Comprehensive Elucidation of the Role of L and 2A Security Proteins on Cell Death during EMCV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
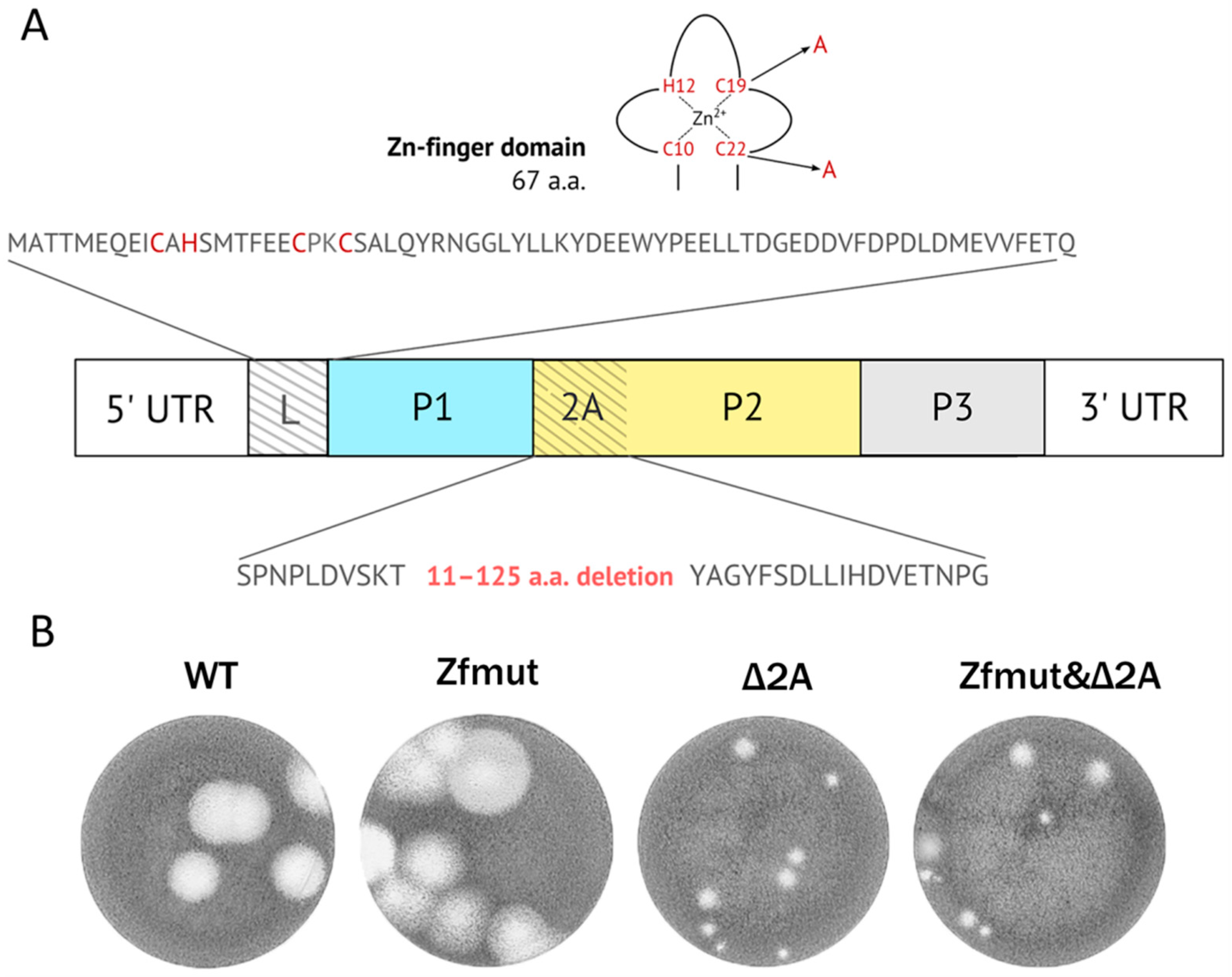
2.2. Construction of pM16.1Δ2A and pM16.1Zfmut&Δ2A
2.3. In Vitro Transcription and Transfection
2.4. Plaque Titration Assay
2.5. Plaque Cloning Procedure
2.6. Single Cycle Infection Experiments
2.7. TUNEL Assay and Propidium Iodide (PI) Staining
2.8. RNA Extraction and RT-qPCR
2.9. Western Blot Analysis
3. Results
3.1. Simultaneous Inactivation of Both L and 2A Security Proteins Does Not Abolish Viral Replication
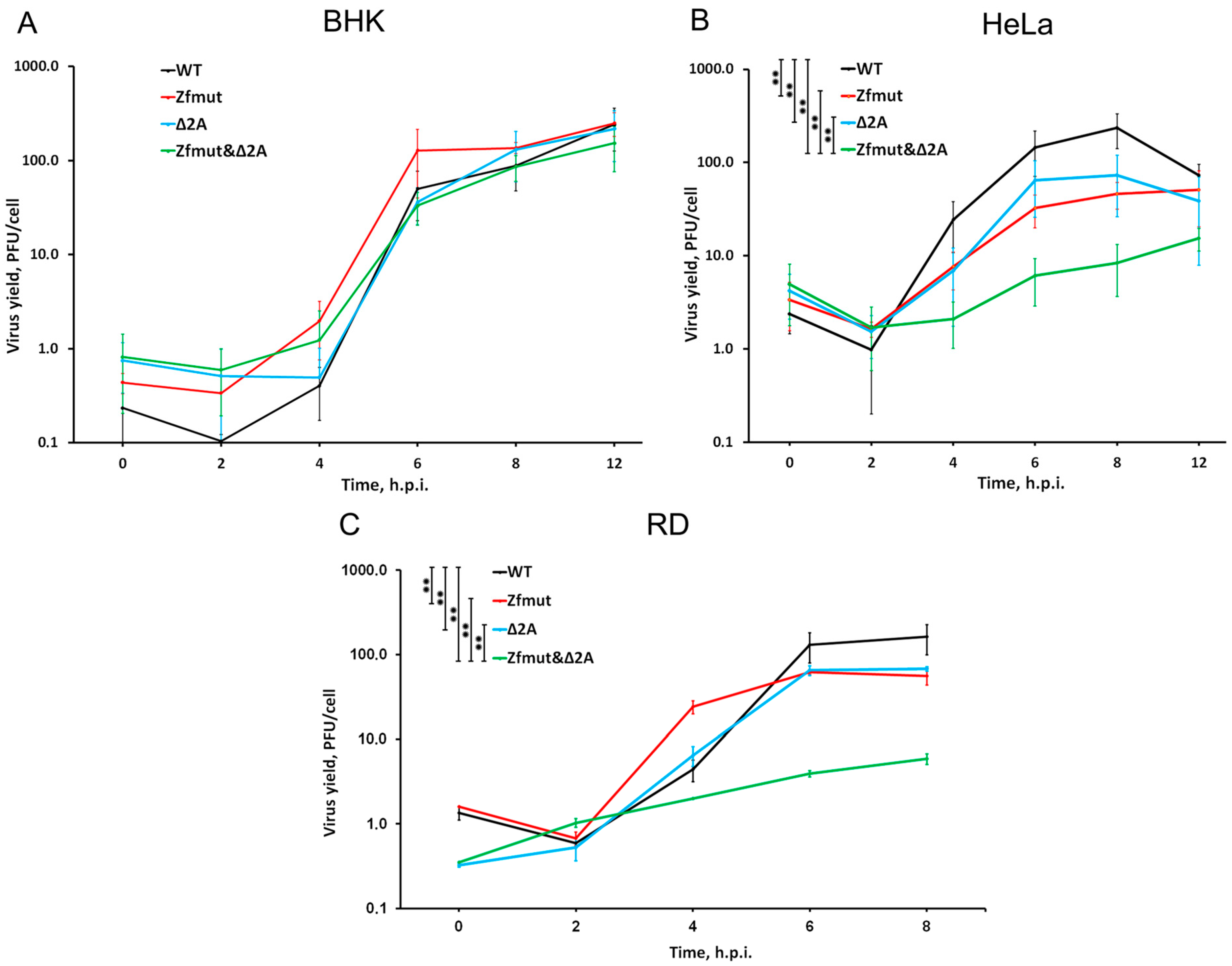
3.2. Functional Inactivation of L and 2A Proteins of EMCV Reduces Viral Yield in HeLa and RD Cell Lines in a Cumulative Manner
3.3. Functional Inactivation of L, but Not 2A, Leads to Reduced Viral Genome Replication during Infection
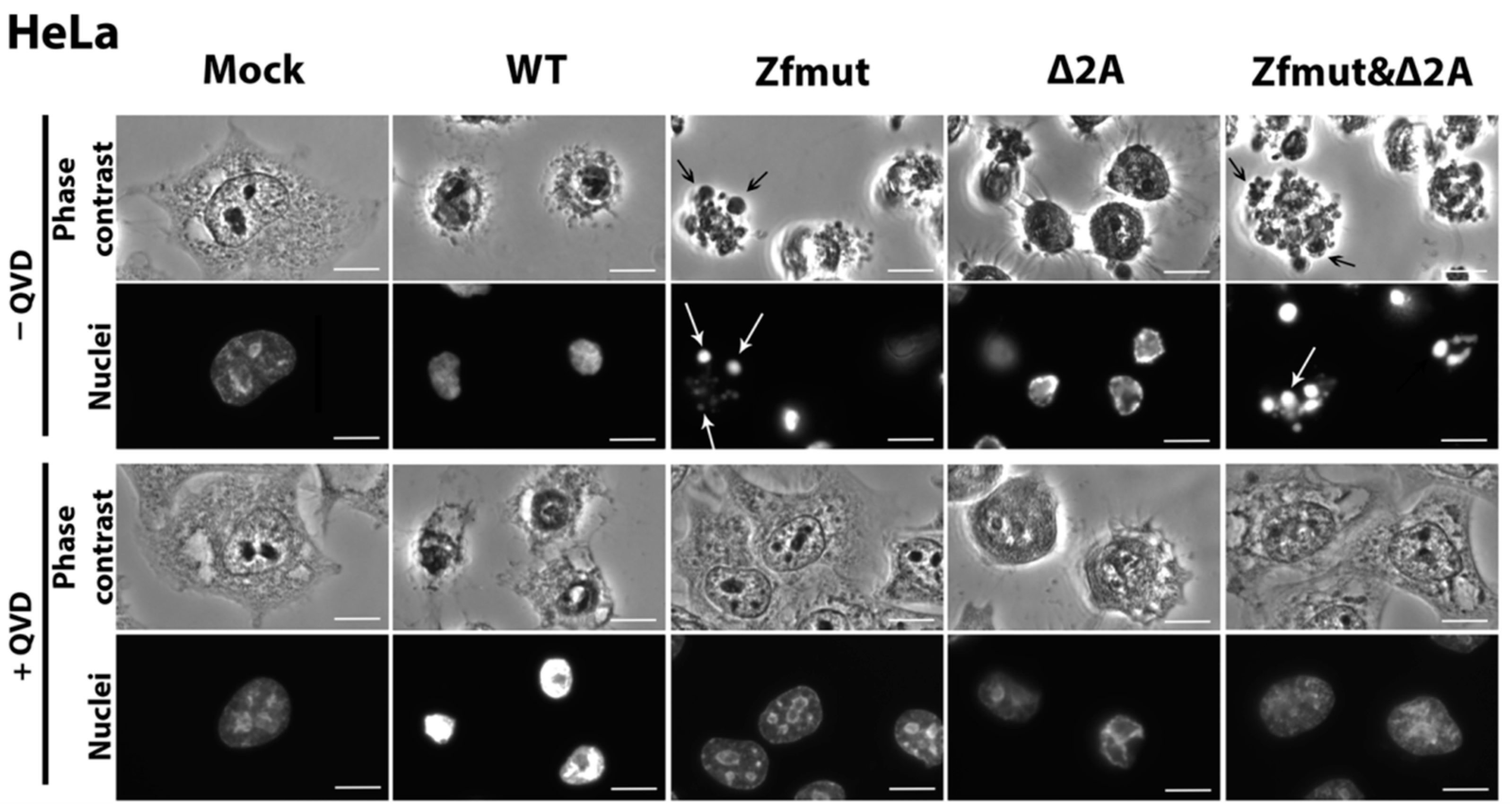
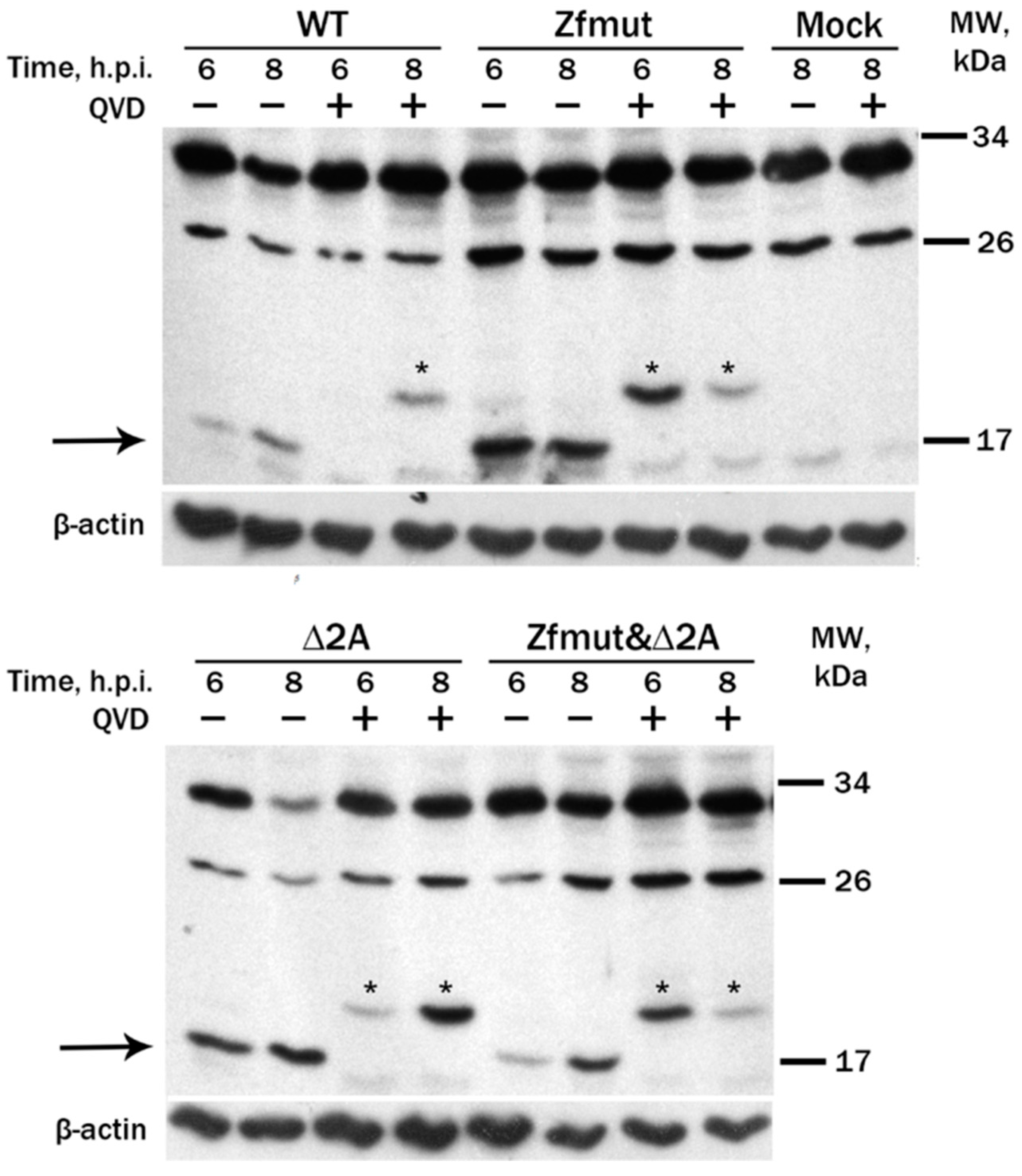
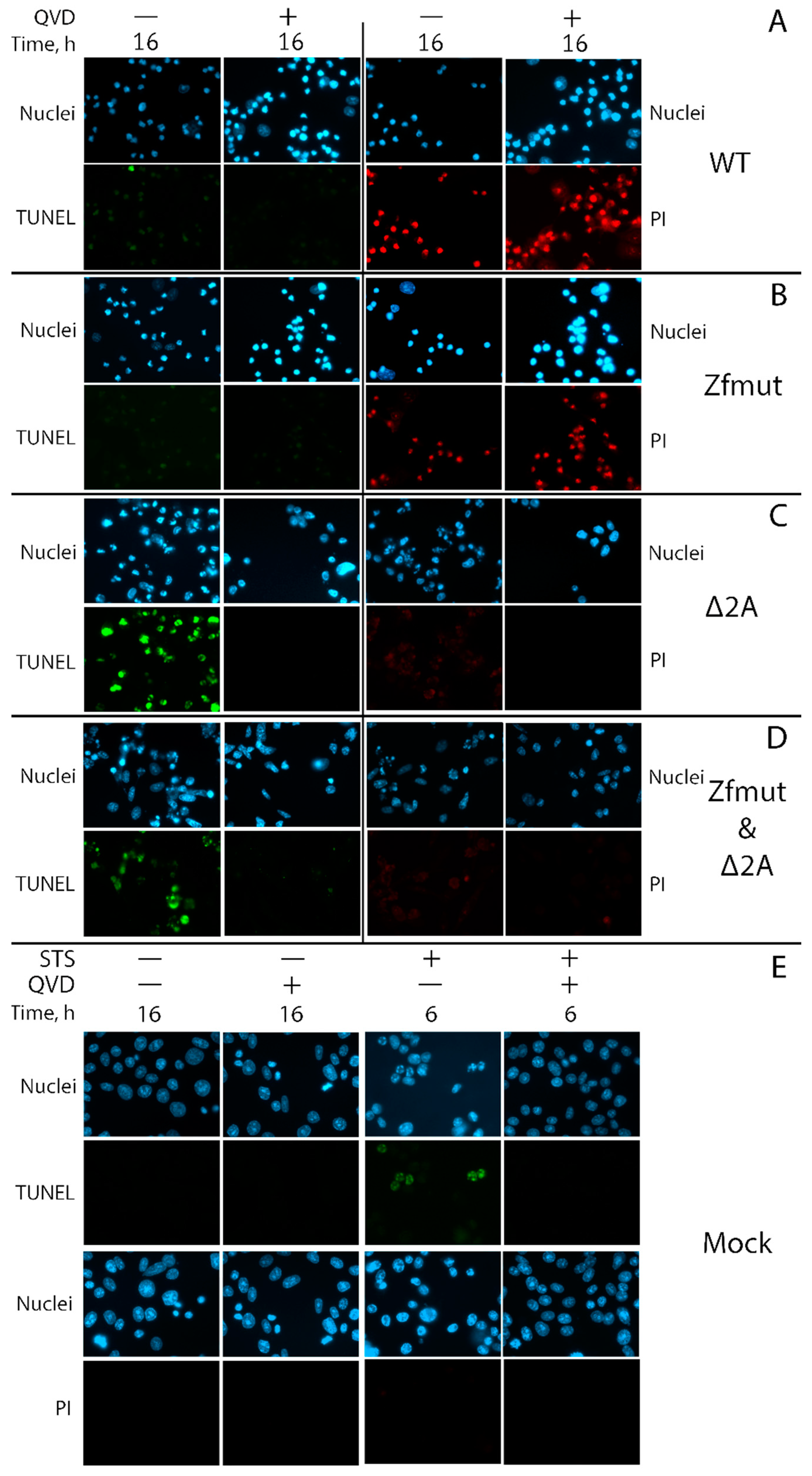
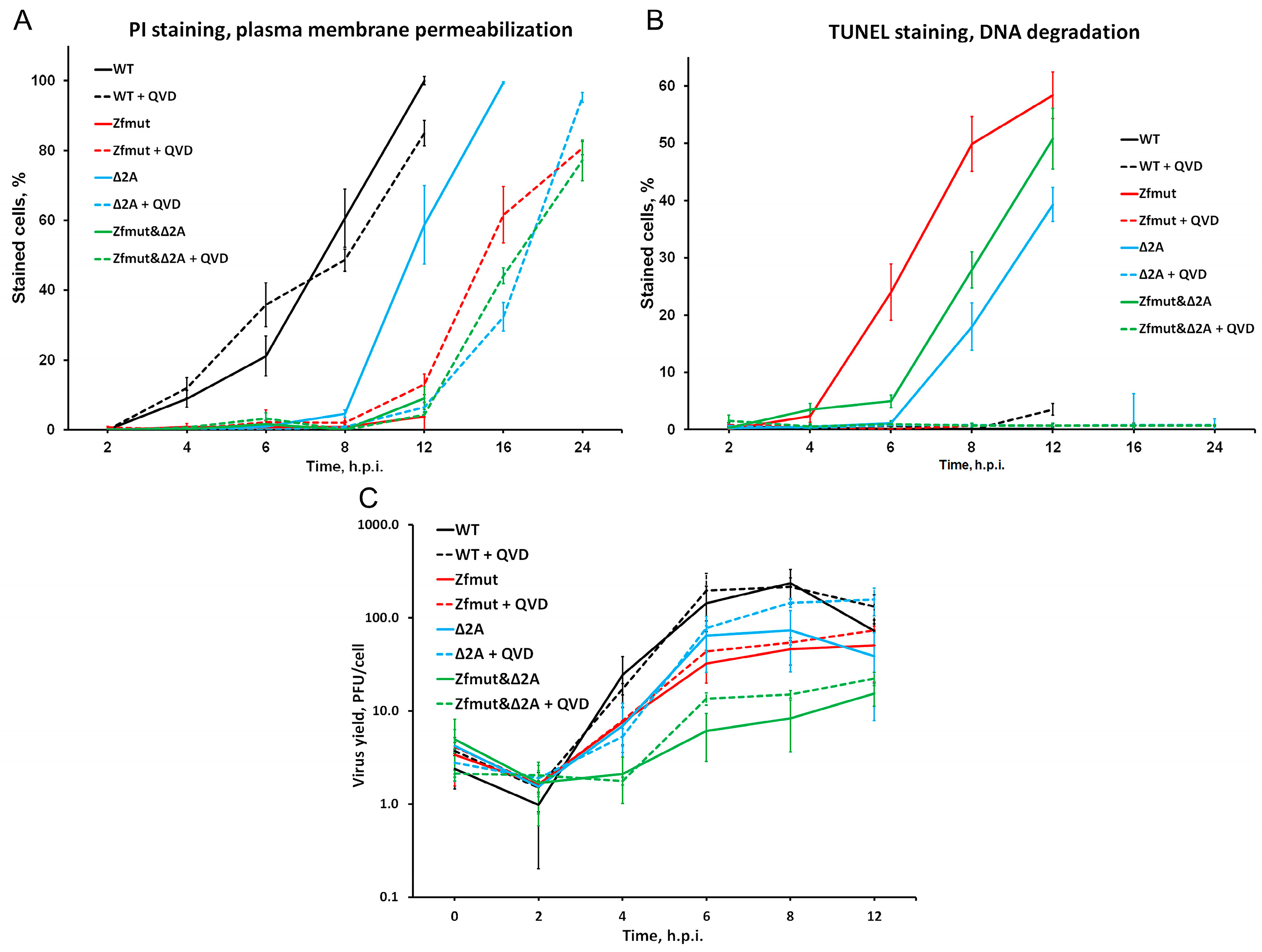
3.4. Infection with Both L- and 2A-Deficient EMCV Mutants Induces Caspase-Dependent Cell Death in Infected HeLa Cells
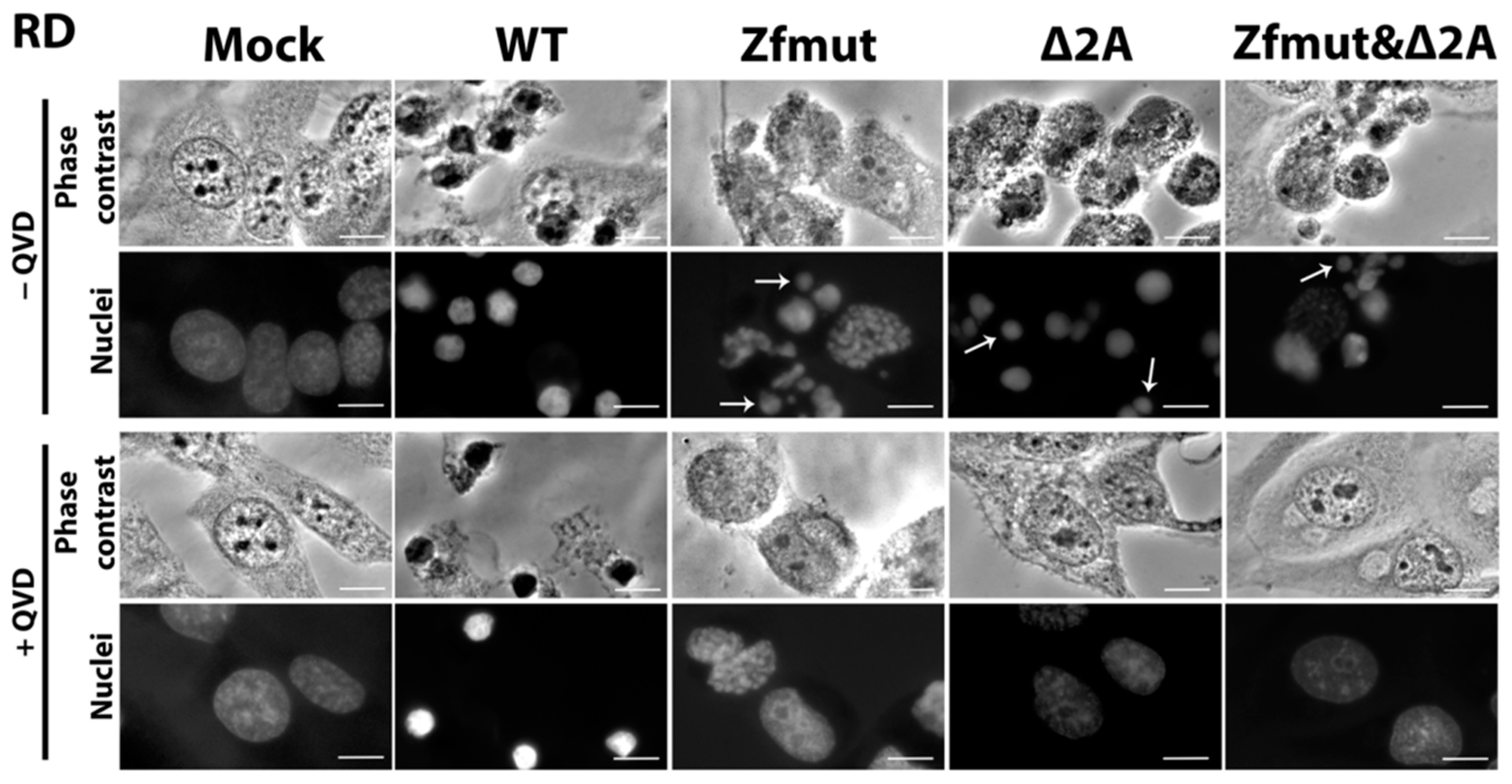
3.5. Infection with Both L- and 2A-Deficient EMCV Mutants Induces Caspase-Dependent Cell Death in Infected RD Cells
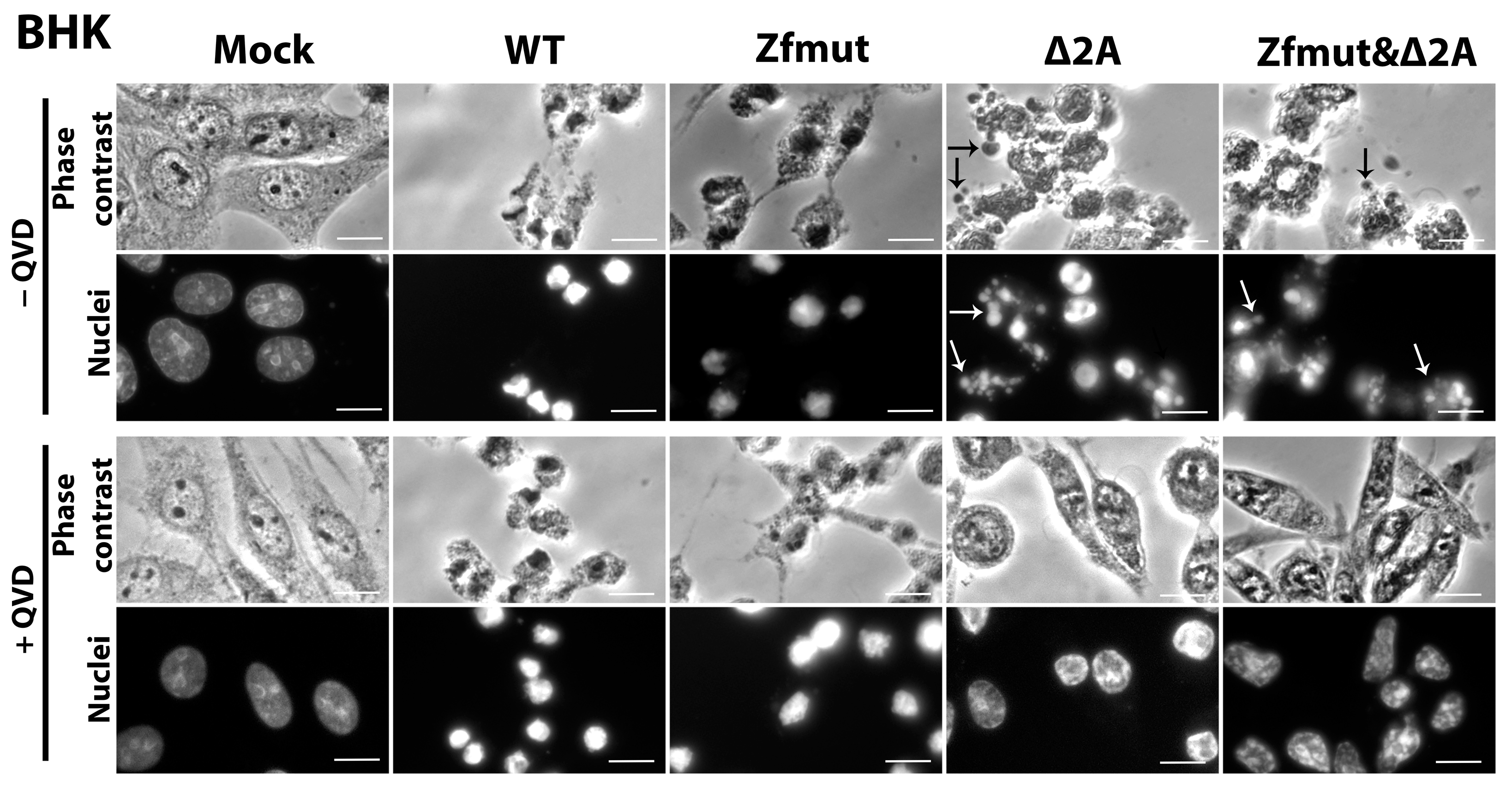
3.6. Functional Inactivation of the 2A Protein, but Not L, Leads to Apoptosis in the BHK-21 Cell Line upon Infection with EMCV
3.7. Simultaneous Inactivation of the L and/or 2A Security Proteins Leads to the Amelioration of Cell Pathology upon Inhibition of Apoptosis
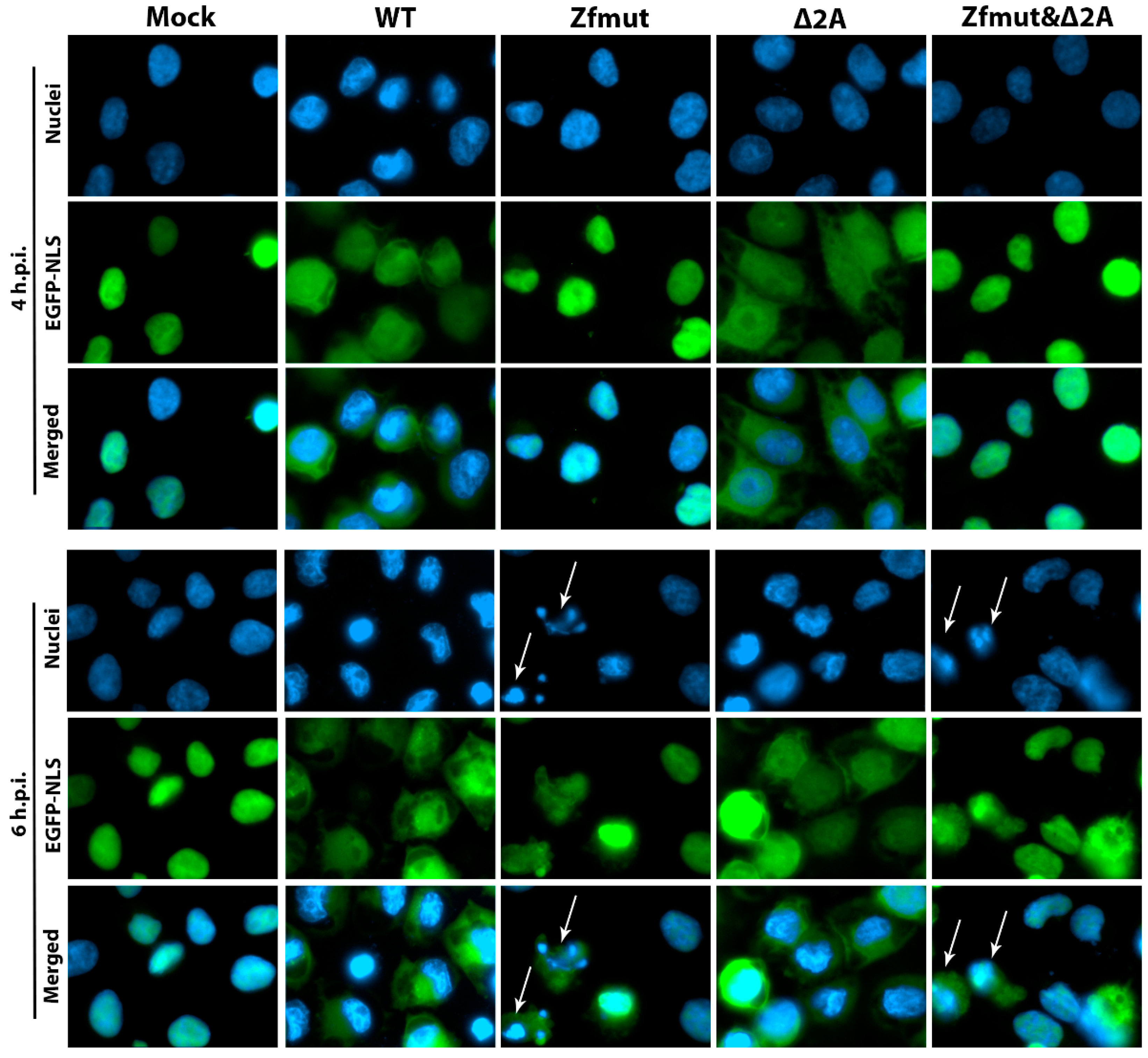
3.8. Security Protein 2A Is Not Involved in Disruption of the Nucleo-Cytoplasmic Traffic
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmenberg, A.C.; Kirby, E.M.; Janda, M.R.; Drake, N.L.; Duke, G.M.; Potratz, K.F.; Collett, M.S. The Nucleotide and Deduced Amino Acid Sequences of the Encephalomyocarditis Viral Polyprotein Coding Region. Nucleic Acids Res. 1984, 12, 2969–2985. [Google Scholar] [CrossRef]
- Loughran, G.; Firth, A.E.; Atkins, J.F. Ribosomal Frameshifting into an Overlapping Gene in the 2B-Encoding Region of the Cardiovirus Genome. Proc. Natl. Acad. Sci. USA 2011, 108, E1111–E1119. [Google Scholar] [CrossRef]
- Carocci, M.; Bakkali-Kassimi, L. The Encephalomyocarditis Virus. Virulence 2012, 3, 351–367. [Google Scholar] [CrossRef]
- Jungeblut, C.W.; Sanders, M.; Feiner, R.R. STUDIES IN RODENT POLIOMYELITIS. J. Exp. Med. 1942, 75, 611–629. [Google Scholar] [CrossRef]
- Helwig, F.C.; Schmidt, C.H. A Filter-Passing Agent Producing Interstitial Myocarditis in Anthropoid Apes and Small Animals. Science 1945, 102, 31–33. [Google Scholar] [CrossRef]
- Murnane, T.G.; Craighead, J.E.; Mondragon, H.; Shelokov, A. Fatal Disease of Swine Due to Encephalomyocarditis Virus. Science 1960, 131, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Smithburn, K.C.; Mengo, A.J.H. Encephalomyelitis Virus. Isolation and Immunological Properties. Br. J. Exp. Pathol. 1948, 29, 547. [Google Scholar]
- Agol, V.I.; Gmyl, A.P. Viral Security Proteins: Counteracting Host Defences. Nat. Rev. Microbiol. 2010, 8, 867–878. [Google Scholar] [CrossRef]
- Agol, V.I. Cytopathic Effects: Virus-Modulated Manifestations of Innate Immunity? Trends Microbiol. 2012, 20, 570–576. [Google Scholar] [CrossRef]
- Mikitas, O.V.; Ivin, Y.Y.; Golyshev, S.A.; Povarova, N.V.; Galkina, S.I.; Pletjushkina, O.Y.; Nadezhdina, E.S.; Gmyl, A.P.; Agol, V.I. Suppression of Injuries Caused by a Lytic RNA Virus (Mengovirus) and Their Uncoupling from Viral Reproduction by Mutual Cell/Virus Disarmament. J. Virol. 2012, 86, 5574–5583. [Google Scholar] [CrossRef][Green Version]
- Romanova, L.I.; Lidsky, P.V.; Kolesnikova, M.S.; Fominykh, K.V.; Gmyl, A.P.; Sheval, E.V.; Hato, S.V.; van Kuppeveld, F.J.M.; Agol, V.I. Antiapoptotic Activity of the Cardiovirus Leader Protein, a Viral “Security” Protein. J. Virol. 2009, 83, 7273–7284. [Google Scholar] [CrossRef][Green Version]
- Tolskaya, E.A.; Romanova, L.I.; Kolesnikova, M.S.; Ivannikova, T.A.; Smirnova, E.A.; Raikhlin, N.T.; Agol, V.I. Apoptosis-Inducing and Apoptosis-Preventing Functions of Poliovirus. J. Virol. 1995, 69, 1181–1189. [Google Scholar] [CrossRef]
- Romanova, L.I.; Belov, G.A.; Lidsky, P.V.; Tolskaya, E.A.; Kolesnikova, M.S.; Evstafieva, A.G.; Vartapetian, A.B.; Egger, D.; Bienz, K.; Agol, V.I. Variability in Apoptotic Response to Poliovirus Infection. Virology 2005, 331, 292–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurosaka, K.; Takahashi, M.; Watanabe, N.; Kobayashi, Y. Silent Cleanup of Very Early Apoptotic Cells by Macrophages. J. Immunol. 2003, 171, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Fadok, V. Corpse Clearance Defines the Meaning of Cell Death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma Membrane Changes during Programmed Cell Deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.D.; Baker, J.C.; Palmenberg, A.C. Proteolytic Cleavage of Encephalomyocarditis Virus Capsid Region Substrates by Precursors to the 3C Enzyme. J. Virol. 1989, 63, 1054–1058. [Google Scholar] [CrossRef]
- Bacot-Davis, V.R.; Ciomperlik, J.J.; Basta, H.A.; Cornilescu, C.C.; Palmenberg, A.C. Solution Structures of Mengovirus Leader Protein, Its Phosphorylated Derivatives, and in Complex with Nuclear Transport Regulatory Protein, RanGTPase. Proc. Natl. Acad. Sci. USA 2014, 111, 15792–15797. [Google Scholar] [CrossRef]
- Lidsky, P.V.; Hato, S.; Bardina, M.V.; Aminev, A.G.; Palmenberg, A.C.; Sheval, E.V.; Polyakov, V.Y.; van Kuppeveld, F.J.M.; Agol, V.I. Nucleocytoplasmic Traffic Disorder Induced by Cardioviruses. J. Virol. 2006, 80, 2705–2717. [Google Scholar] [CrossRef]
- Bardina, M.V.; Lidsky, P.V.; Sheval, E.V.; Fominykh, K.V.; van Kuppeveld, F.J.M.; Polyakov, V.Y.; Agol, V.I. Mengovirus-Induced Rearrangement of the Nuclear Pore Complex: Hijacking Cellular Phosphorylation Machinery. J. Virol. 2009, 83, 3150–3161. [Google Scholar] [CrossRef]
- Porter, F.W.; Palmenberg, A.C. Leader-Induced Phosphorylation of Nucleoporins Correlates with Nuclear Trafficking Inhibition by Cardioviruses. J. Virol. 2009, 83, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Galama, J.M.; van Kuppeveld, F.J.; Melchers, W.J. Mengovirus Leader Is Involved in the Inhibition of Host Cell Protein Synthesis. J. Virol. 1996, 70, 4948–4952. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Melchers, W.J.G.; Galama, J.M.D.; van Kuppeveld, F.J.M. The Mengovirus Leader Protein Suppresses Alpha/Beta Interferon Production by Inhibition of the Iron/Ferritin-Mediated Activation of NF-ΚB. J. Virol. 2002, 76, 9664–9672. [Google Scholar] [CrossRef] [PubMed]
- van Pesch, V.; van Eyll, O.; Michiels, T. The Leader Protein of Theiler’s Virus Inhibits Immediate-Early Alpha/Beta Interferon Production. J. Virol. 2001, 75, 7811–7817. [Google Scholar] [CrossRef] [PubMed]
- Hato, S.V.; Ricour, C.; Schulte, B.M.; Lanke, K.H.W.; de Bruijni, M.; Zoll, J.; Melchers, W.J.G.; Michiels, T.; van Kuppeveld, F.J.M. The Mengovirus Leader Protein Blocks Interferon-Alpha/Beta Gene Transcription and Inhibits Activation of Interferon Regulatory Factor 3. Cell. Microbiol. 2007, 9, 2921–2930. [Google Scholar] [CrossRef]
- Hahn, H.; Palmenberg, A.C. Mutational Analysis of the Encephalomyocarditis Virus Primary Cleavage. J. Virol. 1996, 70, 6870–6875. [Google Scholar] [CrossRef]
- Groppo, R.; Brown, B.A.; Palmenberg, A.C. Mutational Analysis of the EMCV 2A Protein Identifies a Nuclear Localization Signal and an EIF4E Binding Site. Virology 2011, 410, 257–267. [Google Scholar] [CrossRef]
- Petty, R.V.; Basta, H.A.; Bacot-Davis, V.R.; Brown, B.A.; Palmenberg, A.C. Binding Interactions between the Encephalomyocarditis Virus Leader and Protein 2A. J. Virol. 2014, 88, 13503–13509. [Google Scholar] [CrossRef]
- Carocci, M.; Cordonnier, N.; Huet, H.; Romey, A.; Relmy, A.; Gorna, K.; Blaise-Boisseau, S.; Zientara, S.; Kassimi, L.B. Encephalomyocarditis Virus 2A Protein Is Required for Viral Pathogenesis and Inhibition of Apoptosis. J. Virol. 2011, 85, 10741–10754. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liang, L.; Qin, T.; Xiao, S.; Liang, R. Encephalomyocarditis Virus 2A Protein Inhibited Apoptosis by Interaction with Annexin A2 through JNK/c-Jun Pathway. Viruses 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Napthine, S.; Ling, R.; Finch, L.K.; Jones, J.D.; Bell, S.; Brierley, I.; Firth, A.E. Protein-Directed Ribosomal Frameshifting Temporally Regulates Gene Expression. Nat. Commun. 2017, 8, 15582. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.H.; Pekarek, L.; Napthine, S.; Kibe, A.; Firth, A.E.; Graham, S.C.; Caliskan, N.; Brierley, I. Structural and Molecular Basis for Cardiovirus 2A Protein as a Viral Gene Expression Switch. Nat. Commun. 2021, 12, 7166. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Katikaneni, P.; Skern, T.; Gustin, K.E. Differential Targeting of Nuclear Pore Complex Proteins in Poliovirus-Infected Cells. J. Virol. 2008, 82, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Yoshino, Y.; Miyazawa, M.; Horie, H.; Ohka, S.; Nomoto, A. 2A Protease Is Not a Prerequisite for Poliovirus Replication. J. Virol. 2010, 84, 5947–5957. [Google Scholar] [CrossRef]
- Belov, G.A.; Lidsky, P.V.; Mikitas, O.V.; Egger, D.; Lukyanov, K.A.; Bienz, K.; Agol, V.I. Bidirectional Increase in Permeability of Nuclear Envelope upon Poliovirus Infection and Accompanying Alterations of Nuclear Pores. J. Virol. 2004, 78, 10166–10177. [Google Scholar] [CrossRef]
- Duke, G.M.; Palmenberg, A.C. Cloning and Synthesis of Infectious Cardiovirus RNAs Containing Short, Discrete Poly(C) Tracts. J. Virol. 1989, 63, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.M.T.; Hall, D.J.; Hill, M.; Riddle, M.; Pranter, A.; Dillman, J.; Deibel, M.; Palmenberg, A.C. Leader Protein of Encephalomyocarditis Virus Binds Zinc, Is Phosphorylated during Viral Infection, and Affects the Efficiency of Genome Translation. Virology 2001, 290, 261–271. [Google Scholar] [CrossRef]
- Buendia, B.; Santa-Maria, A.; Courvalin, J.C. Caspase-Dependent Proteolysis of Integral and Peripheral Proteins of Nuclear Membranes and Nuclear Pore Complex Proteins during Apoptosis. J. Cell Sci. 1999, 112, 1743–1753. [Google Scholar] [CrossRef]
- Cornilescu, C.C.; Porter, F.W.; Zhao, K.Q.; Palmenberg, A.C.; Markley, J.L. NMR Structure of the Mengovirus Leader Protein Zinc-Finger Domain. FEBS Lett. 2008, 582, 896–900. [Google Scholar] [CrossRef]
- Palmenberg, A.C.; Parks, G.D.; Hall, D.J.; Ingraham, R.H.; Seng, T.W.; Pallai, P.V. Proteolytic Processing of the Cardioviral P2 Region: Primary 2A/2B Cleavage in Clone-Derived Precursors. Virology 1992, 190, 754–762. [Google Scholar] [CrossRef]
- Caliskan, N.; Hill, C.H. Insights from Structural Studies of the Cardiovirus 2A Protein. Biosci. Rep. 2022, 42, BSR20210406. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Hahn, H.; Gingras, A.C.; Palmenberg, A.C.; Sonenberg, N. Rapamycin and Wortmannin Enhance Replication of a Defective Encephalomyocarditis Virus. J. Virol. 1998, 72, 5811–5819. [Google Scholar] [CrossRef]
- Habjan, M.; Penski, N.; Spiegel, M.; Weber, F. T7 RNA Polymerase-Dependent and -Independent Systems for CDNA-Based Rescue of Rift Valley Fever Virus. J. Gen. Virol. 2008, 89, 2157–2166. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5′-Phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Ivin, Y.Y.; Butusova, A.A.; Gladneva, E.E.; Kolomijtseva, G.Y.; Khapchaev, Y.K.; Ishmukhametov, A.A. The Role of the Encephalomyocarditis Virus Type 1 Proteins L and 2A in the Inhibition of the Synthesis of Cellular Proteins and the Accumulation of Viral Proteins during Infection. Vopr. Virusol. 2023, 68, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kim, J.; Tenson, T.; Min, J.-Y.; Kainov, D. Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis. Viruses 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Ghadge, G.; Roos, R.P. Apoptotic and Antiapoptotic Activity of L Protein of Theiler’s Murine Encephalomyelitis Virus. J. Virol. 2011, 85, 7177–7185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Y.; Lu, Y.; Meng, J.; Wang, Z. Pyroptosis and Its Regulation in Diabetic Cardiomyopathy. Front. Physiol. 2022, 12, 791848. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A New Frontier in Cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by Caspase-3 during Apoptosis Mediates Progression to Secondary Necrotic/Pyroptotic Cell Death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Besch, R.; Poeck, H.; Hohenauer, T.; Senft, D.; Häcker, G.; Berking, C.; Hornung, V.; Endres, S.; Ruzicka, T.; Rothenfusser, S.; et al. Proapoptotic Signaling Induced by RIG-I and MDA-5 Results in Type I Interferon-Independent Apoptosis in Human Melanoma Cells. J. Clin. Investig. 2009, 119, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 Heterotetramer: Structure and Function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.K.; Sharma, R.; Raychaudhuri, S.; Kundu, P.; Tsai, W.; Dasgupta, A. The Interaction of Cytoplasmic RNA Viruses with the Nucleus. Virus Res. 2003, 95, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yanagi, Y.; Ichinohe, T. Encephalomyocarditis Virus Viroporin 2B Activates NLRP3 Inflammasome. PLoS Pathog. 2012, 8, e1002857. [Google Scholar] [CrossRef]
- Hato, S.V.; Sorgeloos, F.; Ricour, C.; Zoll, J.; Melchers, W.J.G.; Michiels, T.; van Kuppeveld, F.J.M. Differential IFN-Alpha/Beta Production Suppressing Capacities of the Leader Proteins of Mengovirus and Foot-and-Mouth Disease Virus. Cell. Microbiol. 2010, 12, 310–317. [Google Scholar] [CrossRef]



| Cell Line | WT | L-mutant (Zfmut) | 2A-mutant (Δ2A) | L&2A-mutant (Zfmut&Δ2A) |
|---|---|---|---|---|
| Cell Death Description | ||||
| HeLa | Membrane permeabilization, shrinking of nuclei, caspase independent | DNA degradation, fragmentation of nuclei, formation of apoptotic bodies, caspase dependent | DNA degradation, membrane permeabilization, shrinking of nuclei, caspase dependent | DNA degradation, fragmentation of nuclei, formation of apoptotic bodies, caspase dependent |
| RD | DNA degradation, fragmentation of nuclei, caspase dependent | |||
| BHK-21 | Membrane permeabilization, shrinking of nuclei, caspase independent | DNA degradation, fragmentation of nuclei, formation of apoptotic bodies, caspase dependent | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivin, Y.; Butusova, A.; Gladneva, E.; Gmyl, A.; Ishmukhametov, A. Comprehensive Elucidation of the Role of L and 2A Security Proteins on Cell Death during EMCV Infection. Viruses 2024, 16, 280. https://doi.org/10.3390/v16020280
Ivin Y, Butusova A, Gladneva E, Gmyl A, Ishmukhametov A. Comprehensive Elucidation of the Role of L and 2A Security Proteins on Cell Death during EMCV Infection. Viruses. 2024; 16(2):280. https://doi.org/10.3390/v16020280
Chicago/Turabian StyleIvin, Yury, Anna Butusova, Ekaterina Gladneva, Anatoly Gmyl, and Aydar Ishmukhametov. 2024. "Comprehensive Elucidation of the Role of L and 2A Security Proteins on Cell Death during EMCV Infection" Viruses 16, no. 2: 280. https://doi.org/10.3390/v16020280
APA StyleIvin, Y., Butusova, A., Gladneva, E., Gmyl, A., & Ishmukhametov, A. (2024). Comprehensive Elucidation of the Role of L and 2A Security Proteins on Cell Death during EMCV Infection. Viruses, 16(2), 280. https://doi.org/10.3390/v16020280






