Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways
Abstract
1. Impact Statement
2. Introduction
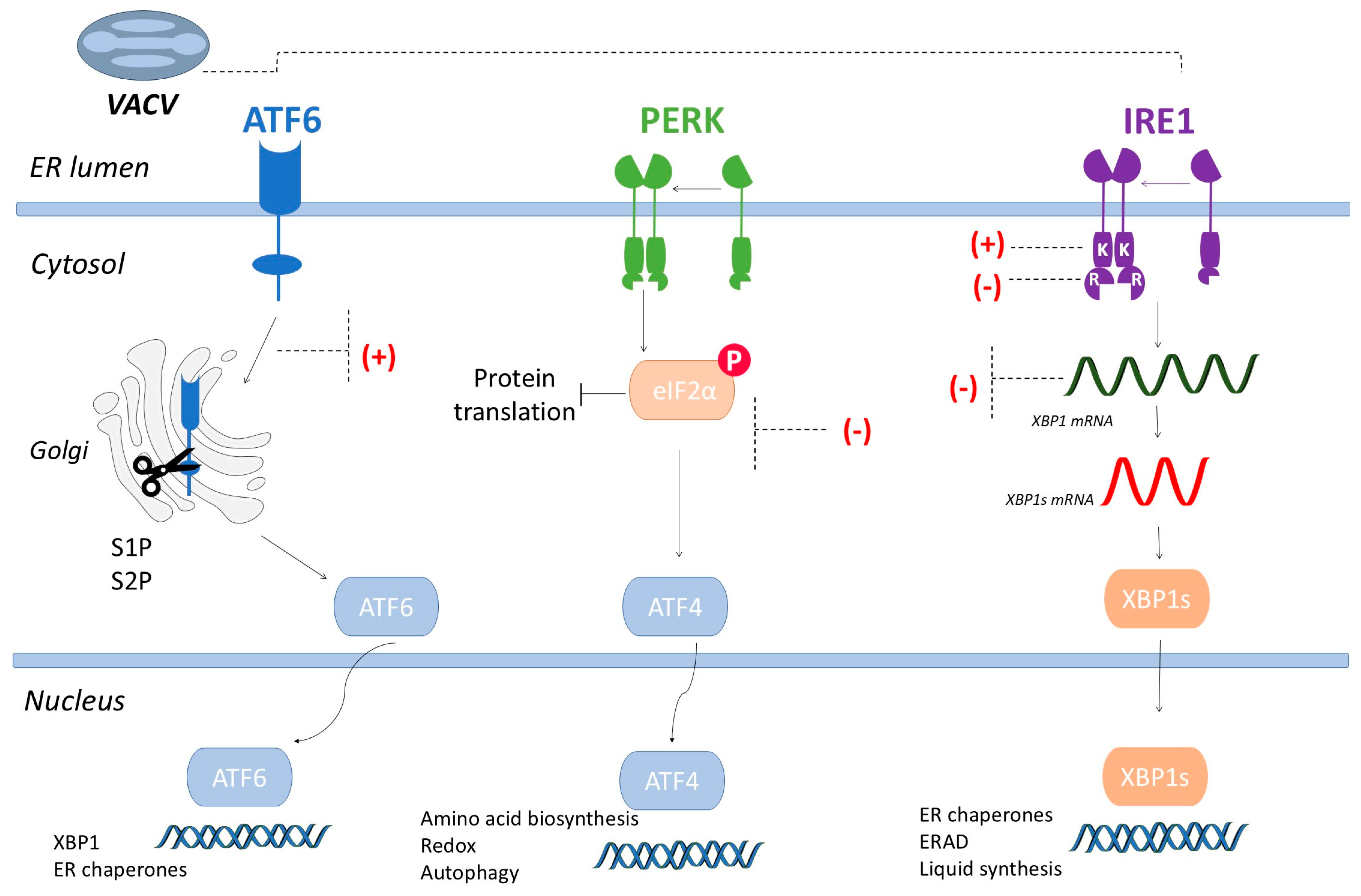
2.1. Endoplasmic Reticulum Stress Sensors
2.2. UPR Pathway and Viral Infections
| Virus * | Viral Protein | Affected UPR Pathway Receptor | Reference |
|---|---|---|---|
| SARS-CoV (Betacoronavirus pandemicum) | 8ab protein | ATF6 activation | [47] |
| Epstein Barr (Lymphocryptovirus humangamma4) | LMP-1 | Activation of PERK, IRE1, and ATF6 | [48,49] |
| Murine cytomegalovirus (Muromegalovirus muridbeta1) | M50 | Negatively regulates IREI | [50] |
| Human cytomegalovirus (Cytomegalovirus humanbeta5) | UL50 | Negatively regulates IREI | [50] |
| Human herpesvirus 1 (Simplexvirus humanalpha1) | UL41 | Reduced XBP1 expression | [52] |
| Dengue virus type 2 (Orthoflavivirus denguei) | NS2B | Induction of XBP1 processing | [56] |
| Adeno-associated virus vectors based on serotype (AAV) 2 | Not described | Activation of IRE1 and PERK | [39] |
| African swine fever virus | K205R | Activation of ATF6, IREI, and PERK | [53] |
| Zika virus (Orthoflavivirus zikaense) | Not described | Activation of IRE1–XBP1 and PERK–ATF4 | [58,59,60] |
| Myxoma virus (Leporipoxvirus myxoma) | Not described | Activation of ATF6 and inhibition of CHOP, ATF4, and XBP1 | [63] |
| Vaccinia virus (Orthopoxvirus vaccínia) | Not described | ATF6 activation, XBP1 inhibition | [67,68] |
2.3. UPR and Future Perspectives: Optimization of Heterologous Expression Using Viral Vectors and Vaccine Production
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, S.H.; von Heijne, G. How translocons select transmembrane helices. Annu. Rev. Biophys. 2008, 37, 23–42. [Google Scholar] [CrossRef]
- Johnson, A.E. The structural and functional coupling of two molecular machines, the ribosome and the translocon. J. Cell Biol. 2009, 185, 765–767. [Google Scholar] [CrossRef][Green Version]
- Fagone, P.; Jackowski, S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009, 50, S311–S316. [Google Scholar] [CrossRef]
- Ravindran, M.S.; Bagchi, P.; Cunningham, C.N.; Tsai, B. Opportunistic intruders: How viruses orchestrate ER functions to infect cells. Nat. Rev. Microbiol. 2016, 14, 407–420. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A. Virus-induced ER stress and the unfolded protein response. Front. Plant Sci. 2012, 3, 293. [Google Scholar] [CrossRef]
- Mei, Y.; Thompson, M.D.; Cohen, R.A.; Tong, X. Endoplasmic reticulum stress and related pathological processes. J. Pharmacol. Biomed. Anal. 2013, 1, 1000107. [Google Scholar]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Hussain, S.G.; Ramaiah, K.V. Endoplasmic reticulum: Stress, signalling and apoptosis. Curr. Sci. 2007, 93, 1684–1696. [Google Scholar]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef]
- Satpute-Krishnan, P.; Ajinkya, M.; Bhat, S.; Itakura, E.; Hegde, R.S.; Lippincott-Schwartz, J. ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell 2014, 158, 522–533. [Google Scholar] [CrossRef]
- Volmer, R.; Ron, D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Biol. 2015, 33, 67–73. [Google Scholar] [CrossRef]
- Mori, K. Signalling pathways in the unfolded protein response: Development from yeast to mammals. J. Biochem. 2009, 146, 743–750. [Google Scholar] [CrossRef]
- Bernales, S.; Papa, F.R.; Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006, 22, 487–508. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef]
- Woehlbier, U.; Hetz, C. Modulating stress responses by the UPRosome: A matter of life and death. Trends Biochem. Sci. 2011, 36, 329–337. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Nishitoh, H. CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef]
- Hosoi, T.; Ozawa, K. Endoplasmic reticulum stress in disease: Mechanisms and therapeutic opportunities. Clin. Sci. 2010, 118, 19–29. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Sadighi Akha, A.A.; Raden, D.; Kaufman, R.J. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006, 4, e374. [Google Scholar] [CrossRef]
- Takayanagi, S.; Fukuda, R.; Takeuchi, Y.; Tsukada, S.; Yoshida, K. Gene regulatory network of unfolded protein response genes in endoplasmic reticulum stress. Cell Stress Chaperones 2013, 18, 11–23. [Google Scholar] [CrossRef]
- He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006, 13, 393–403. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Kamimura, D.; Bevan, M.J. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. J. Immunol. 2008, 181, 5433–5441. [Google Scholar] [CrossRef]
- Roach, J.C.; Smith, K.D.; Strobe, K.L.; Nissen, S.M.; Haudenschild, C.D.; Zhou, D.; Vasicek, T.J.; Held, G.A.; Stolovitzky, G.A.; Hood, L.E. Transcription factor expression in lipopolysaccharide-activated peripheral-blood-derived mononuclear cells. Proc. Natl. Acad. Sci. USA 2007, 104, 16245–16250. [Google Scholar] [CrossRef]
- Richardson, C.E.; Kooistra, T.; Kim, D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010, 463, 1092–1095. [Google Scholar] [CrossRef]
- Jiang, H.; Zou, J.; Zhang, H.; Fu, W.; Zeng, T.; Huang, H.; Zhou, F.; Hou, J. Unfolded protein response inducers tunicamycin and dithiothreitol promote myeloma cell differentiation mediated by XBP-1. Clin. Exp. Med. 2015, 15, 85–96. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006, 86, 1133–1149. [Google Scholar] [CrossRef]
- Shen, J.; Snapp, E.L.; Lippincott-Schwartz, J.; Prywes, R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol. Cell. Biol. 2005, 25, 921–932. [Google Scholar] [CrossRef]
- Pincus, D.; Chevalier, M.W.; Aragón, T.; van Anken, E.; Vidal, S.E.; El-Samad, H.; Walter, P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010, 8, e1000415. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Zhang, Y.; Ron, D.; Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 2009, 4, e4170. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Kaser, A.; Kaufman, R.J.; Blumberg, R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016, 16, 469–484. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.-S.; Lee, H.J.; Kim, D.H.; Noh, Y.H.; Yu, K.; Jung, H.-Y.; Lee, S.H.; Lee, J.Y.; Youn, Y.C. Activation of PERK signaling attenuates Aβ-mediated ER stress. PLoS ONE 2010, 5, e10489. [Google Scholar] [CrossRef]
- Janssens, S.; Pulendran, B.; Lambrecht, B.N. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 2014, 15, 910–919. [Google Scholar] [CrossRef]
- Hooper, P.L.; Hightower, L.E.; Hooper, P.L. Loss of stress response as a consequence of viral infection: Implications for disease and therapy. Cell Stress Chaperones 2012, 17, 647–655. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Sen, D.; Hareendran, S.; Roshini, V.; David, S.; Srivastava, A.; Jayandharan, G.R. Activation of the cellular unfolded protein response by recombinant adeno-associated virus vectors. PLoS ONE 2013, 8, e53845. [Google Scholar] [CrossRef]
- Galindo Barreales, I.; Hernáez, B.; Muñoz-Moreno, R.; Cuesta-Geijo, M.A.; Dalmau-Mena, I.; Alonso, C. The ATF6 branch of unfolded protein response and apoptosis are activated to promote African swine fever virus infection. Cell Death Dis. 2012, 3, e341. [Google Scholar] [CrossRef]
- Versteeg, G.A.; Van De Nes, P.S.; Bredenbeek, P.J.; Spaan, W.J. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J. Virol. 2007, 81, 10981–10990. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Harris, E. Early dengue virus protein synthesis induces extensive rearrangement of the endoplasmic reticulum independent of the UPR and SREBP-2 pathway. PLoS ONE 2012, 7, e38202. [Google Scholar] [CrossRef] [PubMed]
- Burnett, H.F.; Audas, T.E.; Liang, G.; Lu, R.R. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones 2012, 17, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, J.L.; Sorondo, O.; Alcala, A.; Vizzi, E.; Diaz, Y.; Ruiz, M.C.; Michelangeli, F.; Liprandi, F.; Ludert, J.E. Rotavirus infection of cells in culture induces activation of RhoA and changes in the actin and tubulin cytoskeleton. PLoS ONE 2012, 7, e47612. [Google Scholar] [CrossRef] [PubMed]
- Borsa, M. Avaliação da ativação da via UPR (Unfolded Protein Response) em células de indivíduos HIV Positivos sob Diferentes Esquemas Terapêuticos. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2012. [Google Scholar]
- Borsa, M.; Ferreira, P.L.; Petry, A.; Ferreira, L.G.; Camargo, M.M.; Bou-Habib, D.C.; Pinto, A.R. HIV infection and antiretroviral therapy lead to unfolded protein response activation. Virol. J. 2015, 12, 77. [Google Scholar] [CrossRef]
- Sung, S.-C.; Chao, C.-Y.; Jeng, K.-S.; Yang, J.-Y.; Lai, M.M. The 8ab protein of SARS-CoV is a luminal ER membrane-associated protein and induces the activation of ATF6. Virology 2009, 387, 402–413. [Google Scholar] [CrossRef]
- Granato, M.; Romeo, M.A.; Tiano, M.S.; Santarelli, R.; Gonnella, R.; Montani, M.S.G.; Faggioni, A.; Cirone, M. Bortezomib promotes KHSV and EBV lytic cycle by activating JNK and autophagy. Sci. Rep. 2017, 7, 13052. [Google Scholar] [CrossRef]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A.S. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef]
- Stahl, S.; Burkhart, J.M.; Hinte, F.; Tirosh, B.; Mohr, H.; Zahedi, R.P.; Sickmann, A.; Ruzsics, Z.; Budt, M.; Brune, W. Cytomegalovirus downregulates IRE1 to repress the unfolded protein response. PLoS Pathog. 2013, 9, e1003544. [Google Scholar] [CrossRef]
- Shigemi, Z.; Baba, Y.; Hara, N.; Matsuhiro, J.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Effects of ER stress on unfolded protein responses, cell survival, and viral replication in primary effusion lymphoma. Biochem. Biophys. Res. Commun. 2016, 469, 565–572. [Google Scholar] [CrossRef]
- Zhang, P.; Su, C.; Jiang, Z.; Zheng, C. Herpes simplex virus 1 UL41 protein suppresses the IRE1/XBP1 signal pathway of the unfolded protein response via its RNase activity. J. Virol. 2017, 91, e02056-16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, L.; Wang, J.; Su, D.; Li, D.; Du, Y.; Yang, G.; Zhang, G.; Chu, B. African swine fever virus K205r induces ER stress and consequently activates autophagy and the NF-κB signaling pathway. Viruses 2022, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Umareddy, I.; Pluquet, O.; Wang, Q.Y.; Vasudevan, S.G.; Chevet, E.; Gu, F. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol. J. 2007, 4, 91. [Google Scholar] [CrossRef]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M. A nuclear transport inhibitor that modulates the unfolded protein response and provides in vivo protection against lethal dengue virus infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Hsu, Y.-W.; Liao, C.-L.; Lin, Y.-L. Flavivirus Infection Activates the XBP1 Pathway of the Unfolded Protein Response To Cope with Endoplasmic Reticulum Stress. J. Virol. 2006, 80, 11868–11880. [Google Scholar] [CrossRef]
- Roth, H.; Magg, V.; Uch, F.; Mutz, P.; Klein, P.; Haneke, K.; Lohmann, V.; Bartenschlager, R.; Fackler, O.T.; Locker, N. Flavivirus infection uncouples translation suppression from cellular stress responses. mBio 2017, 8, e02150-16. [Google Scholar] [CrossRef]
- Amorim, R.; Temzi, A.; Griffin, B.D.; Mouland, A.J. Zika virus inhibits eIF2α-dependent stress granule assembly. PLoS Neglected Trop. Dis. 2017, 11, e0005775. [Google Scholar] [CrossRef] [PubMed]
- Gladwyn-Ng, I.; Cordón-Barris, L.; Alfano, C.; Creppe, C.; Couderc, T.; Morelli, G.; Thelen, N.; America, M.; Bessières, B.; Encha-Razavi, F. Stress-induced unfolded protein response contributes to Zika virus–associated microcephaly. Nat. Neurosci. 2018, 21, 63–71. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, W.; Sun, J.; Fu, Z.; Ke, X.; Zheng, C.; Zhang, Y.; Li, P.; Liu, Y.; Hu, Q. ZIKV infection activates the IRE1-XBP1 and ATF6 pathways of unfolded protein response in neural cells. J. Neuroinflamm. 2018, 15, 275. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, P.; Yuan, L.; Lian, Z.; Hu, D.; Yao, X.; Li, X. Induction of UPR promotes interferon response to inhibit PRRSV replication via PKR and NF-κB pathway. Front. Microbiol. 2021, 12, 757690. [Google Scholar] [CrossRef]
- Tardif, K.D.; Mori, K.; Kaufman, R.J.; Siddiqui, A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J. Biol. Chem. 2004, 279, 17158–17164. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.M.; Bartee, M.Y.; Bartee, E. Myxoma virus attenuates expression of activating transcription factor 4 (ATF4) which has implications for the treatment of proteasome inhibitor–resistant multiple myeloma. Oncolytic Virotherapy 2015, 4, 1–11. [Google Scholar] [PubMed]
- Jindal, S.; Young, R.A. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J. Virol. 1992, 66, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, N.; Doglio, L.; Schleich, S.; Locker, J.K. Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei. Mol. Biol. Cell 2001, 12, 2031–2046. [Google Scholar] [CrossRef]
- Knipe, D.M.; Howley, P.M. Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Leão, T.L.; Lourenço, K.L.; De Oliveira Queiroz, C.; Serufo, Â.V.; Da Silva, A.M.; Barbosa-Stancioli, E.F.; Da Fonseca, F.G. Vaccinia virus induces endoplasmic reticulum stress and activates unfolded protein responses through the ATF6α transcription factor. J. Virol. 2023, 20, 145. [Google Scholar] [CrossRef]
- Lourenço, K.L.; Leão, T.L.; Queiroz, C.O.D.; Serufo, Â.V.; Da Fonseca, F.G. Manipulation of unfolded protein response by zoonotic vaccinia virus strains Guarani P1 and Passatempo. Exp. Biol. Med. 2023, 248, 1684–1693. [Google Scholar] [CrossRef]
- Barry, G.; Fragkoudis, R.; Ferguson, M.C.; Lulla, A.; Merits, A.; Kohl, A.; Fazakerley, J.K. Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells. J. Virol. 2010, 84, 7369–7377. [Google Scholar] [CrossRef]
- Paremskaia, A.I.; Kogan, A.A.; Murashkina, A.; Naumova, D.A.; Satish, A.; Abramov, I.S.; Feoktistova, S.G.; Mityaeva, O.N.; Deviatkin, A.A.; Volchkov, P.Y. Codon-optimization in gene therapy: Promises, prospects and challenges. Front. Bioeng. Biotechnol. 2024, 12, 1371596. [Google Scholar] [CrossRef]
- Abrahamian, P.; Hammond, J.; Hammond, R.W. Development and optimization of a pepino mosaic virus-based vector for rapid expression of heterologous proteins in plants. Appl. Microbiol. Biotechnol. 2021, 105, 627–645. [Google Scholar] [CrossRef]
- Bykonia, E.N.; Kleymenov, D.A.; Gushchin, V.A.; Siniavin, A.E.; Mazunina, E.P.; Kozlova, S.R.; Zolotar, A.N.; Usachev, E.V.; Kuznetsova, N.A.; Shidlovskaya, E.V. Major role of S-glycoprotein in providing immunogenicity and protective immunity in mRNA lipid nanoparticle vaccines based on SARS-CoV-2 structural proteins. Vaccines 2024, 12, 379. [Google Scholar] [CrossRef]
- Loomis, R.J.; DiPiazza, A.T.; Falcone, S.; Ruckwardt, T.J.; Morabito, K.M.; Abiona, O.M.; Chang, L.A.; Caringal, R.T.; Presnyak, V.; Narayanan, E. Chimeric fusion (F) and attachment (G) glycoprotein antigen delivery by mRNA as a candidate Nipah vaccine. Front. Immunol. 2021, 12, 772864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca, F.G.; Serufo, Â.V.; Leão, T.L.; Lourenço, K.L. Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways. Viruses 2024, 16, 1555. https://doi.org/10.3390/v16101555
da Fonseca FG, Serufo ÂV, Leão TL, Lourenço KL. Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways. Viruses. 2024; 16(10):1555. https://doi.org/10.3390/v16101555
Chicago/Turabian Styleda Fonseca, Flávio Guimarães, Ângela Vieira Serufo, Thiago Lima Leão, and Karine Lima Lourenço. 2024. "Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways" Viruses 16, no. 10: 1555. https://doi.org/10.3390/v16101555
APA Styleda Fonseca, F. G., Serufo, Â. V., Leão, T. L., & Lourenço, K. L. (2024). Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways. Viruses, 16(10), 1555. https://doi.org/10.3390/v16101555







