Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts
Abstract
1. Introduction
2. Recent Bovine Influenza A Virus Outbreak in Domestic Ruminants in the US
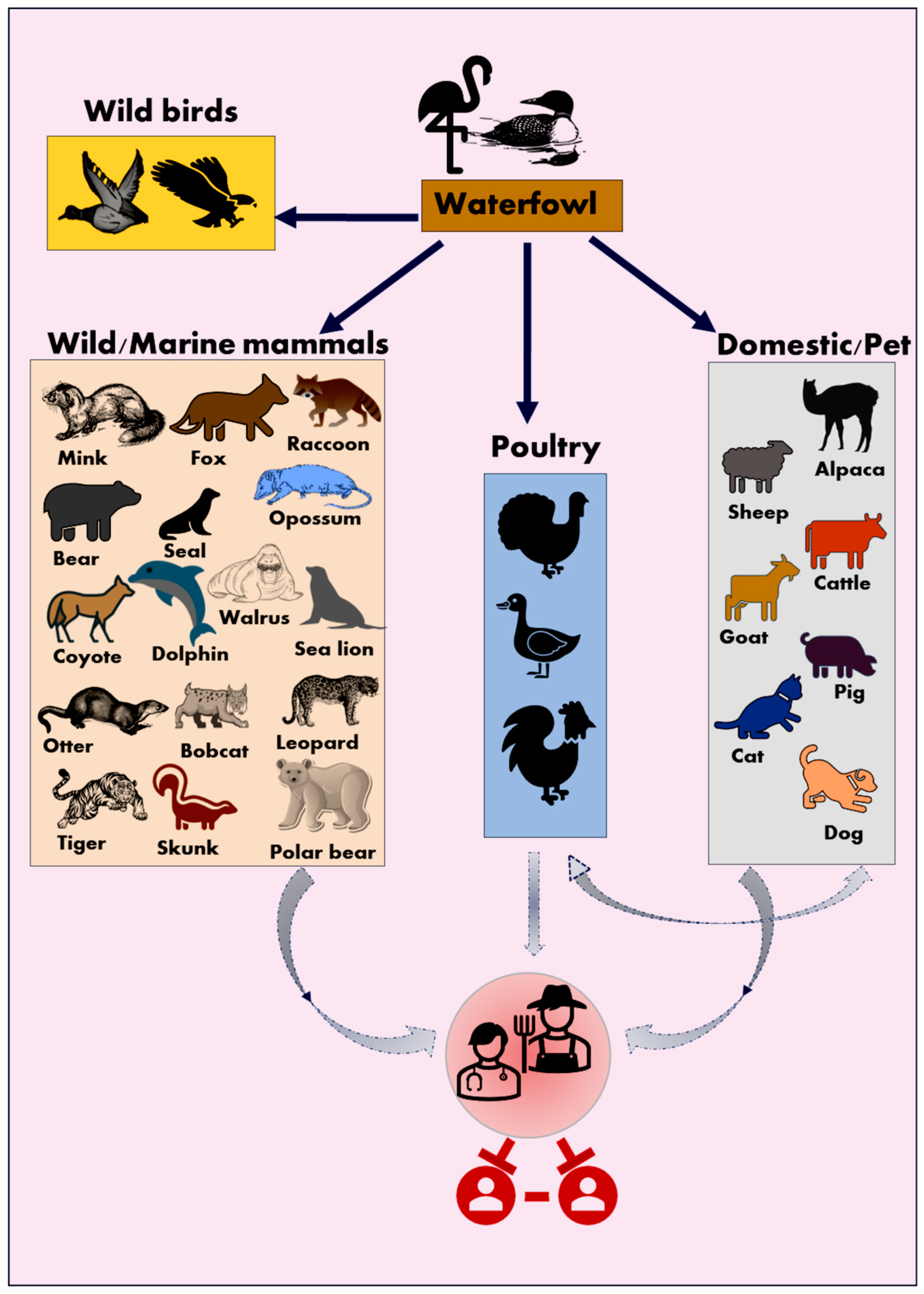
2.1. Natural Infection of HPAI A(H5N1) in Wild Mammals
2.2. Natural Infection of HPAI A(H5N1) in Marine Mammals
2.3. Natural Infection of HPAI A(H5N1) in Domestic/Companion Animals
2.4. Experimental Infection of HPAI A(H5N1) in Mammalian Hosts
3. Neuropathophysiology of H5Nx Infections in Mammals
4. HPAI A(H5N1) Virus Infection in Humans
5. HA, NA, and NS Also Determine Virus Tropism and Host Range
6. Vaccines Against HPAI A(H5N1)
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr. Top. Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014, 22, 183–191. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Pathogenicity and virulence of influenza. Virulence 2023, 14, 2223057. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Katsushima, N.; Nagai, Y.; Shoji, M.; Itagaki, T.; Sakamoto, M.; Kitaoka, S.; Mizuta, K.; Nishimura, H. Clinical features of influenza C virus infection in children. J. Infect. Dis. 2006, 193, 1229–1235. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Z.; Lin, T.; Sreenivasan, C.; Gao, R.; Thomas, M.; Druce, J.; Hause, B.M.; Kaushik, R.S.; Li, F.; et al. Genetic and antigenic characteristics of a human influenza C virus clinical isolate. J. Med. Virol. 2020, 92, 161–166. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Z.; Huang, C.; Wang, D.; Li, F. Influenza D virus. Curr. Opin. Virol. 2020, 44, 154–161. [Google Scholar] [CrossRef]
- Yu, J.; Liu, R.; Zhou, B.; Chou, T.W.; Ghedin, E.; Sheng, Z.; Gao, R.; Zhai, S.L.; Wang, D.; Li, F. Development and Characterization of a Reverse-Genetics System for Influenza D Virus. J. Virol. 2019, 93, e01186-19. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Liu, R.; Gao, R.; Guo, Y.; Hause, B.M.; Thomas, M.; Naveed, A.; Clement, T.; Rausch, D.; Christopher-Hennings, J.; et al. Influenza C and D Viruses Demonstrated a Differential Respiratory Tissue Tropism in a Comparative Pathogenesis Study in Guinea Pigs. J. Virol. 2023, 97, e00356-23. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Thomas, M.; Kaushik, R.S.; Wang, D.; Li, F. Influenza A in Bovine Species: A Narrative Literature Review. Viruses 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Porter, E.; Lohman, M.; Lu, N.; Peddireddi, L.; Hanzlicek, G.; Marthaler, D.; Liu, X.; Bai, J. Influenza C Virus in Cattle with Respiratory Disease, United States, 2016–2018. Emerg. Infect. Dis. 2018, 24, 1926–1929. [Google Scholar] [CrossRef]
- Zhang, H.; Porter, E.P.; Lohman, M.; Lu, N.; Peddireddi, L.; Hanzlicek, G.; Marthaler, D.; Liu, X.; Bai, J. Complete Genome Sequence of an Influenza C Virus Strain Identified from a Sick Calf in the United States. Microbiol. Resour. Announc. 2018, 7, e00828-18. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Schultz-Cherry, S. Immunology of avian influenza virus: A review. Dev. Comp. Immunol. 2000, 24, 269–283. [Google Scholar] [CrossRef]
- Lee, D.H.; Criado, M.F.; Swayne, D.E. Pathobiological Origins and Evolutionary History of Highly Pathogenic Avian Influenza Viruses. Cold Spring Harb. Perspect. Med. 2021, 11. [Google Scholar] [CrossRef]
- Luczo, J.M.; Stambas, J.; Durr, P.A.; Michalski, W.P.; Bingham, J. Molecular pathogenesis of H5 highly pathogenic avian influenza: The role of the haemagglutinin cleavage site motif. Rev. Med. Virol. 2015, 25, 406–430. [Google Scholar] [CrossRef]
- Bóna, M.; Kiss, I.; Dénes, L.; Szilasi, A.; Mándoki, M. Tissue Tropism of H9N2 Low-Pathogenic Avian Influenza Virus in Broiler Chickens by Immunohistochemistry. Animals 2023, 13, 1052. [Google Scholar] [CrossRef]
- Bogs, J.; Veits, J.; Gohrbandt, S.; Hundt, J.; Stech, O.; Breithaupt, A.; Teifke, J.P.; Mettenleiter, T.C.; Stech, J. Highly Pathogenic H5N1 Influenza Viruses Carry Virulence Determinants beyond the Polybasic Hemagglutinin Cleavage Site. PLoS ONE 2010, 5, e11826. [Google Scholar] [CrossRef]
- Avian Influenza in Birds. Available online: https://www.cdc.gov/flu/avianflu/avian-in-birds.htm (accessed on 23 April 2024).
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Jeong, S.; Otgontogtokh, N.; Lee, D.-H.; Davganyam, B.; Lee, S.-H.; Cho, A.; Tseren-Ochir, E.-O.; Song, C.-S. Highly Pathogenic Avian Influenza Clade 2.3.4.4 Subtype H5N6 Viruses Isolated from Wild Whooper Swans, Mongolia, 2020. Emerg. Infect. Dis. J. 2021, 27, 1181. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.; Zecchin, B.; Giussani, E.; Palumbo, E.; Agüero-García, M.; Bachofen, C.; Bálint, Á.; Banihashem, F.; Banyard, A.C.; Beerens, N.; et al. High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe—Why trends of virus evolution are more difficult to predict. Virus Evol. 2024, 10, veae027. [Google Scholar] [CrossRef]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Donis, R.O.; World Health Organization/World Organisation for Animal Health/Food and Agriculture Organization (WHO/OIE/FAO) H5 Evolution Working Group. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Kandeil, A.; Patton, C.; Jones, J.C.; Jeevan, T.; Harrington, W.N.; Trifkovic, S.; Seiler, J.P.; Fabrizio, T.; Woodard, K.; Turner, J.C.; et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat. Commun. 2023, 14, 3082. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef]
- H5N1 Bird Flu Detections across the United States (Backyard and Commercial). Available online: https://www.cdc.gov/flu/avianflu/data-map-commercial.html (accessed on 23 April 2024).
- AVMA. Goat in Minnesota tests positive for HPAI. Available online: https://www.avma.org/news/goat-minnesota-tests-positive-hpai (accessed on 24 April 2024).
- United States of America—Influenza A Viruses of High Pathogenicity (Inf. with) (Non-Poultry Including Wild Birds) (2017-)—Follow Up Report 43. Available online: https://wahis.woah.org/#/in-review/4451?reportId=166488&fromPage=event-dashboard-url (accessed on 24 April 2024).
- United States of America—Influenza A Viruses of High Pathogenicity (Inf. with) (Non-Poultry Including Wild Birds) (2017-)—Follow Up Report 44. Available online: https://wahis.woah.org/#/in-review/4451?reportId=166639&fromPage=event-dashboard-url (accessed on 24 April 2024).
- Highly Pathogenic Avian Influenza (HPAI) Detections in Livestock. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock (accessed on 2 May 2024).
- USDA. Highly Pathogenic Avian Influenza (HPAI) H5N1 Detections in Alpacas. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals/highly-pathogenic-avian (accessed on 14 October 2024).
- Burrough, E.; Magstadt, D.; Petersen, B.; Timmermans, S.; Gauger, P.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. J. 2024, 30, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, L.; Fusaro, A.; Kuiken, T.; Mirinavičiūtė, G.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; Delacourt, R.; et al. Avian influenza overview March-June 2024. Efsa J. 2024, 22, e8930. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakamura, K.; Mase, M. Survival of Highly Pathogenic Avian Influenza H5N1 Virus in Tissues Derived from Experimentally Infected Chickens. Appl. Environ. Microbiol. 2017, 83, e00604-17. [Google Scholar] [CrossRef]
- Bauer, L.; Benavides, F.F.W.; Veldhuis Kroeze, E.J.B.; de Wit, E.; van Riel, D. The neuropathogenesis of highly pathogenic avian influenza H5Nx viruses in mammalian species including humans. Trends Neurosci. 2023, 46, 953–970. [Google Scholar] [CrossRef]
- Kristensen, C.; Larsen, L.E.; Trebbien, R.; Jensen, H.E. The avian influenza A virus receptor SA-alpha2,3-Gal is expressed in the porcine nasal mucosa sustaining the pig as a mixing vessel for new influenza viruses. Virus Res. 2024, 340, 199304. [Google Scholar] [CrossRef] [PubMed]
- Gunning, R.F.; Brown, I.H.; Crawshaw, T.R. Evidence of influenza A virus infection in dairy cows with sporadic milk drop syndrome. Vet. Rec. 1999, 145, 556–557. [Google Scholar] [CrossRef]
- Brown, I.H.; Crawshaw, T.R.; Harris, P.A.; Alexander, D.J. Detection of antibodies to influenza A virus in cattle in association with respiratory disease and reduced milk yield. Vet. Rec. 1998, 143, 637–638. [Google Scholar] [PubMed]
- Crawshaw, T.R.; Brown, I.H.; Essen, S.C.; Young, S.C. Significant rising antibody titres to influenza A are associated with an acute reduction in milk yield in cattle. Vet. J. 2008, 178, 98–102. [Google Scholar] [CrossRef]
- Fujita, R.; Tachi, T.; Hino, M.; Nagata, K.; Saiki, M.; Inumaru, M.; Higa, Y.; Itokawa, K.; Uemura, N.; Matsumura, R.; et al. Blowflies are potential vector for avian influenza virus at enzootic area in Japan. Sci. Rep. 2024, 14, 10285. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.I.; Gamarra-Toledo, V.; Euguí, J.R.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg. Infect. Dis. 2024, 30, 444–452. [Google Scholar] [CrossRef]
- Plaza, P.I.; Gamarra-Toledo, V.; Rodriguez Eugui, J.; Rosciano, N.; Lambertucci, S.A. Pacific and Atlantic sea lion mortality caused by highly pathogenic Avian Influenza A(H5N1) in South America. Travel. Med. Infect. Dis. 2024, 59, 102712. [Google Scholar] [CrossRef]
- Oguzie, J.; Marushchak, L.; Shittu, I.; Lednicky, J.; Miller, A.; Hao, H.; Nelson, M.; Gray, G. Avian Influenza A(H5N1) Virus among Dairy Cattle, Texas, USA. Emerg. Infect. Dis. J. 2024, 30, 1425. [Google Scholar] [CrossRef]
- Reperant, L.A.; van Amerongen, G.; van de Bildt, M.W.; Rimmelzwaan, G.F.; Dobson, A.P.; Osterhaus, A.D.; Kuiken, T. Highly pathogenic avian influenza virus (H5N1) infection in red foxes fed infected bird carcasses. Emerg. Infect. Dis. 2008, 14, 1835–1841. [Google Scholar] [CrossRef]
- Ulloa, M.; Fernandez, A.; Ariyama, N.; Colom-Rivero, A.; Rivera, C.; Nunez, P.; Sanhueza, P.; Johow, M.; Araya, H.; Torres, J.C.; et al. Mass mortality event in South American sea lions (Otaria flavescens) correlated to highly pathogenic avian influenza (HPAI) H5N1 outbreak in Chile. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Murawski, A.; Fabrizio, T.; Ossiboff, R.; Kackos, C.; Jeevan, T.; Jones, J.C.; Kandeil, A.; Walker, D.; Turner, J.C.M.; Patton, C.; et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Commun. Biol. 2024, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 2004, 10, 2189–2191. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, B.T.; Berhane, Y.; Nadeau, M.S.; Embury-Hyatt, C.; Lung, O.; Xu, W.; Lair, S. Influenza A(H5N1) Virus Infections in 2 Free-Ranging Black Bears (Ursus americanus), Quebec, Canada. Emerg. Infect. Dis. 2023, 29, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef]
- Elsmo, E.J.; Wunschmann, A.; Beckmen, K.B.; Broughton-Neiswanger, L.E.; Buckles, E.L.; Ellis, J.; Fitzgerald, S.D.; Gerlach, R.; Hawkins, S.; Ip, H.S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b Infections in Wild Terrestrial Mammals, United States, 2022. Emerg. Infect. Dis. 2023, 29, 2451–2460. [Google Scholar] [CrossRef]
- Wildlife, California department of Fish and Wildlife Avian Influenza Detected in Deceased Bobcat. 2023. Available online: https://wildlife.ca.gov/News/Archive/avian-influenza-detected-in-deceased-bobcat (accessed on 6 August 2024).
- USDA. Detections of Highly Pathogenic Avian Influenza in Mammals. 2024. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (accessed on 7 August 2024).
- Thanawongnuwech, R.; Amonsin, A.; Tantilertcharoen, R.; Damrongwatanapokin, S.; Theamboonlers, A.; Payungporn, S.; Nanthapornphiphat, K.; Ratanamungklanon, S.; Tunak, E.; Songserm, T.; et al. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg. Infect. Dis. 2005, 11, 699–701. [Google Scholar] [CrossRef]
- Tammiranta, N.; Isomursu, M.; Fusaro, A.; Nylund, M.; Nokireki, T.; Giussani, E.; Zecchin, B.; Terregino, C.; Gadd, T. Highly pathogenic avian influenza A (H5N1) virus infections in wild carnivores connected to mass mortalities of pheasants in Finland. Infect. Genet. Evol. 2023, 111, 105423. [Google Scholar] [CrossRef]
- Hu, T.; Zhao, H.; Zhang, Y.; Zhang, W.; Kong, Q.; Zhang, Z.; Cui, Q.; Qiu, W.; Deng, B.; Fan, Q.; et al. Fatal influenza A (H5N1) virus Infection in zoo-housed Tigers in Yunnan Province, China. Sci. Rep. 2016, 6, 25845. [Google Scholar] [CrossRef]
- Hall, J.S.; Bentler, K.T.; Landolt, G.; Elmore, S.A.; Minnis, R.B.; Campbell, T.A.; Barras, S.C.; Root, J.J.; Pilon, J.; Pabilonia, K.; et al. Influenza Infection in Wild Raccoons. Emerg. Infect. Dis. 2008, 14, 1842–1848. [Google Scholar] [CrossRef]
- Wildlife, California department of Fish and Wildlife, Avian Influenza Detected in Deceased Mountain Lions. 2023. Available online: https://wildlife.ca.gov/News/Archive/avian-influenza-detected-in-deceased-mountain-lions (accessed on 7 August 2024).
- Aguero, M.; Monne, I.; Sanchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernandez-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 2023, 28. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, L.; Xiong, J.; Wang, C.; Chen, L.; Yang, P.; Yu, H.; Yan, Q.; Cheng, Y.; Jiang, L.; et al. Semiaquatic mammals might be intermediate hosts to spread avian influenza viruses from avian to human. Sci. Rep. 2019, 9, 11641. [Google Scholar] [CrossRef] [PubMed]
- Lindh, E.; Lounela, H.; Ikonen, N.; Kantala, T.; Savolainen-Kopra, C.; Kauppinen, A.; Österlund, P.; Kareinen, L.; Katz, A.; Nokireki, T.; et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Eurosurveillance 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol. Spectr. 2023, 11, e0286722. [Google Scholar] [CrossRef]
- Leguia, M.; Garcia-Glaessner, A.; Munoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef]
- Thorsson, E.; Zohari, S.; Roos, A.; Banihashem, F.; Bröjer, C.; Neimanis, A. Highly Pathogenic Avian Influenza A(H5N1) Virus in a Harbor Porpoise, Sweden. Emerg. Infect. Dis. 2023, 29, 852–855. [Google Scholar] [CrossRef]
- Stimmelmayr, R.; Rotstein, D.; Torchetti, M.K.; Gerlach, R. Highly Pathogenic Avian Influenza Virus A(H5N1) Clade 2.3.4.4b Infection in Free-Ranging Polar Bear, Alaska, USA. Emerg. Infect. Dis. J. 2024, 30, 1660. [Google Scholar] [CrossRef] [PubMed]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Ly, H. Highly pathogenic avian influenza H5N1 virus infection of companion animals. Virulence 2024, 15, 2289780. [Google Scholar] [CrossRef]
- Thiry, E.; Zicola, A.; Addie, D.; Egberink, H.; Hartmann, K.; Lutz, H.; Poulet, H.; Horzinek, M.C. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet. Microbiol. 2007, 122, 25–31. [Google Scholar] [CrossRef]
- Szalus-Jordanow, O.; Golke, A.; Dzieciatkowski, T.; Czopowicz, M.; Kardas, M.; Mickiewicz, M.; Moroz-Fik, A.; Lobaczewski, A.; Markowska-Daniel, I.; Frymus, T. Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms 2024, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Rabalski, L.; Milewska, A.; Pohlmann, A.; Gackowska, K.; Lepionka, T.; Szczepaniak, K.; Swiatalska, A.; Sieminska, I.; Arent, Z.; Beer, M.; et al. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Eurosurveillance 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Wolf, P.U.; Uhl, W.; Gerst, S.; Harder, T.; Starick, E.; Vahlenkamp, T.W.; Mettenleiter, T.C.; Teifke, J.P. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet. Pathol. 2007, 44, 261–268. [Google Scholar] [CrossRef]
- Lee, K.; Yeom, M.; Vu, T.T.H.; Do, H.Q.; Na, W.; Lee, M.; Jeong, D.G.; Cheon, D.S.; Song, D. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg. Microbes Infect. 2024, 13, 2290835. [Google Scholar] [CrossRef] [PubMed]
- Sillman, S.J.; Drozd, M.; Loy, D.; Harris, S.P. Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J. Comp. Pathol. 2023, 205, 17–23. [Google Scholar] [CrossRef]
- Kuiken, T.; Rimmelzwaan, G.; van Riel, D.; van Amerongen, G.; Baars, M.; Fouchier, R.; Osterhaus, A. Avian H5N1 influenza in cats. Science 2004, 306, 241. [Google Scholar] [CrossRef]
- Domestic Dog Tests Positive for Avian Influenza in Canada. Available online: https://www.canada.ca/en/food-inspection-agency/news/2023/04/domestic-dog-tests-positive-for-avian-influenza-in-canada.html (accessed on 25 April 2024).
- Moreno, A.; Bonfante, F.; Bortolami, A.; Cassaniti, I.; Caruana, A.; Cottini, V.; Cereda, D.; Farioli, M.; Fusaro, A.; Lavazza, A.; et al. Asymptomatic infection with clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) in carnivore pets, Italy, April 2023. Eurosurveillance 2023, 28, 2300441. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.L.; Arruda, B.; Palmer, M.V.; Boggiatto, P.; Sarlo Davila, K.; Buckley, A.; Ciacci Zanella, G.; Snyder, C.A.; Anderson, T.K.; Hutter, C.; et al. Experimental reproduction of viral replication and disease in dairy calves and lactating cows inoculated with highly pathogenic avian influenza H5N1 clade 2.3.4.4b. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kalthoff, D.; Hoffmann, B.; Harder, T.; Durban, M.; Beer, M. Experimental infection of cattle with highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, 1132–1134. [Google Scholar] [CrossRef]
- Halwe, N.J.; Cool, K.; Breithaupt, A.; Schön, J.; Trujillo, J.D.; Nooruzzaman, M.; Kwon, T.; Ahrens, A.K.; Britzke, T.; McDowell, C.D.; et al. Outcome of H5N1 clade 2.3.4.4b virus infection in calves and lactating cows. bioRxiv 2024. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Biswas, A.; Guan, L.; Gu, C.; Maemura, T.; Trifkovic, S.; Wang, T.; Babujee, L.; Dahn, R.; Halfmann, P.J.; et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 2024, 633, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Graaf, A.; Piesche, R.; Sehl-Ewert, J.; Grund, C.; Pohlmann, A.; Beer, M.; Harder, T. Low Susceptibility of Pigs against Experimental Infection with HPAI Virus H5N1 Clade 2.3.4.4b. Emerg. Infect. Dis. 2023, 29, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Trujillo, J.D.; Carossino, M.; Lyoo, E.L.; McDowell, C.D.; Cool, K.; Matias-Ferreyra, F.S.; Jeevan, T.; Morozov, I.; Gaudreault, N.N.; et al. Pigs are highly susceptible to but do not transmit mink-derived highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b. Emerg. Microbes Infect. 2024, 13, 2353292. [Google Scholar] [CrossRef]
- Siegers, J.Y.; Ferreri, L.; Eggink, D.; Veldhuis Kroeze, E.J.B.; Te Velthuis, A.J.W.; van de Bildt, M.; Leijten, L.; van Run, P.; de Meulder, D.; Bestebroer, T.; et al. Evolution of highly pathogenic H5N1 influenza A virus in the central nervous system of ferrets. PLoS Pathog. 2023, 19, e1011214. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.; Sun, X.; Pulit-Penaloza, J.; Maines, T. Fatal Infection in Ferrets after Ocular Inoculation with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg. Infect. Dis. J. 2024, 30, 1484. [Google Scholar] [CrossRef]
- Restori, K.H.; Septer, K.M.; Field, C.J.; Patel, D.R.; VanInsberghe, D.; Raghunathan, V.; Lowen, A.C.; Sutton, T.C. Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat. Commun. 2024, 15, 4112. [Google Scholar] [CrossRef]
- Hatta, M.; Hatta, Y.; Kim, J.H.; Watanabe, S.; Shinya, K.; Nguyen, T.; Lien, P.S.; Le, Q.M.; Kawaoka, Y. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog. 2007, 3, 1374–1379. [Google Scholar] [CrossRef]
- Human Infection Caused by Avian Influenza A (H5N1)-Chile. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON461 (accessed on 25 April 2024).
- de Jong, M.D.; Bach, V.C.; Phan, T.Q.; Vo, M.H.; Tran, T.T.; Nguyen, B.H.; Beld, M.; Le, T.P.; Truong, H.K.; Nguyen, V.V.; et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005, 352, 686–691. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N. Engl. J. Med. 2024, 390, 2028–2029. [Google Scholar] [CrossRef]
- CDC. Past Reported Global Human Cases with Highly Pathogenic Avian Influenza A(H5N1) (HPAI H5N1) by Country, 1997–2024. 2024. Available online: https://www.cdc.gov/bird-flu/php/avian-flu-summary/chart-epi-curve-ah5n1.html (accessed on 12 August 2024).
- State Health Officials Investigate a Detection of H5 Influenza Virus in a Human in Colorado. Available online: https://cdphe.colorado.gov/press-release/state-health-officials-investigate-a-detection-of-h5-influenza-virus-in-a-human (accessed on 25 April 2024).
- Fouchier, R.A.; Schneeberger, P.M.; Rozendaal, F.W.; Broekman, J.M.; Kemink, S.A.; Munster, V.; Kuiken, T.; Rimmelzwaan, G.F.; Schutten, M.; Van Doornum, G.J.; et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 1356–1361. [Google Scholar] [CrossRef]
- Belser, J.A.; Lash, R.R.; Garg, S.; Tumpey, T.M.; Maines, T.R. The eyes have it: Influenza virus infection beyond the respiratory tract. Lancet Infect. Dis. 2018, 18, e220–e227. [Google Scholar] [CrossRef] [PubMed]
- CDC. Technical Update: Summary Analysis of Genetic Sequences of Highly Pathogenic Avian Influenza A(H5N1) Viruses in Texas. 2024. Available online: https://www.cdc.gov/bird-flu/spotlights/h5n1-analysis-texas.html (accessed on 13 August 2024).
- Taft, A.S.; Ozawa, M.; Fitch, A.; Depasse, J.V.; Halfmann, P.J.; Hill-Batorski, L.; Hatta, M.; Friedrich, T.C.; Lopes, T.J.; Maher, E.A.; et al. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat. Commun. 2015, 6, 7491. [Google Scholar] [CrossRef] [PubMed]
- Human Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus in Chile. Available online: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/chile-first-case-h5n1-addendum.htm (accessed on 25 April 2024).
- Gabriel, G.; Dauber, B.; Wolff, T.; Planz, O.; Klenk, H.D.; Stech, J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. USA 2005, 102, 18590–18595. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.A.; Bousse, T.L.; Desmet, E.A.; Kim, B.; Takimoto, T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J. Virol. 2010, 84, 4395–4406. [Google Scholar] [CrossRef]
- Bean, W.J.; Schell, M.; Katz, J.; Kawaoka, Y.; Naeve, C.; Gorman, O.; Webster, R.G. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J. Virol. 1992, 66, 1129–1138. [Google Scholar] [CrossRef]
- Gao, R.; Gu, M.; Liu, K.; Li, Q.; Li, J.; Shi, L.; Li, X.; Wang, X.; Hu, J.; Liu, X.; et al. T160A mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Vet. Microbiol. 2018, 217, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.G.; Kim, Y.I.; Casel, M.A.B.; Choi, J.H.; Gil, J.R.; Rollon, R.; Kim, E.H.; Kim, S.M.; Ji, H.Y.; Park, D.B.; et al. HA N193D substitution in the HPAI H5N1 virus alters receptor binding affinity and enhances virulence in mammalian hosts. Emerg. Microbes Infect. 2024, 13, 2302854. [Google Scholar] [CrossRef]
- Shelton, H.; Roberts, K.L.; Molesti, E.; Temperton, N.; Barclay, W.S. Mutations in haemagglutinin that affect receptor binding and pH stability increase replication of a PR8 influenza virus with H5 HA in the upper respiratory tract of ferrets and may contribute to transmissibility. J. Gen. Virol. 2013, 94, 1220–1229. [Google Scholar] [CrossRef]
- Du, W.; de Vries, E.; van Kuppeveld, F.J.M.; Matrosovich, M.; de Haan, C.A.M. Second sialic acid-binding site of influenza A virus neuraminidase: Binding receptors for efficient release. FEBS J. 2021, 288, 5598–5612. [Google Scholar] [CrossRef]
- de Vries, E.; de Haan, C.A. Letter to the editor: Highly pathogenic influenza A(H5N1) viruses in farmed mink outbreak contain a disrupted second sialic acid binding site in neuraminidase, similar to human influenza A viruses. Eurosurveillance 2023, 28, 2300085. [Google Scholar] [CrossRef]
- Ma, W.; Brenner, D.; Wang, Z.; Dauber, B.; Ehrhardt, C.; Hogner, K.; Herold, S.; Ludwig, S.; Wolff, T.; Yu, K.; et al. The NS segment of an H5N1 highly pathogenic avian influenza virus (HPAIV) is sufficient to alter replication efficiency, cell tropism, and host range of an H7N1 HPAIV. J. Virol. 2010, 84, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Jiao, P.; Wang, A.; Zhao, F.; Tian, G.; Wang, X.; Yu, K.; Bu, Z.; Chen, H. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 2006, 80, 11115–11123. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Z.; Uprety, T.; Sreenivasan, C.C.; Sheng, Z.; Hause, B.M.; Brunick, C.; Wu, H.; Luke, T.; Bausch, C.L.; et al. A fully human monoclonal antibody possesses antibody-dependent cellular cytotoxicity (ADCC) activity against the H1 subtype of influenza A virus by targeting a conserved epitope at the HA1 protomer interface. J. Med. Virol. 2023, 95, e28901. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Pascua, P.N.Q.; Nguyen, H.T.; Chesnokov, A.; Champion, C.; Mishin, V.P.; Wentworth, D.E.; Gubareva, L.V. New insights into the neuraminidase-mediated hemagglutination activity of influenza A(H3N2) viruses. Antivir. Res. 2023, 218, 105719. [Google Scholar] [CrossRef]
- Yu, J.; Sreenivasan, C.; Sheng, Z.; Zhai, S.L.; Wollman, J.W.; Luo, S.; Huang, C.; Gao, R.; Wang, Z.; Kaushik, R.S.; et al. A recombinant chimeric influenza virus vaccine expressing the consensus H3 hemagglutinin elicits broad hemagglutination inhibition antibodies against divergent swine H3N2 influenza viruses. Vaccine 2023, 41, 6318–6326. [Google Scholar] [CrossRef]
- Yang, D.; Sun, C.; Gao, R.; Wang, H.; Liu, W.; Yu, K.; Zhou, G.; Zhao, B.; Yu, L. A Temperature-Dependent Translation Defect Caused by Internal Ribosome Entry Site Mutation Attenuates Foot-and-Mouth Disease Virus: Implications for Rational Vaccine Design. J. Virol. 2020, 94, 00990-20. [Google Scholar] [CrossRef]
- Sun, C.; Yang, D.; Gao, R.; Liang, T.; Wang, H.; Zhou, G.; Yu, L. Modification of the internal ribosome entry site element impairs the growth of foot-and-mouth disease virus in porcine-derived cells. J. Gen. Virol. 2016, 97, 901–911. [Google Scholar] [CrossRef]
- Gao, R.; Sreenivasan, C.C.; Sheng, Z.; Hause, B.M.; Zhou, B.; Wentworth, D.E.; Clement, T.; Rausch, D.; Brunick, C.; Christopher-Hennings, J.; et al. Human Monoclonal Antibody Derived from Transchromosomic Cattle Neutralizes Multiple H1 Clades of Influenza A Virus by Recognizing a Novel Conformational Epitope in the Hemagglutinin Head Domain. J. Virol. 2020, 94, 00945-20. [Google Scholar] [CrossRef]
- Nogales, A.; Martinez-Sobrido, L. Reverse Genetics Approaches for the Development of Influenza Vaccines. Int. J. Mol. Sci. 2016, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Webby, R.J.; Humberd, J.; Seiler, J.P.; Webster, R.G. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 2006, 194, 159–167. [Google Scholar] [CrossRef]
- Neumann, G.; Fujii, K.; Kino, Y.; Kawaoka, Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc. Natl. Acad. Sci. USA 2005, 102, 16825–16829. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.J.; Perez, D.R.; Coleman, J.S.; Guan, Y.; Knight, J.H.; Govorkova, E.A.; McClain-Moss, L.R.; Peiris, J.S.; Rehg, J.E.; Tuomanen, E.I.; et al. Responsiveness to a pandemic alert: Use of reverse genetics for rapid development of influenza vaccines. Lancet 2004, 363, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Panickan, S.; Bhatia, S.; Bhat, S.; Bhandari, N.; Pateriya, A.K.; Kalaiyarasu, S.; Sood, R.; Tripathi, M. Reverse genetics based H5N2 vaccine provides clinical protection against H5N1, H5N8 and H9N2 avian influenza infection in chickens. Vaccine 2022, 40, 6998–7008. [Google Scholar] [CrossRef]
- Neumann, G. Influenza Reverse Genetics-Historical Perspective. Cold Spring Harb. Perspect. Med. 2021, 11. [Google Scholar] [CrossRef]
- Tian, G.; Zeng, X.; Li, Y.; Shi, J.; Chen, H. Protective efficacy of the H5 inactivated vaccine against different highly pathogenic H5N1 avian influenza viruses isolated in China and Vietnam. Avian Dis. 2010, 54, 287–289. [Google Scholar] [CrossRef]
- Kong, H.; Zhang, Q.; Gu, C.; Shi, J.; Deng, G.; Ma, S.; Liu, J.; Chen, P.; Guan, Y.; Jiang, Y.; et al. A live attenuated vaccine prevents replication and transmission of H7N9 virus in mammals. Sci. Rep. 2015, 5, 11233. [Google Scholar] [CrossRef]
- Kozlov, M. US will vaccinate birds against avian flu for first time—What researchers think. Nature 2023, 618, 220–221. [Google Scholar] [CrossRef]
- Swayne, D.E.; Spackman, E.; Pantin-Jackwood, M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird-agricultural interface. Ecohealth 2014, 11, 94–108. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Hafez, H.M. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: Epidemiology and control challenges. Epidemiol. Infect. 2011, 139, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, S.; Grosbois, V.; Pham, T.T.; Dao, D.T.; Nguyen, T.D.; Fenwick, S.; Roger, F.; Ellis, T.; Peyre, M. Evaluation of the vaccination efficacy against H5N1 in domestic poultry in the Red River Delta in Vietnam. Epidemiol. Infect. 2013, 141, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bai, X.; Li, M.; Zeng, X.; Xu, J.; Li, P.; Wang, M.; Song, X.; Zhao, Z.; Tian, G.; et al. Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021. Emerg. Infect. Dis. 2023, 29, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Principles for vaccine protection in chickens and domestic waterfowl against avian influenza: Emphasis on Asian H5N1 high pathogenicity avian influenza. Ann. N. Y. Acad. Sci. 2006, 1081, 174–181. [Google Scholar] [CrossRef]
- Fact Sheet: USDA Continues Partner Engagement to Mitigate Highly Pathogenic Avian Influenza for 2023 Season. Available online: https://www.usda.gov/media/press-releases/2023/04/14/fact-sheet-usda-continues-partner-engagement-mitigate-highly (accessed on 27 April 2024).
- Prevention and Antiviral Treatment of Bird Flu Viruses in People. Available online: https://www.cdc.gov/flu/avianflu/prevention.htm#anchor_1647619820462 (accessed on 27 April 2024).
- Wan, X.F.; Ferguson, L.; Oliva, J.; Rubrum, A.; Eckard, L.; Zhang, X.; Woolums, A.R.; Lion, A.; Meyer, G.; Murakami, S.; et al. Limited Cross-Protection Provided by Prior Infection Contributes to High Prevalence of Influenza D Viruses in Cattle. J. Virol. 2020, 94, 00240-20. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16, 1703. https://doi.org/10.3390/v16111703
Sreenivasan CC, Li F, Wang D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses. 2024; 16(11):1703. https://doi.org/10.3390/v16111703
Chicago/Turabian StyleSreenivasan, Chithra C., Feng Li, and Dan Wang. 2024. "Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts" Viruses 16, no. 11: 1703. https://doi.org/10.3390/v16111703
APA StyleSreenivasan, C. C., Li, F., & Wang, D. (2024). Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses, 16(11), 1703. https://doi.org/10.3390/v16111703






