 |
| putative topoisomerase II large subunit | DNA/RNA | 5 |
| DNA topoisomerase II small subunit | DNA/RNA | 6 |
| putative tRNA processing enzyme | DNA/RNA | 7 |
| putative ADP-ribose binding protein | DNA/RNA | 8 |
| putative DexA exonuclease | DNA/RNA | 9 |
| dCMP deaminase | DNA/RNA | 10 |
| membrane-flanked domain protein | Virulence | 11 |
| putative head completion protein | Assembly | 12 |
| baseplate tail tube cap | Structural | 13 |
| baseplate wedge subunit | Structural | 14 |
| putative baseplate hub subunit | Structural | 15 |
| putative tape measure protein | Structural | 16 |
| DNA helicase loader | DNA/RNA | 17 |
| putative DNA ligase | DNA/RNA | 18 |
| transcriptional regulator | DNA/RNA | 19 |
| DNA primase-helicase subunit | DNA/RNA | 20 |
| putative RecA protein | DNA/RNA | 21 |
| putative dUTP + B23:B63 diphosphatase | DNA/RNA | 22 |
| putative dNMP kinase | DNA/RNA | 23 |
| putative thymidylate synthase | DNA/RNA | 24 |
| putative thymidylate kinase | DNA/RNA | 25 |
| putative DNA end protector protein | DNA/RNA | 26 |
| putative baseplate tail tube protein | Structural | 27 |
| putative ssDNA binding protein | DNA/RNA | 28 |
| putative late promoter transcription accessory | DNA/RNA | 29 |
| zinc ribbon domain-containing protein | DNA/RNA | 30 |
| RuvC-like Holliday junction resolvase | DNA/RNA | 31 |
| putative baseplate hub subunit | Structural | 32 |
| baseplate hub subunit and tail lysozyme | Structural | 33 |
| baseplate wedge subunit | Structural | 34 |
| Glutaredoxin | DNA/RNA | 35 |
| putative Ribonucleotide-diphosphate reductase beta subunit | DNA/RNA | 36 |
| ribonucleoside-diphosphate reductase subunit alpha | DNA/RNA | 37 |
| PhoH-like phosphate starvation-inducible gene | Virulence | 38 |
| endolysin N-acetylmuramidase | Lysis | 39 |
| putative DNA primase | DNA/RNA | 40 |
| putative adenylosuccinate synthase | DNA/RNA | 41 |
| putative RNA endonuclease | DNA/RNA | 42 |
| putative recombination endonuclease subunit | DNA/RNA | 43 |
| putative recombination/repair endonuclease subunit | DNA/RNA | 44 |
| putative sigma factor for late transcription | DNA/RNA | 45 |
| Ribonuclease | DNA/RNA | 46 |
| putative ATP-dependent helicase | DNA/RNA | 47 |
| putative DNA binding protein | DNA/RNA | 48 |
| Rz-like spanin | Virulence | 49 |
| putative i-spanin | Virulence | 50 |
| putative von Willebrand factor type A domain | Virulence | 51 |
| zinc-finger-containing domain protein | DNA/RNA | 52 |
| nucleoside triphosphate pyrophosphohydrolase | DNA/RNA | 53 |
| RegA-like translation repressor protein | DNA/RNA | 54 |
| putative clamp holder for DNA polymerase | DNA/RNA | 55 |
| putative clamp loader, small subunit | DNA/RNA | 56 |
| putative sliding clamp holder protein | DNA/RNA | 57 |
| DNA helicase | DNA/RNA | 58 |
| Exonuclease | DNA/RNA | 59 |
| UvsY-like recombination mediator | DNA/RNA | 60 |
| putative tail completion protein | Assembly | 61 |
| Major capsid protein | Structural | 62 |
| prohead core scaffold protein | Assembly | 63 |
| head maturation protease | Assembly | 64 |
| putative prohead core protein | Structural | 65 |
| putative portal vertex protein | Structural | 66 |
| putative tail tube protein | Structural | 67 |
| putative tail sheath protein | Structural | 68 |
| terminase large subunit precursor | DNA/RNA | 69 |
| putative terminase small subunit | DNA/RNA | 70 |
| putative proximal tail sheath stabilizer | Assembly | 71 |
| putative neck and head completion protein | Assembly | 72 |
| putative neck protein | Structural | 73 |
| neck protein | Structural | 74 |
| virion structural protein | Structural | 75 |
| putative VrlC protein | Virulence | 76 |
| putative tail fibers protein | Structural | 77 |
| tail spike protein | Structural | 78 |
| putative tail fiber | Structural | 79 |
| baseplate wedge subunit | Structural | 80 |
| baseplate wedge subunit | Structural | 81 |
| putative pyridoxal-phosphate dependent enzyme | DNA/RNA | 82 |
| putative DNA polymerase | DNA/RNA | 83 |
| guanylate kinase | DNA/RNA | 84 |
| guanylate kinase | DNA/RNA | 85 |
| Sajous1 Gene Product | Phage Structural Proteins | # Spectra Retrieved |
| gp157 | Major capsid protein | 924 |
| gp136 | Tail fiber protein | 125 |
| gp139 | Exo-alpha-sialidase and tail protein | 113 |
| gp137 | Tail spike protein | 96 |
| gp149 | Tail sheath protein monomer | 89 |
| gp152 | Portal vertex protein of the head | 89 |
| gp138 | Putative tail fiber protein | 89 |
| gp55 | Phage baseplate wedge protein | 80 |
| gp135 | Putative tail fiber protein | 74 |
| gp53 | Putative tape measure protein | 52 |
| gp151 | Tail tube protein monomer | 52 |
| gp83 | Putative tail fiber protein | 43 |
| gp203 | Putative structural protein | 39 |
| gp143 | Putative neck protein | 33 |
| gp115 | Putative structural protein | 31 |
| gp25 | Baseplate tail tube | 24 |
| gp134 | Head closure | 21 |
| gp13 | Tail associated lysozyme | 20 |
| gp56 | T4-like baseplate tail tube cap | 17 |
| gp146 | Proximal tail completion and sheath stabilization | 16 |
| gp119 | Putative tail needle knob | 16 |
| gp145 | Neck and head completion protein | 16 |
| gp54 | Putative baseplate hub subunit | 13 |
| gp55 | Baseplate wedge subunit | 12 |
| gp116 | Phage structural protein | 10 |
| gp166 | Phage putative structural protein | 8 |
| gp168 | Tail completion protein | 8 |
| gp4 | Phage-encoded peptidoglycan binding protein | 6 |
| gp12 | Putative baseplate wedge protein | 6 |
| gp142 | putative virion structural protein | 2 |
| gp154 | Putative prohead core protein | 2 |
| Sajous1 Gene Product | Phage DNA/RNA Processes | # Retrieved |
| gp211 | RegB site-specific RNA endonuclease | 31 |
| gp192 | ParB N-terminal domain containing protein | 11 |
| gp24 | Single-stranded DNA binding protein | 5 |
| gp8 | Glutaredoxin | 4 |
| gp26 | DNA end protector protein | 3 |
| gp179 | Putative DNA-directed RNA polymerase | 3 |
| gp66 | QueC-like queuosine biosynthesis protein | 1 |
| gp31 | Putative thymidylate synthase | 1 |
| gp33 | Putative dUTP diphosphatase | 1 |
| gp174 | Sliding clamp loader | 1 |
| gp180 | Putative DNA-directed RNA polymerase | 1 |
| gp185 | VWA domain-containing protein | 1 |
| Sajous1 Gene Product | Lysis Proteins | # Retrieved |
| gp87 | RIIB protector from prophage-induced early lysis | 2 |
| gp88 | RllB lysis inhibitor | 1 |
| gp59 | Membrane protein | 1 |
| Sajous1 Gene Product | Phage Assembly Proteins | # Retrieved |
| gp140 | Putative virulence-associated VriC protein | 311 |
| gp214 | Tail completion protein | 196 |
| gp155 | Putative prohead protease | 13 |
| gp156 | Prohead core assembly scaffold | 4 |
| gp148 | Terminase DNA packaging enzyme large subunit | 4 |
| gp147 | Terminase DNA packaging enzyme small subunit | 1 |
| Sajous1 Gene Product | Miscellaneous Functions | # Retrieved |
| gp200 | SPFH domain band 7 family lipoprotein | 3 |
| gp160 | Pyruvate: Ferredoxin oxidoreductase | 1 |
| gp186 | Putative acyl carrier protein | 1 |
| Sajous1 Gene Product | Hypothetical proteins | # Retrieved |
| gp29 | Hypothetical protein | 10 |
| gp120 | Hypothetical protein | 4 |
| gp153 | Hypothetical protein | 2 |
| gp215 | Hypothetical protein | 32 |
| gp216 | Hypothetical protein | 20 |
| gp217 | Hypothetical protein | 10 |
| Sajous1 Gene Product | Putative Function | Retrieval # | Confidence | % Identity (% Aligned **) |
|---|---|---|---|---|
| gp29 | DNA Polymerase zeta-subunit | 10 | 58.2 * | 25 (18) |
| gp120 | Tapasin | 4 | 8.0 * | 40 (59) |
| gp153 | Methionine synthase | 2 | 47.2 * | 13 (82) |
| gp215 | BiMOP duplicated molybdate-binding domain | 32 | 63 | 16 (23) |
| gp216 | ATP-dependent DNA helicase, hydrolase | 20 | 27.3 * | 43 (27.3) |
| gp217 | Ferritin | 10 | 96.7 | 22 (96.7) |
| Bacteria Name | AR2819 | FrontPhage- News | SilasIsHot | Sajous1 | ChubbyThor |
|---|---|---|---|---|---|
| WT Salmonella enterica IR715 | 1.5 × 1010 | 8.0 × 1010 | 6.9 × 1010 | 8.1 × 1010 | 5.4 × 1013 |
| tonB Salmonella IR715 | 1.8 × 109 | 7.9 × 1010 | 6.6 × 1010 | 7.2 × 1010 | 4.9 × 1013 |
| feoB Salmonella IR715 | 1.5 × 109 | 5.8 × 1010 | 8.1 × 109 | 8.9 × 109 | 8.4 × 1012 |
| tonB feoB Salmonella IR715 | 1.6 × 1010 | 9.0 × 1010 | 5.9 × 1010 | 8.4 × 1010 | 5.3 × 1013 |
| Salmonella Typhimurium LT2 | 3.4 × 1011 | 2.0 × 1010 | 2.6 × 1010 | 6.0 × 1010 | 4.7 × 1010 |
| Citrobacter freundii ATCC 8090 | 1.6 × 1010 | 6.0 × 109 | 2.0 × 109 | 3.4 × 108 | 1.7 × 109 |
| Cronobacter sakazakii ATCC 29544 | ND | ND | ND | ND | ND |
| Enterobacter cloacae ATCC 13047 | ND | ND | ND | ND | ND |
| Escherichia coli K12 | ND | ND | ND | 1.2 × 108 | * |
| Erwinia amylovora ATCC 29780 | ND | ND | ND | ND | ND |
| Serratia marcescens ATCC 27143 | ND | ND | ND | ND | ND |
| Shigella boydii ATCC 9207 | ND | ND | ND | ND | 7.2 × 1011 |
| Klebsiella pneumoniae ATCC 10031 | ND | ND | ND | ND | ND |
| Bacterium * | Strain | AR2819 | Front- PhageNews | SilasIsHot | Sajous1 | Chubby-Thor |
|---|---|---|---|---|---|---|
| Salmonella enterica | #0031 | 3.1 × 1012 | 1.6 × 1012 | 1.0 × 1012 | 1.5 × 1011 | 7.6 × 104 |
| Salmonella enterica | #0409 | 2.5 × 1012 | 2.7 × 108 | 1.1 × 108 | 3.1 × 1011 | 1.2 × 109 |
| Salmonella heidelberg | #0404 | 2.4 × 1012 | ND | 1.80 × 1011 | 3.1 × 1012 | 1.12 × 105 |
| Salmonella albert | #0401 | ND | ND | ND | ND | ND |
| Salmonella cubana | #0402 | ND | ND | ND | ND | ND |
| Salmonella infantis | #0410 | ND | ND | ND | ND | ND |
| Salmonella senftenberg | #0405 | ND | ND | ND | ND | ND |
| Salmonella corvallis | #0406 | ND | ND | ND | ND | ND |
| Shigella flexneri | #0421 | ND | ND | ND | ND | ND |
| Shigella flexneri | #0423 | ND | ND | ND | ND | ND |
| Shigella flexneri | #0424 | ND | ND | ND | ND | ND |
| Shigella flexneri | #0425 | ND | ND | ND | ND | ND |
| Shigella sonnei | #0030 | ND | ND | ND | ND | ND |
| Shigella sonnei | #0422 | ND | ND | ND | 1.6 × 106 | ND |
| Shigella sonnei | #0426 | ND | ND | ND | 8.7 × 105 | ND |
| Citrobacter freundii | #0021 | ND | ND | ND | ND | ND |
| Citrobacter freundii | #0023 | ND | ND | ND | ND | ND |
| Citrobacter freundii | #0022 | ND | ND | ND | ND | ND |
| Citrobacter koseri | #0024 | ND | ND | ND | ND | ND |
| Citrobacter koseri | #0025 | ND | ND | ND | ND | ND |
| Salmonella enterica | LT2 | 1.1 × 1011 | 3.4 × 1010 | 5.4 × 1010 | 8.8 × 108 | 3.3 × 107 |
| Escherichia coli O157:H7 | 300748 | 3.6 × 1010 | 1.7 × 109 | ND | 7.0 × 103 | 1.5 × 109 |
| Escherichia coli O157:H7 | 300598 | 1.7 × 1011 | 1.3 × 1010 | ND | 1.4 × 107 | 8.0 × 109 |
| Escherichia coli O157:H7 | 298559 | 2.6 × 1010 | 4.0 × 109 | ND | 1.5 × 108 | 2.1 × 1010 |
| Escherichia coli O157:H7 | 298521 | 3.7 × 1011 | 2.0 × 1010 | ND | 3.5 × 108 | 9.4 × 108 |
| Escherichia coli O157:H7 | 290116 | 8.0 × 109 | 1.2 × 1010 | ND | 2.4 × 107 | 4.7 × 1010 |
| TSP | AR2819 | Front- PhageNews | Guerrero | SilasIsHot | Sajous1 | ChubbyThor |
|---|---|---|---|---|---|---|
| TSP1 | gp56 TSP1-20 (ECML-4, gp189) | gp214 TSP1-20 (ECML-4, gp189) | gp170 TSP1-20 (ECML-4, gp189) | gp221 TSP1-18 (SA157lW, gp4) | gp139 TSP1-20 (ECML-4, gp189) | gp207 TSP1-24 (AG3, gp207) gp208 TSP1-22 (P46FS4, gp5) * |
| TSP2 | gp55 TSP2-1 (ECML-4, gp190) | gp215 TSP2-1 (ECML-4, gp190) | gp171 TSP2-1 (ECML-4, gp190) | gp220 TSP2-5 (Bering, gp7) | gp138 TSP2-1 (SFP10, gp161) | gp1 TSP2-1 (SA157lW, gp3) |
| TSP3 | gp5 TSP3-1 (Moolton gp44) | gp216 TSP3-1 (Moolton, gp44) | gp172 TSP3-1 (Moolton, gp44) | gp219 TSP3-1 (Moolton, gp44) | gp137 TSP3-1 (Moolton, gp44) | ? |
| TSP4 | gp53 TSP4-12 (Maynard, gp38) | gp217 TSP4-2 (CBA120, gp213) | gp173 TSP4-9 (DET7, gp206) | gp218 TSP4-4 (Barely, gp5) | gp136 TSP4-9 (DET7, gp206) | gp2 TSP4-14 (AG3, gp213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, E.B.; Ewool, K.K.K.; Bowden, L.C.; Fierro, J.; Johnson, D.; Meinzer, M.; Tayler, S.; Grose, J.H. Genomic and Proteomic Analysis of Six Vi01-like Phages Reveals Wide Host Range and Multiple Tail Spike Proteins. Viruses 2024, 16, 289. https://doi.org/10.3390/v16020289
Harris EB, Ewool KKK, Bowden LC, Fierro J, Johnson D, Meinzer M, Tayler S, Grose JH. Genomic and Proteomic Analysis of Six Vi01-like Phages Reveals Wide Host Range and Multiple Tail Spike Proteins. Viruses. 2024; 16(2):289. https://doi.org/10.3390/v16020289
Chicago/Turabian StyleHarris, Evan B., Kenneth K. K. Ewool, Lucy C. Bowden, Jonatan Fierro, Daniel Johnson, McKay Meinzer, Sadie Tayler, and Julianne H. Grose. 2024. "Genomic and Proteomic Analysis of Six Vi01-like Phages Reveals Wide Host Range and Multiple Tail Spike Proteins" Viruses 16, no. 2: 289. https://doi.org/10.3390/v16020289
APA StyleHarris, E. B., Ewool, K. K. K., Bowden, L. C., Fierro, J., Johnson, D., Meinzer, M., Tayler, S., & Grose, J. H. (2024). Genomic and Proteomic Analysis of Six Vi01-like Phages Reveals Wide Host Range and Multiple Tail Spike Proteins. Viruses, 16(2), 289. https://doi.org/10.3390/v16020289







