Isolation and Characterization of Yunnan Variants of the Pseudorabies Virus and Their Pathogenicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Sources
2.2. Enzyme-Linked Immunosorbent Assay
2.3. Detection of PRV
2.4. Virus Isolation
2.5. Analysis of Genetic Variations
2.6. Pathogenicity Test in Rats
2.7. Measurement of Blood Physiological Indices
2.8. Organ Index
2.9. Viral Load Calculation
2.10. Histological Analysis
2.11. Data Processing and Analysis
3. Results
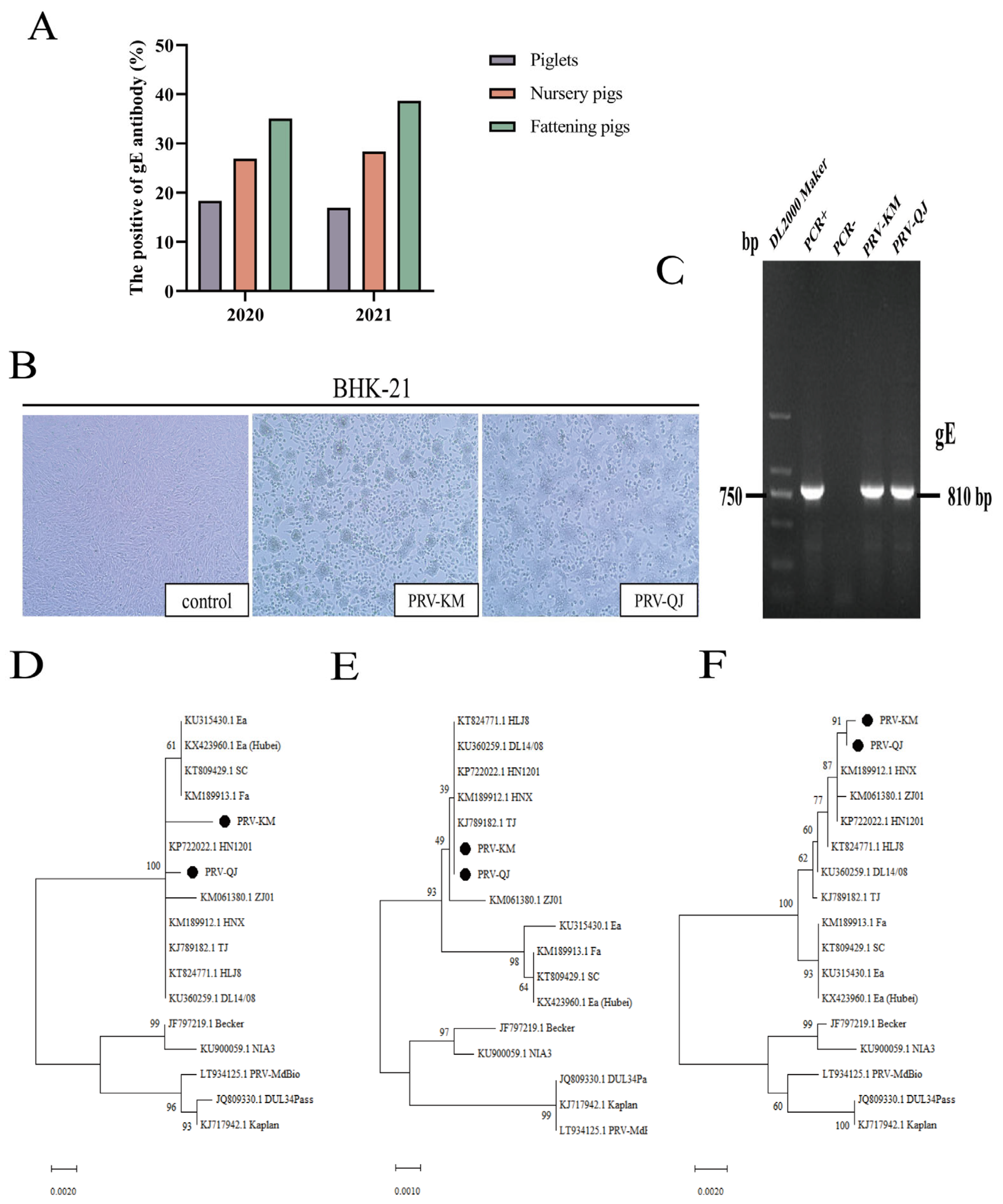
3.1. Epidemiological Analysis of Porcine PRV-gE Serum in Yunnan Province in 2020–2021
3.2. Virus Isolation and PCR Identification
3.3. Phylogenetic Analysis
3.4. Nucleotide Sequence Identity Analysis of the PRV gB, gD, and gE Genes
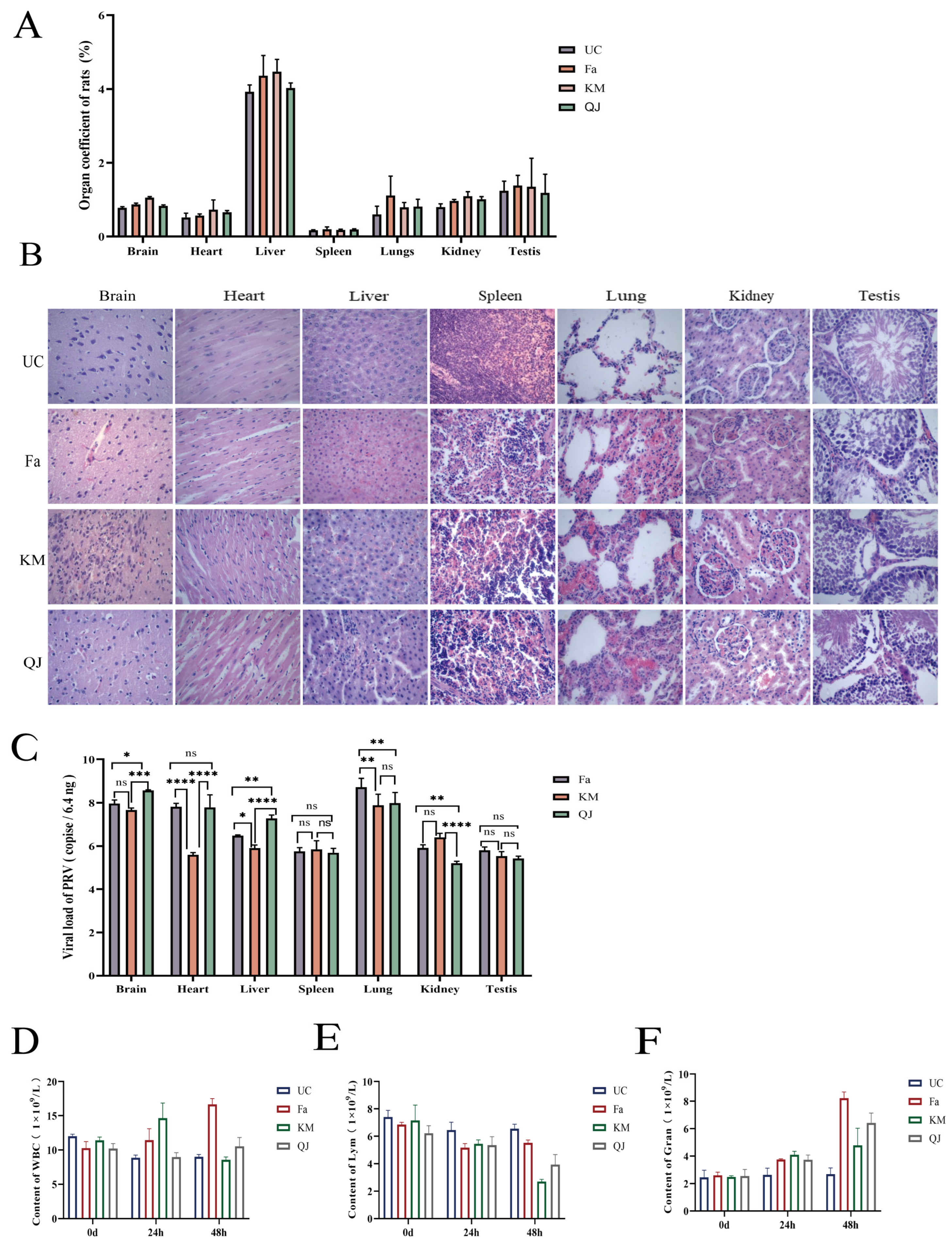
3.5. Pathogenicity of the PRV-KM and PRV-QJ Isolates and the Classical Fa Strain in Rats
3.6. Dynamic Determination of Detoxification Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.P. The history of pseudorabies in the United States. J. Am. Vet. Med. Assoc. 1954, 124, 259–261. [Google Scholar] [PubMed]
- Lee, J.Y.; Wilson, M.R. A review of pseudorabies (Aujeszky’s disease) in pigs. Can. Vet. J. 1979, 20, 65–69. [Google Scholar] [PubMed]
- van Nes, A. Mathematical modelling of pseudorabies virus (syn. Aujeszky’s disease virus) outbreaks aids eradication programmes: A review. Vet. Q. 2001, 23, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.; López Peña, M.; Quiroga, M.I.; Bermúdez, R.; Nieto, J.M.; Alemañ, N. Pseudorabies virus infection in mink: A host-specific pathogenesis. Vet. Immunol. Immunopathol. 2008, 124, 264–273. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Auclert, L.Z.; Zhai, X.; Wong, G.; Zhang, C.; Zhu, H.; Xing, G.; Wang, S.; He, W.; Li, K.; et al. Interspecies Transmission, Genetic Diversity, and Evolutionary Dynamics of Pseudorabies Virus. J. Infect. Dis. 2019, 219, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tan, L.; Wang, C.; He, S.; Fang, L.; Wang, Z.; Zhong, Y.; Zhang, K.; Liu, D.; Yang, Q.; et al. Serological Investigation and Genetic Characteristics of Pseudorabies Virus in Hunan Province of China from 2016 to 2020. Front. Vet. Sci. 2021, 8, 762326. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, J.; Gao, L.; He, Y.; Xie, J.; Zhu, P.; Zhang, Y.; Zhang, X.; Duan, L.; Yang, S.; et al. Epidemiological Investigation and Genetic Analysis of Pseudorabies Virus in Yunnan Province of China from 2017 to 2021. Viruses 2022, 14, 895. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, Z.; Hu, D.; Zhang, Q.; Han, T.; Li, X.; Gu, X.; Yuan, L.; Zhang, S.; Wang, B.; et al. Pathogenic pseudorabies virus, China, 2012. Emerg. Infect. Dis. 2014, 20, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Sun, Z.; Tan, F.F.; Guo, L.H.; Wang, Y.Z.; Wang, J.; Wang, Z.Y.; Wang, L.L.; Li, X.D.; Xiao, Y.; et al. Pathogenicity of a currently circulating Chinese variant pseudorabies virus in pigs. World J. Virol. 2016, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, R.; Hu, H.; Chen, X.; Yin, Z.; Liang, X.; He, C.; Yin, L.; Ye, G.; Zou, Y.; et al. The antiviral activity of kaempferol against pseudorabies virus in mice. BMC Vet. Res. 2021, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, Q.; Fu, Q.; Song, X.; Jia, R.; Yang, Y.; Zou, Y.; Li, L.; He, C.; Liang, X.; et al. Antiviral properties of resveratrol against pseudorabies virus are associated with the inhibition of IκB kinase activation. Sci. Rep. 2017, 7, 8782. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ren, H.; Gu, J.; Wang, J.; Jiang, L.; Gao, S. The Epidemiological Analysis of Pseudorabies Virus and Pathogenicity of the Variant Strain in Shandong Province. Front. Vet. Sci. 2022, 9, 806824. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhu, Q.; Chen, H.; Wang, Z.; Zheng, L.; Liu, F.; Wei, Z. Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China. Viruses 2022, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Hou, C.; Sun, H.; Yang, W.; Dong, J.; Bai, J.; Jiang, P. Emergence of highly virulent pseudorabies virus in southern China. Can. J. Vet. Res. 2015, 79, 221–228. [Google Scholar] [PubMed]
- Zhou, Q.; Zhang, L.; Liu, H.; Ye, G.; Huang, L.; Weng, C. Isolation and Characterization of Two Pseudorabies Virus and Evaluation of Their Effects on Host Natural Immune Responses and Pathogenicity. Viruses 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]

| Virus | Primer Sequence (5′–3′) | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| PRV PRV | gE F-CCCAACGACACACGGGGCCTCTA gE R-GCACAGCACGCAGAGCCAGA gB 1F-GCAGTCTTCAGGTCCGTCTTCC | 810 893 | 58.5 58.6 |
| gB 1R-GGTGTAGGTGTCGTTGGTGGTG | |||
| gB 2F-CGGCAAGTGCGTCTCCAA | 2012 | 58.5 | |
| gB 2R-GCCCGCTGTTCTTCTTGC | |||
| PRV | gD 1F-CGCGTACCCGTACACCGAGTC | 1329 | 60 |
| gD 1R-CAGAAACAGCAGCGTCCCGTC | |||
| gD 2F-GCATCGTCATCATGTGCCTCCAT | 867 | 60.5 | |
| gD 2R-TTGTACGTGCGGTGCTGGTCC | |||
| PRV | gE 1F-GCCACCATCGCAGAGGAACA | 1198 | 58.9 |
| gE 1R-GGTCATCACGAGCACGTACAGC | |||
| gE 2F-CCGTCACCGAGGTCCCGAGT | 1583 | 60.5 | |
| PRV | gE 2R-CCCATTCGTCACTTCCGGTTTCT gE F-CTACAGCGAGAGAGCGACAACGA gE R-CGACAGCGAGCAGATGACCA GCACAG | 139 | 60 |
| Number | Strain | GenBank Accession Number | Time | Area |
|---|---|---|---|---|
| 1 | TJ | KJ789182.1 | 2012 | China |
| 2 | ZJ01 | KM061380.1 | 2012 | China |
| 3 | HNX | KM189912.1 | 2012 | China |
| 4 | HN1201 | KP722022.1 | 2012 | China |
| 5 | HLJ8 | KT824771.1 | 2013 | China |
| 6 | DL14/08 | KU360259.1 | 2014 | China |
| 7 | SC | KT809429.1 | 1986 | China |
| 8 | Ea | KU315430.1 | 1990 | China |
| 9 | Ea (Hubei) | KX423960.1 | 1993 | China |
| 10 | Fa | KM189913.1 | 2012 | China |
| 11 | Becker | JF797219.1 | 2011 | USA |
| 12 | DUL34Pass | JQ809330.1 | 2012 | Germany |
| 13 | Kaplan | KJ717942.1 | 2011 | Hungary |
| 14 | NIA3 | KU900059.1 | 2016 | UK |
| 15 | PRV-MdBio | LT934125.1 | 2017 | Hungary |
| gB | gD | gE | |
|---|---|---|---|
| Comparison of two PRV strains obtained in this study | 99.7% | 99.7% | 99.6% |
| Compared with PRV variants | 99.6–99.9% | 99.5–100% | 99.5–99.7% |
| Compared with classical PRV strains | 99.1–99.4% | 98.7–99.1% | 99.1–99.2% |
| Compared with European and American PRV strains | 98.3–99.1% | 98.2–98.9% | 97.4–97.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ye, H.; Zhang, X.; Zhang, Y.; Li, Y.; Yao, J.; Gao, L.; Wang, S.; Yu, Y.; Shu, X. Isolation and Characterization of Yunnan Variants of the Pseudorabies Virus and Their Pathogenicity in Rats. Viruses 2024, 16, 233. https://doi.org/10.3390/v16020233
Song C, Ye H, Zhang X, Zhang Y, Li Y, Yao J, Gao L, Wang S, Yu Y, Shu X. Isolation and Characterization of Yunnan Variants of the Pseudorabies Virus and Their Pathogenicity in Rats. Viruses. 2024; 16(2):233. https://doi.org/10.3390/v16020233
Chicago/Turabian StyleSong, Chunlian, Hua Ye, Xue Zhang, Yalun Zhang, Yonghui Li, Jun Yao, Lin Gao, Shanqiang Wang, Yougeng Yu, and Xianghua Shu. 2024. "Isolation and Characterization of Yunnan Variants of the Pseudorabies Virus and Their Pathogenicity in Rats" Viruses 16, no. 2: 233. https://doi.org/10.3390/v16020233
APA StyleSong, C., Ye, H., Zhang, X., Zhang, Y., Li, Y., Yao, J., Gao, L., Wang, S., Yu, Y., & Shu, X. (2024). Isolation and Characterization of Yunnan Variants of the Pseudorabies Virus and Their Pathogenicity in Rats. Viruses, 16(2), 233. https://doi.org/10.3390/v16020233





