Understanding the Omicron Variant Impact in Healthcare Workers: Insights from the Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf) on Risk Factors for Breakthrough and Reinfections
Abstract
1. Introduction
2. Materials and Methods
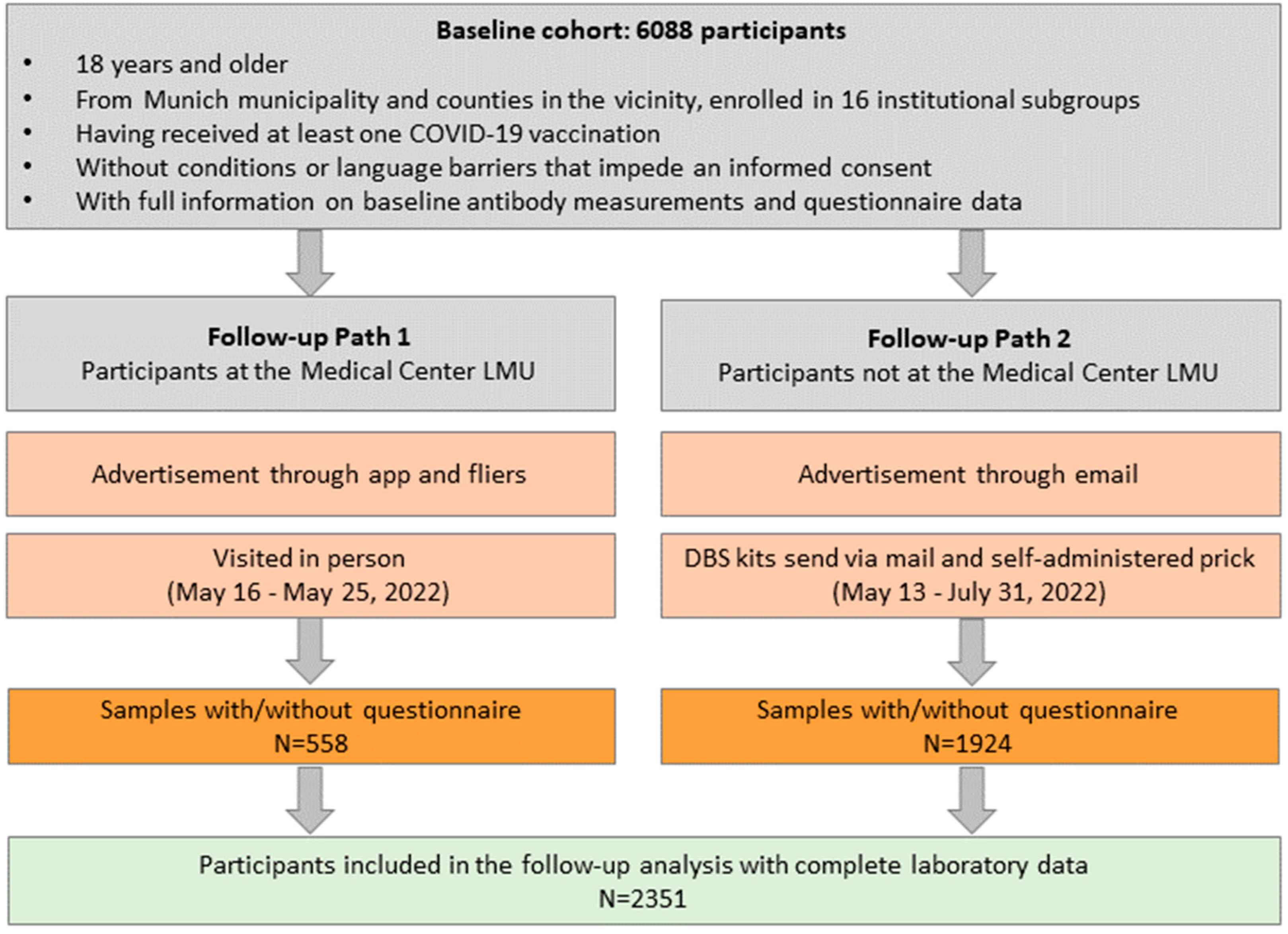
2.1. The Follow-Up Logistics for the KoCo-Impf
2.2. Specimen Collection and Laboratory Analyses
2.3. Data and Statistical Analysis
3. Results
3.1. Non-Responder Mechanism and Follow-Up Cohort Description
3.2. Development of the Antibodies over Time: Group Characterization and Vanishing Effect of Vaccination
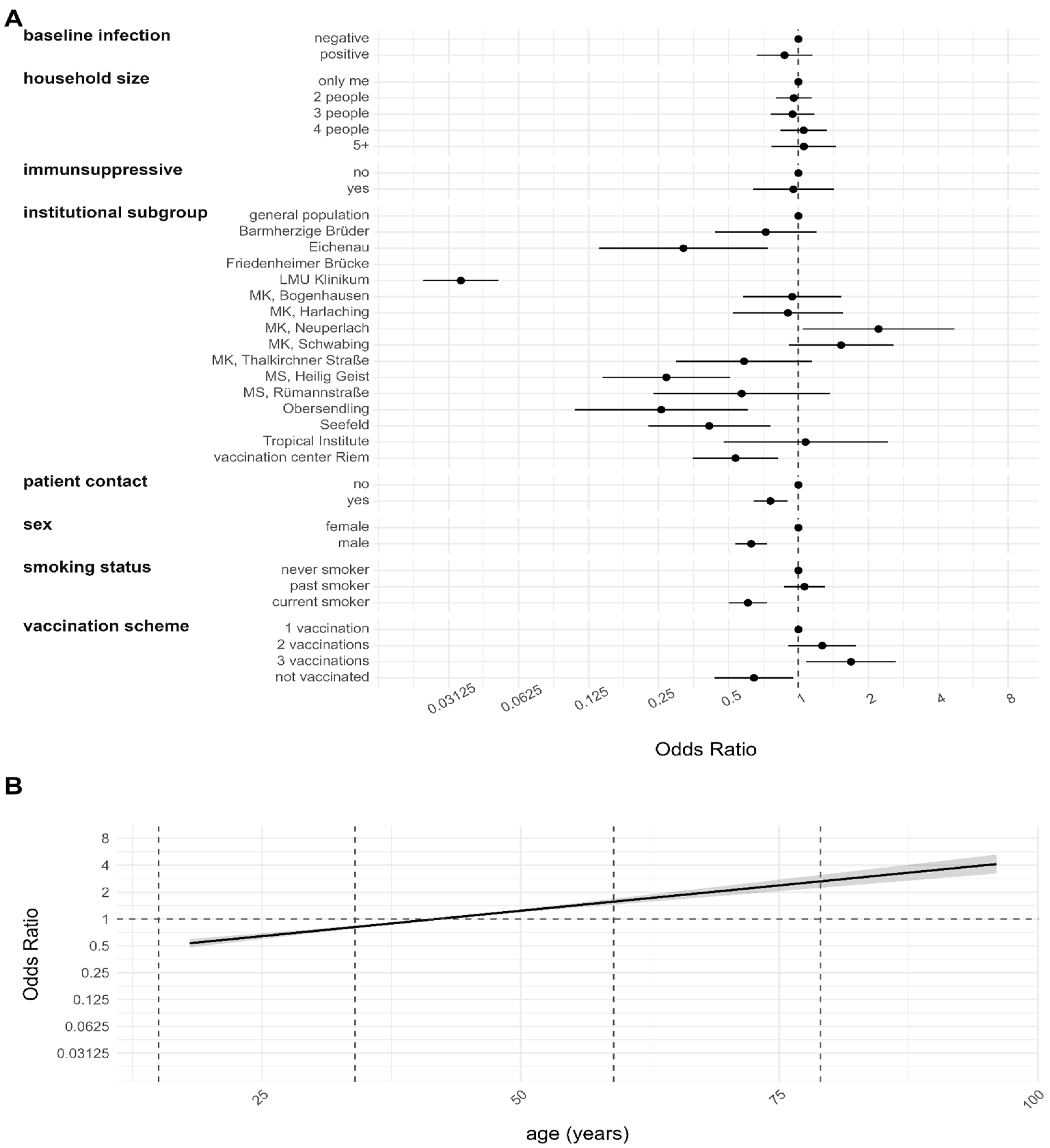
3.3. Risk Factor Analysis for the Anti-N Sero-Positivity during Different Observation Periods
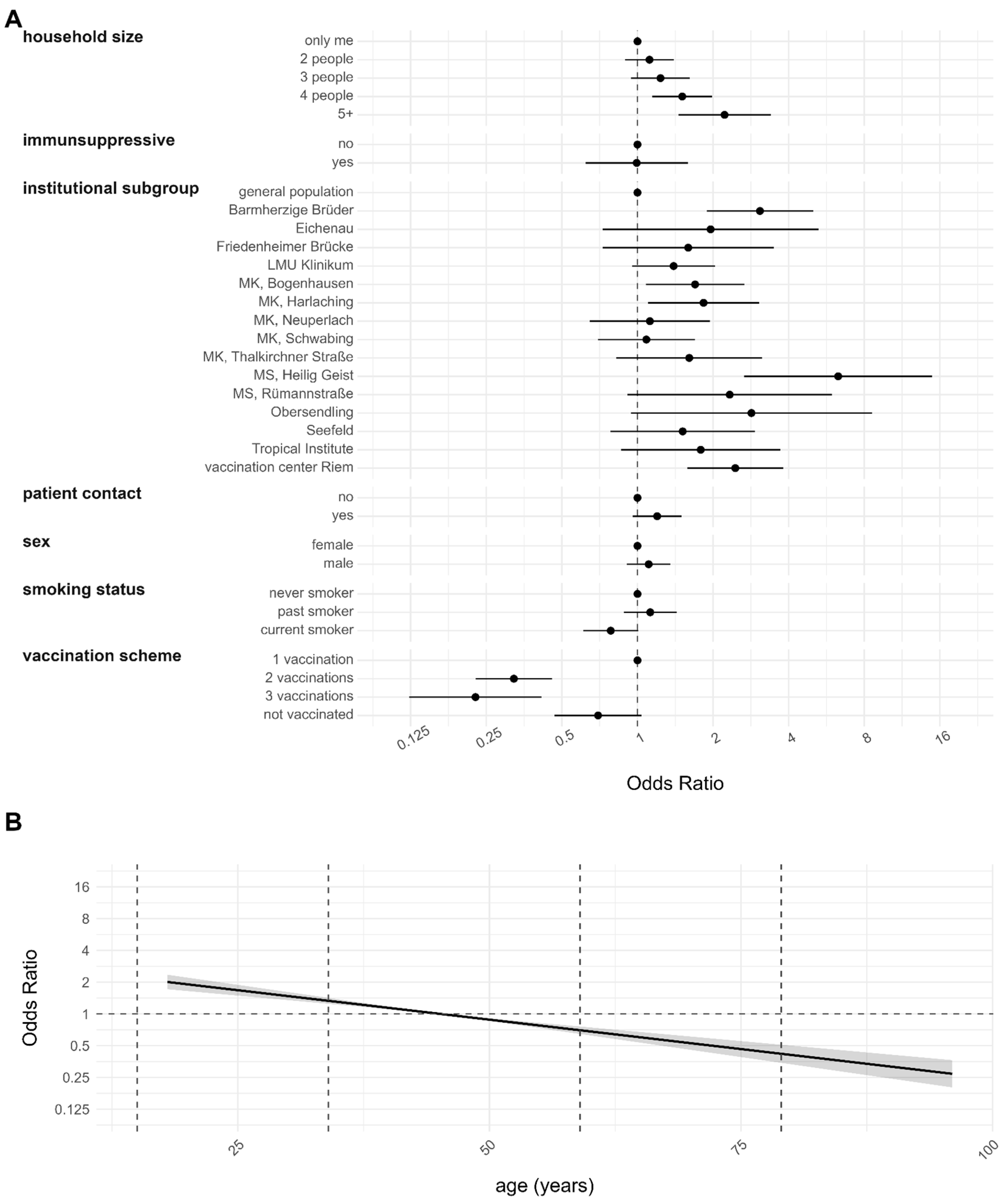
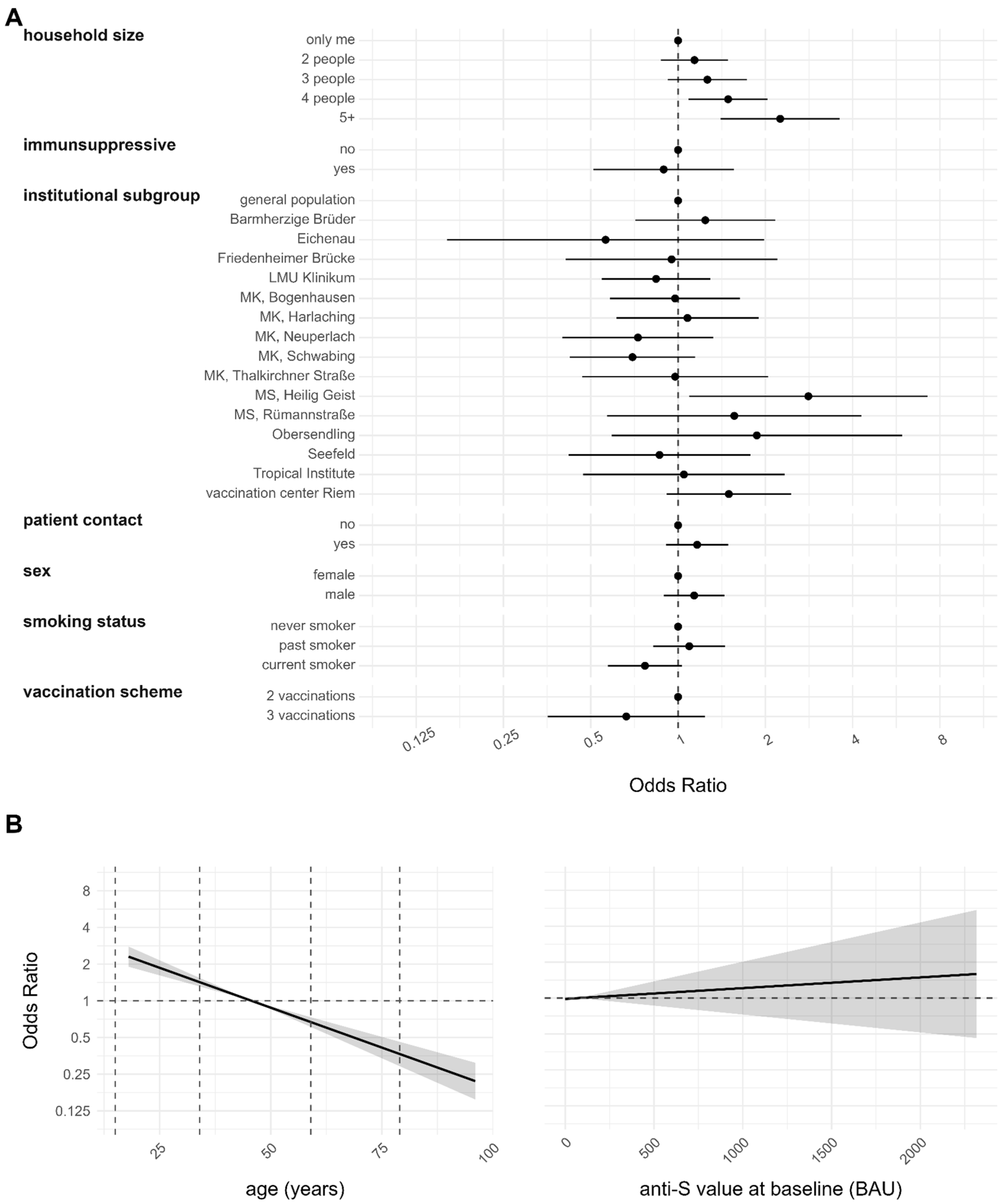
3.4. Risk Factor Analyses for Infection after Complete Vaccination and Reinfection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- WHO. Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 18 February 2024).
- WHO. COVID-19 Dashboard Showing Total Cumulative Reported COVID-19 Cases and Reported COVID-19 Deaths. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 18 February 2024).
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Soegiarto, G.; Mahdi, B.A.; Wulandari, L.; Fahmita, K.D.; Hadmoko, S.T.; Gautama, H.I.; Prasetyaningtyas, D.; Prasetyo, M.E.; Negoro, P.P.; Arafah, N.; et al. Evaluation of Antibody Response and Adverse Effects Following Heterologous COVID-19 Vaccine Booster with mRNA Vaccine among Healthcare Workers in Indonesia. Vaccines 2023, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Doernberg, S.B.; Holubar, M.; Jain, V.; Weng, Y.; Lu, D.; Bollyky, J.B.; Sample, H.; Huang, B.; Craik, C.S.; Desai, M.; et al. Incidence and Prevalence of Coronavirus Disease 2019 Within a Healthcare Worker Cohort During the First Year of the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Inghels, M.; Kane, R.; Lall, P.; Nelson, D.; Nanyonjo, A.; Asghar, Z.; Ward, D.; McCranor, T.; Kavanagh, T.; Hogue, T.; et al. Ethnicity and Risk for SARS-CoV-2 Infection among the Healthcare Workforce: Results of a Retrospective Cohort Study in Rural United Kingdom. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 122, 115–122. [Google Scholar] [CrossRef]
- Platten, M.; Nienhaus, A.; Peters, C.; Cranen, R.; Wisplinghoff, H.; Kersten, J.F.; Bach, A.D.; Michels, G. Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis. Int. J. Environ. Res. Public Health 2022, 19, 2429. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among Front-Line Health-Care Workers and the General Community: A Prospective Cohort Study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Iversen, K.; Bundgaard, H.; Hasselbalch, R.B.; Kristensen, J.H.; Nielsen, P.B.; Pries-Heje, M.; Knudsen, A.D.; Christensen, C.E.; Fogh, K.; Norsk, J.B.; et al. Risk of COVID-19 in Health-Care Workers in Denmark: An Observational Cohort Study. Lancet Infect. Dis. 2020, 20, 1401–1408. [Google Scholar] [CrossRef]
- Brophy, J.T.; Keith, M.M.; Hurley, M.; McArthur, J.E. Sacrificed: Ontario Healthcare Workers in the Time of COVID-19. New Solut. J. Environ. Occup. Health Policy NS 2021, 30, 267–281. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Brehm, T.T.; Schwinge, D.; Lampalzer, S.; Schlicker, V.; Küchen, J.; Thompson, M.; Ullrich, F.; Huber, S.; Schmiedel, S.; Addo, M.M.; et al. Seroprevalence of SARS-CoV-2 Antibodies among Hospital Workers in a German Tertiary Care Center: A Sequential Follow-up Study. Int. J. Hyg. Environ. Health 2021, 232, 113671. [Google Scholar] [CrossRef] [PubMed]
- Reinkemeyer, C.; Khazaei, Y.; Weigert, M.; Hannes, M.; Le Gleut, R.; Plank, M.; Winter, S.; Noreña, I.; Meier, T.; Xu, L.; et al. The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers. Viruses 2023, 15, 1574. [Google Scholar] [CrossRef] [PubMed]
- Sabetian, G.; Moghadami, M.; Hashemizadeh Fard Haghighi, L.; Shahriarirad, R.; Fallahi, M.J.; Asmarian, N.; Moeini, Y.S. COVID-19 Infection among Healthcare Workers: A Cross-Sectional Study in Southwest Iran. Virol. J. 2021, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.V.; Mohren, J.; Erren, T.C. COVID-19 and Healthcare Workers: A Rapid Systematic Review into Risks and Preventive Measures. BMJ Open 2021, 11, e042270. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.; Hassan Khamis, A.; Ho, S.B. COVID-19 and Healthcare Workers: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef]
- McGrath, J.; McAloon, C.G.; More, S.J.; Garrett, S.; Reidy, C.; Geary, U.; Noonan, N.; Bergin, C. Risk Factors for SARS-CoV-2 Infection in Healthcare Workers Following an Identified Nosocomial COVID-19 Exposure during Waves 1-3 of the Pandemic in Ireland. Epidemiol. Infect. 2022, 150, e186. [Google Scholar] [CrossRef]
- Le Gleut, R.; Plank, M.; Pütz, P.; Radon, K.; Bakuli, A.; Rubio-Acero, R.; Paunovic, I.; Rieß, F.; Winter, S.; Reinkemeyer, C.; et al. The Representative COVID-19 Cohort Munich (KoCo19): From the Beginning of the Pandemic to the Delta Virus Variant. BMC Infect. Dis. 2023, 23, 466. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron Is an Immune Escape Variant with an Altered Cell Entry Pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 Omicron Variants BA.1 to BA.5: Implications for Immune Escape and Transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Acero, R.; Castelletti, N.; Fingerle, V.; Olbrich, L.; Bakuli, A.; Wölfel, R.; Girl, P.; Müller, K.; Jochum, S.; Strobl, M.; et al. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-Characterized Oligo-/Asymptomatic Patients. Infect. Dis. Ther. 2021, 10, 1505–1518. [Google Scholar] [CrossRef]
- Olbrich, L.; Castelletti, N.; Schälte, Y.; Garí, M.; Pütz, P.; Bakuli, A.; Pritsch, M.; Kroidl, I.; Saathoff, E.; Guggenbuehl Noller, J.M.; et al. Head-to-Head Evaluation of Seven Different Seroassays Including Direct Viral Neutralisation in a Representative Cohort for SARS-CoV-2. J. Gen. Virol. 2021, 102, 001653. [Google Scholar] [CrossRef] [PubMed]
- Beyerl, J.; Rubio-Acero, R.; Castelletti, N.; Paunovic, I.; Kroidl, I.; Khan, Z.N.; Bakuli, A.; Tautz, A.; Oft, J.; Hoelscher, M.; et al. A Dried Blood Spot Protocol for High Throughput Analysis of SARS-CoV-2 Serology Based on the Roche Elecsys Anti-N Assay. EBioMedicine 2021, 70, 103502. [Google Scholar] [CrossRef]
- Castelletti, N.; Paunovic, I.; Rubio-Acero, R.; Beyerl, J.; Plank, M.; Reinkemeyer, C.; Kroidl, I.; Noreña, I.; Winter, S.; Olbrich, L.; et al. A Dried Blood Spot Protocol for High-Throughput Quantitative Analysis of SARS-CoV-2 RBD Serology Based on the Roche Elecsys System. Microbiol. Spectr. 2024, 12, e02885-23. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Donders, R.A.R.T.; Stijnen, T.; Harrell, F.E. Using the Outcome for Imputation of Missing Predictor Values Was Preferred. J. Clin. Epidemiol. 2006, 59, 1092–1101. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Bauer, A.; Weigert, M.; Jalal, H. APCtools: Descriptive and Model-Based Age-Period-CohortAnalysis. J. Open Source Softw. 2022, 7, 4056. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Pethe, S.; Kefayati, S.; Srinivasan, R.; Hake, P.; Deshpande, A.; Liu, X.; Hoang, E.; Davila, M.; Bianco, S.; et al. Globally Local: Hyper-Local Modeling for Accurate Forecast of COVID-19. Epidemics 2021, 37, 100510. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Visci, G.; Violante, F.S.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Larese Fillon, F.; Negro, C.; Janke, C.; Castelletti, N.; et al. Determinants of Anti-S Immune Response at 6 Months after COVID-19 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Front. Immunol. 2022, 13, 986085. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Lodi, V.; Feola, D.; De Palma, G.; Sansone, E.; Sala, E.; Janke, C.; Castelletti, N.; Porru, S.; Spiteri, G.; et al. Determinants of Anti-S Immune Response at 9 Months after COVID-19 Vaccination in a Multicentric European Cohort of Healthcare Workers-ORCHESTRA Project. Viruses 2022, 14, 2657. [Google Scholar] [CrossRef]
- Leomanni, L.; Collatuzzo, G.; Sansone, E.; Sala, E.; De Palma, G.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Basso, D.; Pavanello, S.; et al. Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Vaccines 2023, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Bakuli, A.; Pütz, P.; Le Gleut, R.; Guggenbuehl Noller, J.M.; Olbrich, L.; Saathoff, E.; Garí, M.; Schälte, Y.; Frahnow, T.; et al. From First to Second Wave: Follow-up of the Prospective COVID-19 Cohort (KoCo19) in Munich (Germany). BMC Infect. Dis. 2021, 21, 925. [Google Scholar] [CrossRef] [PubMed]
- Garfin, D.R.; Fischhoff, B.; Holman, E.A.; Silver, R.C. Risk Perceptions and Health Behaviors as COVID-19 Emerged in the United States: Results from a Probability-Based Nationally Representative Sample. J. Exp. Psychol. Appl. 2021, 27, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, S.; Andreghetti, G.R.; Mioni, G. Risk Perception towards COVID-19: A Systematic Review and Qualitative Synthesis. Int. J. Environ. Res. Public Health 2022, 19, 4649. [Google Scholar] [CrossRef]
- Sprengholz, P.; Bruckmann, R.; Wiedermann, M.; Brockmann, D.; Betsch, C. From Delta to Omicron: The Role of Individual Factors and Social Context in Self-Reported Compliance with Pandemic Regulations and Recommendations. Soc. Sci. Med. 1982 2023, 317, 115633. [Google Scholar] [CrossRef]
- Li, Y.; Yamamoto, S.; Oshiro, Y.; Inamura, N.; Nemoto, T.; Horii, K.; Takeuchi, J.S.; Mizoue, T.; Konishi, M.; Ozeki, M.; et al. Comparison of Risk Factors for SARS-CoV-2 Infection among Healthcare Workers during Omicron and Delta Dominance Periods in Japan. J. Hosp. Infect. 2023, 134, 97–107. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Slama, N.; Alexeeff, S.E.; Sakoda, L.C.; Fogelberg, R.; Myers, L.C.; Campbell, C.I.; Adams, A.S.; Prochaska, J.J. Tobacco Smoking and Risk of SARS-CoV-2 Infection and Disease Severity Among Adults in an Integrated Healthcare System in California. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2023, 25, 211–220. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Slama, N.; Sakoda, L.C.; Prochaska, J.J.; Fogelberg, R.; Alexeeff, S.E. Current Tobacco Smoking and Risk of SARS-CoV-2 Infection and Hospitalization: Evaluating the Role of Socio-Demographic Factors and Comorbidities. Prev. Med. 2023, 172, 107523. [Google Scholar] [CrossRef]
- Paleiron, N.; Mayet, A.; Marbac, V.; Perisse, A.; Barazzutti, H.; Brocq, F.-X.; Janvier, F.; Dautzenberg, B.; Bylicki, O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2021, 23, 1398–1404. [Google Scholar] [CrossRef]
- Li, W.; Zheng, R.; Liang, R.; Wu, B.; Wang, C.; Zhuo, L.; Wu, M.; Jie, Y.; Lin, B.; Chang, L.; et al. Effects of Smoking on ACE2 Expression Pattern: Risk and Severity of SARS-CoV-2 Infection. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Günther, F.; Einhauser, S.; Peterhoff, D.; Wiegrebe, S.; Niller, H.H.; Beileke, S.; Steininger, P.; Burkhardt, R.; Küchenhoff, H.; Gefeller, O.; et al. Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study. Int. J. Environ. Res. Public. Health 2022, 19, 16996. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Le Thi, T.G.; Osterman, A.; Badell, I.; Huber, M.; Zhelyazkova, A.; Wichert, S.P.; Litwin, A.; Hörmansdorfer, S.; Strobl, F.; et al. Dietary Habits, Traveling and the Living Situation Potentially Influence the Susceptibility to SARS-CoV-2 Infection: Results from Healthcare Workers Participating in the RisCoin Study. Infection 2024, 52, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Misra-Hebert, A.D.; Jehi, L.; Ji, X.; Nowacki, A.S.; Gordon, S.; Terpeluk, P.; Chung, M.K.; Mehra, R.; Dell, K.M.; Pennell, N.; et al. Impact of the COVID-19 Pandemic on Healthcare Workers’ Risk of Infection and Outcomes in a Large, Integrated Health System. J. Gen. Intern. Med. 2020, 35, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Langlete, P.; Tesli, M.; Veneti, L.; Starrfelt, J.; Elstrøm, P.; Meijerink, H. Estimated Vaccine Effectiveness against SARS-CoV-2 Delta and Omicron Infections among Health Care Workers and the General Adult Population in Norway, August 2021–January 2022. Vaccine 2023, 41, 3923–3929. [Google Scholar] [CrossRef]
- Wong, S.-C.; Chan, V.W.-M.; Yuen, L.L.-H.; AuYeung, C.H.-Y.; Leung, J.O.-Y.; Li, C.-K.; Kwok, M.O.-T.; So, S.Y.-C.; Chen, J.H.-K.; Chiu, K.H.-Y.; et al. Infection of Healthcare Workers despite a High Vaccination Rate during the Fifth Wave of COVID-19 Due to Omicron Variant in Hong Kong. Infect. Prev. Pract. 2023, 5, 100261. [Google Scholar] [CrossRef]
- Cegolon, L.; Negro, C.; Mastrangelo, G.; Filon, F.L.; ORCHESTRA Working Group. Primary SARS-CoV-2 Infections, Re-Infections and Vaccine Effectiveness during the Omicron Transmission Period in Healthcare Workers of Trieste and Gorizia (Northeast Italy), 1 December 2021–31 May 2022. Viruses 2022, 14, 2688. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Atti, A.; Insalata, F.; Carr, E.J.; Otter, A.D.; Castillo-Olivares, J.; Wu, M.; Harvey, R.; Howell, M.; Chan, A.; Lyall, J.; et al. Antibody Correlates of Protection from SARS-CoV-2 Reinfection Prior to Vaccination: A Nested Case-Control within the SIREN Study. J. Infect. 2022, 85, 545–556. [Google Scholar] [CrossRef]
- Porru, S.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Caliskan, G.; Violán, C.; Torán-Monserrat, P.; Vimercati, L.; Tafuri, S.; Boffetta, P.; et al. Incidence and Determinants of Symptomatic and Asymptomatic SARS-CoV-2 Breakthrough Infections After Booster Dose in a Large European Multicentric Cohort of Health Workers-ORCHESTRA Project. J. Epidemiol. Glob. Health 2023, 13, 577–588. [Google Scholar] [CrossRef]
- COVID-19 Forecasting Team. Past SARS-CoV-2 Infection Protection against Re-Infection: A Systematic Review and Meta-Analysis. Lancet Lond. Engl. 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Dowell, A.C.; Waiblinger, D.; Wright, J.; Ladhani, S.N.; Moss, P.; sKIDS Investigation Team. Nucleocapsid-Specific Antibodies as a Correlate of Protection against SARS-CoV-2 Reinfection in Children. J. Infect. 2023, 87, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Batra, M.; Tian, R.; Zhang, C.; Clarence, E.; Sacher, C.S.; Miranda, J.N.; De La Fuente, J.R.O.; Mathew, M.; Green, D.; Patel, S.; et al. Role of IgG against N-Protein of SARS-CoV2 in COVID19 Clinical Outcomes. Sci. Rep. 2021, 11, 3455. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 Specific IgM and IgG Responses in COVID-19 Patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Kroidl, I.; Winter, S.; Rubio-Acero, R.; Bakuli, A.; Geldmacher, C.; Eser, T.M.; Déak, F.; Horn, S.; Zielke, A.; Ahmed, M.I.M.; et al. Studying Temporal Titre Evolution of Commercial SARS-CoV-2 Assays Reveals Significant Shortcomings of Using BAU Standardization for Comparison. Virol. J. 2023, 20, 200. [Google Scholar] [CrossRef]
- Gómez-Gonzales, W.; Chihuantito-Abal, L.A.; Gamarra-Bustillos, C.; Morón-Valenzuela, J.; Zavaleta-Oliver, J.; Gomez-Livias, M.; Vargas-Pancorbo, L.; Auqui-Canchari, M.E.; Mejía-Zambrano, H. Risk Factors Contributing to Reinfection by SARS-CoV-2: A Systematic Review. Adv. Respir. Med. 2023, 91, 560–570. [Google Scholar] [CrossRef]
- Conant, R.C.; Ross Ashby, W. Every Good Regulator of a System Must Be a Model of That System †. Int. J. Syst. Sci. 1970, 1, 89–97. [Google Scholar] [CrossRef]

| Covariate | Category | Number of Participants n (%) | Qualitative Anti-N n (%) | Breakthrough Infection n (%) | Reinfection n (%) | |||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Yes | No | Yes | No | |||
| Overall cohort | 2351 (100.0%) | 1036 (44.1%) | 1315 (55.9%) | 695 (38.8%) | 1098 (61.2%) | 84 (48.0%) | 91 (52.0%) | |
| Sex | Female | 1740 (74.0%) | 741 (42.6%) | 999 (57.4%) | 520 (37.3%) | 874 (62.7%) | 59 (48.8%) | 62 (51.2%) |
| Male | 611 (26.0%) | 295 (48.3%) | 316 (51.7%) | 175 (43.9%) | 224 (56.1%) | 25 (46.3%) | 29 (53.7%) | |
| Institutional subgroup | Barmherzige Brüder | 141 (6.0%) | 83 (58.9%) | 58 (41.1%) | 50 (46.3%) | 58 (53.7%) | 13 (39.4%) | 20 (60.6%) |
| Eichenau | 22 (0.9%) | 9 (40.9%) | 13 (59.1%) | 4 (23.5%) | 13 (76.5%) | 2 (40.0%) | 3 (60.0%) | |
| Friedenheimer Brücke | 34 (1.4%) | 14 (41.2%) | 20 (58.8%) | 12 (37.5%) | 20 (62.5%) | 1 (100.0%) | 0 (0.0%) | |
| General population | 504 (21.4%) | 232 (46.0%) | 272 (54.0%) | 50 (39.1%) | 78 (60.9%) | 17 (53.1%) | 15 (46.9%) | |
| Medical Center of LMU | 527 (22.4%) | 200 (38.0%) | 327 (62.0%) | 175 (35.2%) | 322 (64.8%) | 8 (32.0%) | 17 (68.0%) | |
| MK, Bogenhausen | 193 (8.2%) | 87 (45.1%) | 106 (54.9%) | 64 (38.6%) | 102 (61.4%) | 10 (55.6%) | 8 (44.4%) | |
| MK, Harlaching | 124 (5.3%) | 58 (46.8%) | 66 (53.2%) | 46 (41.8%) | 64 (58.2%) | 4 (33.3%) | 8 (66.7%) | |
| MK, Neuperlach | 102 (4.3%) | 34 (33.3%) | 68 (66.7%) | 30 (30.9%) | 67 (69.1%) | 3 (75.0%) | 1 (25.0%) | |
| MK, Schwabing | 248 (10.5%) | 78 (31.5%) | 170 (68.5%) | 66 (28.6%) | 165 (71.4%) | 7 (58.3%) | 5 (41.7%) | |
| MK, Thalkirchner St. | 51 (2.2%) | 20 (39.2%) | 31 (60.8%) | 16 (34.8%) | 30 (65.2%) | 1 (50.0%) | 1 (50.0%) | |
| MS, Heilig Geist | 32 (1.4%) | 23 (71.9%) | 9 (28.1%) | 14 (60.9%) | 9 (39.1%) | 4 (66.7%) | 2 (33.3%) | |
| MS, Rümannstraße | 27 (1.1%) | 12 (44.4%) | 15 (55.6%) | 10 (41.7%) | 14 (58.3%) | 0 (0.0%) | 2 (100.0%) | |
| Obersendling | 15 (0.6%) | 8 (53.3%) | 7 (46.7%) | 7 (50.0%) | 7 (50.0%) | 0 (0.0%) | 1 (100.0%) | |
| Seefeld | 57 (2.4%) | 22 (38.6%) | 35 (61.4%) | 18 (34.0%) | 35 (66.0%) | 3 (75.0%) | 1 (25.0%) | |
| Tropical Institute | 39 (1.7%) | 20 (51.3%) | 19 (48.7%) | 16 (47.1%) | 18 (52.9%) | 1 (50.0%) | 1 (50.0%) | |
| Vaccination center Riem | 235 (10.0%) | 136 (57.9%) | 99 (42.1%) | 117 (54.9%) | 96 (45.1%) | 10 (62.5%) | 6 (37.5%) | |
| Contact with patients | Yes | 1211 (51.5%) | 544 (44.9%) | 667 (55.1%) | 431 (39.9%) | 648 (60.1%) | 43 (44.3%) | 54 (55.7%) |
| No | 762 (32.4%) | 308 (40.4%) | 454 (59.6%) | 178 (35.2%) | 328 (64.8%) | 23 (51.1%) | 22 (48.9%) | |
| Unknown * | 378 (16.1%) | 184 (48.7%) | 194 (51.3%) | 86 (41.3%) | 122 (58.7%) | 18 (54.5%) | 15 (45.5%) | |
| Smoking status | Never smoker | 1636 (69.6%) | 735 (44.9%) | 901 (55.1%) | 500 (40.0%) | 750 (60.0%) | 60 (50.0%) | 60 (50.0%) |
| Current smoker | 343 (14.6%) | 136 (39.7%) | 207 (60.3%) | 91 (34.1%) | 176 (65.9%) | 9 (36.0%) | 16 (64.0%) | |
| Past smoker | 367 (15.6%) | 164 (44.7%) | 203 (55.3%) | 103 (38.0%) | 168 (62.0%) | 15 (50.0%) | 15 (50.0%) | |
| Unknown * | 5 (0.2%) | 1 (20.0%) | 4 (80.0%) | 1 (20.0%) | 4 (80.0%) | - | - | |
| Vaccination scheme | No vaccination ** | 242 (10.3%) | 119 (49.2%) | 123 (50.8%) | - | - | 13 (54.2%) | 11 (45.8%) |
| One vaccination | 226 (9.6%) | 139 (61.5%) | 87 (38.5%) | - | - | 33 (54.1%) | 28 (45.9%) | |
| Two vaccinations | 1779 (75.7%) | 744 (41.8%) | 1035 (58.2%) | 665 (39.3%) | 1028 (60.7%) | 36 (41.9%) | 50 (58.1%) | |
| Three vaccinations | 104 (4.4%) | 34 (32.7%) | 70 (67.3%) | 30 (30.0%) | 70 (70.0%) | 2 (50.0%) | 2 (50.0%) | |
| Household size | One person | 667 (28.4%) | 262 (39.3%) | 405 (60.7%) | 163 (33.3%) | 327 (66.7%) | 23 (43.4%) | 30 (56.6%) |
| 2 people | 803 (34.2%) | 333 (41.5%) | 470 (58.5%) | 219 (35.9%) | 391 (64.1%) | 27 (47.4%) | 30 (52.6%) | |
| 3 people | 367 (15.6%) | 169 (46.0%) | 198 (54.0%) | 121 (41.3%) | 172 (58.7%) | 12 (50.0%) | 12 (50.0%) | |
| 4 people | 349 (14.8%) | 176 (50.4%) | 173 (49.6%) | 119 (44.1%) | 151 (55.9%) | 15 (50.0%) | 15 (50.0%) | |
| 5+ people | 119 (5.1%) | 73 (61.3%) | 46 (38.7%) | 54 (58.1%) | 39 (41.9%) | 5 (62.5%) | 3 (37.5%) | |
| Unknown * | 46 (2.0%) | 23 (50.0%) | 23 (50.0%) | 19 (51.4%) | 18 (48.6%) | 2 (66.7%) | 1 (33.3%) | |
| Intake of immunosupp. drugs | Yes | 83 (3.5%) | 34 (41.0%) | 49 (59.0%) | 20 (31.7%) | 43 (68.3%) | 2 (40.0%) | 3 (60.0%) |
| No | 2252 (95.8%) | 996 (44.2%) | 1256 (55.8%) | 672 (39.0%) | 1049 (61.0%) | 81 (48.2%) | 87 (51.8%) | |
| Unknown * | 16 (0.7%) | 6 (37.5%) | 10 (62.5%) | 3 (33.3%) | 6 (66.7%) | 1 (50.0%) | 1 (50.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janke, C.; Rubio-Acero, R.; Weigert, M.; Reinkemeyer, C.; Khazaei, Y.; Kleinlein, L.; Le Gleut, R.; Radon, K.; Hannes, M.; Picasso, F.; et al. Understanding the Omicron Variant Impact in Healthcare Workers: Insights from the Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf) on Risk Factors for Breakthrough and Reinfections. Viruses 2024, 16, 1556. https://doi.org/10.3390/v16101556
Janke C, Rubio-Acero R, Weigert M, Reinkemeyer C, Khazaei Y, Kleinlein L, Le Gleut R, Radon K, Hannes M, Picasso F, et al. Understanding the Omicron Variant Impact in Healthcare Workers: Insights from the Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf) on Risk Factors for Breakthrough and Reinfections. Viruses. 2024; 16(10):1556. https://doi.org/10.3390/v16101556
Chicago/Turabian StyleJanke, Christian, Raquel Rubio-Acero, Maximilian Weigert, Christina Reinkemeyer, Yeganeh Khazaei, Lisa Kleinlein, Ronan Le Gleut, Katja Radon, Marlene Hannes, Francesco Picasso, and et al. 2024. "Understanding the Omicron Variant Impact in Healthcare Workers: Insights from the Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf) on Risk Factors for Breakthrough and Reinfections" Viruses 16, no. 10: 1556. https://doi.org/10.3390/v16101556
APA StyleJanke, C., Rubio-Acero, R., Weigert, M., Reinkemeyer, C., Khazaei, Y., Kleinlein, L., Le Gleut, R., Radon, K., Hannes, M., Picasso, F., Lucke, A. E., Plank, M., Kotta, I. C., Paunovic, I., Zhelyazkova, A., Noreña, I., Winter, S., Hoelscher, M., Wieser, A., ... on behalf of the ORCHESTRA Working Group. (2024). Understanding the Omicron Variant Impact in Healthcare Workers: Insights from the Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf) on Risk Factors for Breakthrough and Reinfections. Viruses, 16(10), 1556. https://doi.org/10.3390/v16101556






