Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Analysed Parameters
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Univariate and Multivariate Logistic Regression Models
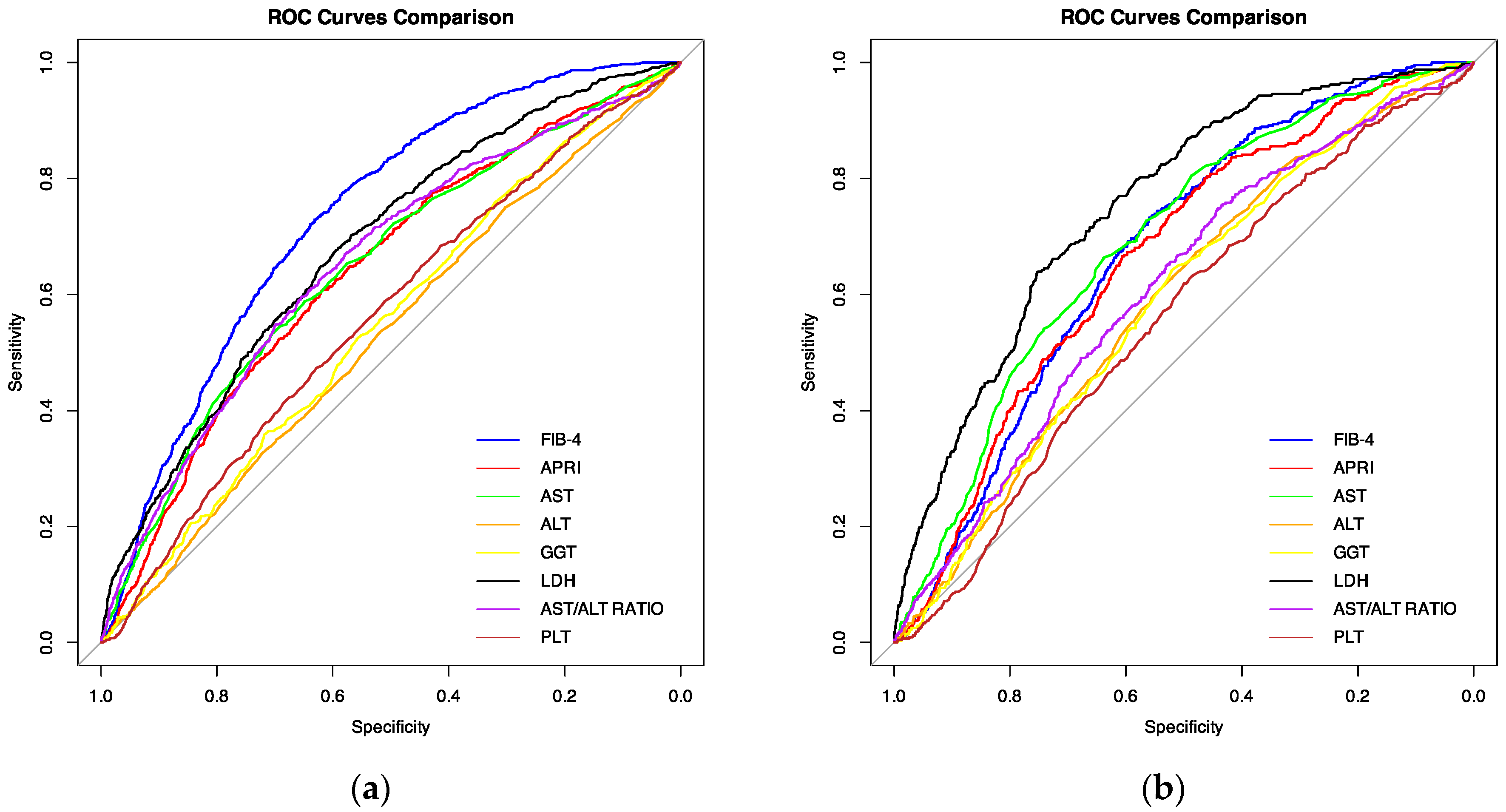
3.3. ROC Analysis
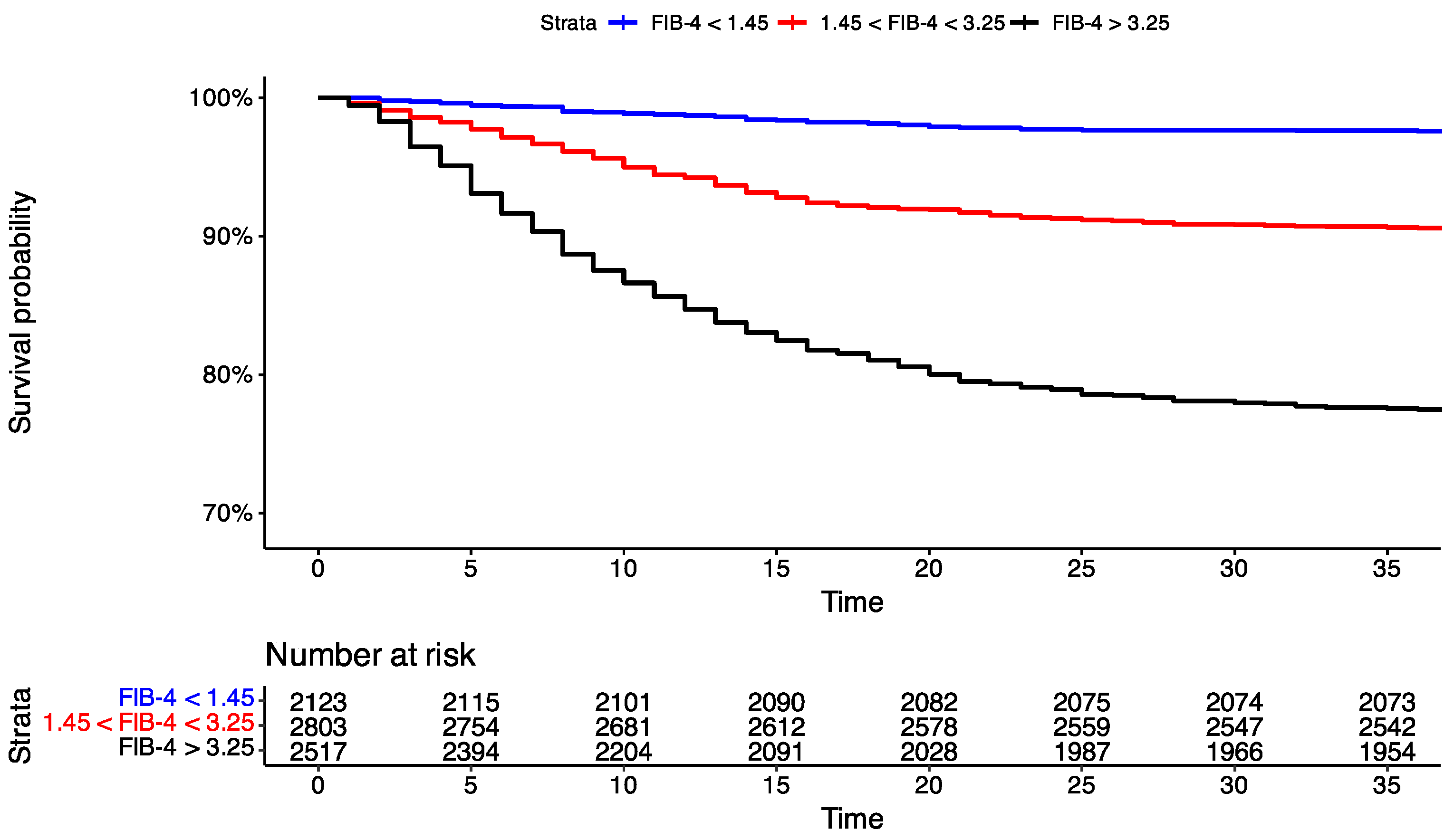
3.4. Time-to-Event Analysis
3.5. Asymptomatic Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 Diagnosis—A Review of Current Methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef] [PubMed]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological Mechanisms of Liver Injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-F.; Marjot, T.; Becchetti, C.; Tilg, H. COVID-19 and Liver Disease. Gut 2022, 71, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Dhiman, R.K.; Limdi, J.K. Evaluation of Abnormal Liver Function Tests. Postgrad. Med. J. 2016, 92, 223–234. [Google Scholar] [CrossRef]
- Read, J.A.; Winter, V.J.; Eszes, C.M.; Sessions, R.B.; Brady, R.L. Structural Basis for Altered Activity of M- and H-Isozyme Forms of Human Lactate Dehydrogenase. Proteins 2001, 43, 175–185. [Google Scholar] [CrossRef]
- Shokri Afra, H.; Amiri-Dashatan, N.; Ghorbani, F.; Maleki, I.; Rezaei-Tavirani, M. Positive Association between Severity of COVID-19 Infection and Liver Damage: A Systematic Review and Meta-Analysis. Gastroenterol. Hepatol. Bed Bench 2020, 13, 292–304. [Google Scholar]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets Contribute to Disease Severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef]
- McConnell, M.J.; Kondo, R.; Kawaguchi, N.; Iwakiri, Y. COVID-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol. Commun. 2022, 6, 255–269. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, L.; Wu, C.; Pan, L.; Lou, Z.; Peng, C.; Dong, Y.; Ruan, B. The Role of Fibrosis Index FIB-4 in Predicting Liver Fibrosis Stage and Clinical Prognosis: A Diagnostic or Screening Tool? J. Formos. Med. Assoc. 2022, 121, 454–466. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Song, T.; Li, Z.; Xia, P.; Tang, X.; Xu, M.; Shen, Y.; Ma, J.; Liu, X.; et al. Liver Fibrosis Scores and Clinical Outcomes in Patients with COVID-19. Front. Med. 2022, 9, 829423. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Kowalska, J.; Pawłowska, M.; Rogalska, M.; Zarębska-Michaluk, D.; Rorat, M.; Lorenc, B.; Czupryna, P.; Sikorska, K.; Piekarska, A.; et al. Remdesivir Decreases Mortality in COVID-19 Patients with Active Malignancy. Cancers 2022, 14, 4720. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; El-Mowafy, M.; El-Mesery, M.; Amin, M.N.; Hussein, M.H.; Killackey, M.T.; Fawzy, M.S.; Kandil, E. Diagnostic and Prognostic Value of Hematological and Immunological Markers in COVID-19 Infection: A Meta-Analysis of 6320 Patients. PLoS ONE 2020, 15, e0238160. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Pan, D.; Shen, W. D-Dimer Levels and Characteristics of Lymphocyte Subsets, Cytokine Profiles in Peripheral Blood of Patients with Severe COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 988666. [Google Scholar] [CrossRef]
- Bradley, J.; Sbaih, N.; Chandler, T.R.; Furmanek, S.; Ramirez, J.A.; Cavallazzi, R. Pneumonia Severity Index and CURB-65 Score Are Good Predictors of Mortality in Hospitalized Patients with SARS-CoV-2 Community-Acquired Pneumonia. Chest 2022, 161, 927–936. [Google Scholar] [CrossRef]
- Gupta, R.K.; Harrison, E.M.; Ho, A.; Docherty, A.B.; Knight, S.R.; van Smeden, M.; Abubakar, I.; Lipman, M.; Quartagno, M.; Pius, R.; et al. Development and Validation of the ISARIC 4C Deterioration Model for Adults Hospitalised with COVID-19: A Prospective Cohort Study. Lancet Respir. Med. 2021, 9, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 Pathophysiology: A Review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Liu, T.; Luo, S.; Libby, P.; Shi, G.-P. Cathepsin L-Selective Inhibitors: A Potentially Promising Treatment for COVID-19 Patients. Pharmacol. Ther. 2020, 213, 107587. [Google Scholar] [CrossRef]
- Ryu, J.K.; Sozmen, E.G.; Dixit, K.; Montano, M.; Matsui, Y.; Liu, Y.; Helmy, E.; Deerinck, T.J.; Yan, Z.; Schuck, R.; et al. SARS-CoV-2 Spike Protein Induces Abnormal Inflammatory Blood Clots Neutralized by Fibrin Immunotherapy. Biorxiv 2021, 13, 2021-10. [Google Scholar]
- Li, D.; Ding, X.; Xie, M.; Tian, D.; Xia, L. COVID-19-Associated Liver Injury: From Bedside to Bench. J. Gastroenterol. 2021, 56, 218–230. [Google Scholar] [CrossRef]
- Ma, C.; Cong, Y.; Zhang, H. COVID-19 and the Digestive System. Am. J. Gastroenterol. 2020, 115, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mei, K.; Tan, Z.; Huang, S.; Liu, F.; Deng, C.; Ma, J.; Yu, P.; Liu, X. Liver Fibrosis Scores and Hospitalization, Mechanical Ventilation, Severity, and Death in Patients with COVID-19: A Systematic Review and Dose-Response Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2022, 2022, e7235860. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Huang, I.; Lim, M.A.; Nasution, S.A.; Kuswardhani, R.A.T. Fibrosis-4 Index and Mortality in Coronavirus Disease 2019: A Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e368. [Google Scholar] [CrossRef]
- Del Zompo, F.; De Siena, M.; Ianiro, G.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Prevalence of Liver Injury and Correlation with Clinical Outcomes in Patients with COVID-19: Systematic Review with Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 13072–13088. [Google Scholar] [CrossRef]
- Cha, M.H.; Regueiro, M.; Sandhu, D.S. Gastrointestinal and Hepatic Manifestations of COVID-19: A Comprehensive Review. World J. Gastroenterol. 2020, 26, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Ibáñez-Samaniego, L.; Bighelli, F.; Usón, C.; Caravaca, C.; Fernández Carrillo, C.; Romero, M.; Barreales, M.; Perelló, C.; Madejón, A.; Marcos, A.C.; et al. Elevation of Liver Fibrosis Index FIB-4 Is Associated with Poor Clinical Outcomes in Patients with COVID-19. J. Infect. Dis. 2020, 222, 726–733. [Google Scholar] [CrossRef]
- Sterling, R.K.; Oakes, T.; Gal, T.S.; Stevens, M.P.; deWit, M.; Sanyal, A.J. The Fibrosis-4 Index Is Associated with Need for Mechanical Ventilation and 30-Day Mortality in Patients Admitted with Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 1794–1797. [Google Scholar] [CrossRef]
- Kolesova, O.; Vanaga, I.; Laivacuma, S.; Derovs, A.; Kolesovs, A.; Radzina, M.; Platkajis, A.; Eglite, J.; Hagina, E.; Arutjunana, S.; et al. Intriguing Findings of Liver Fibrosis Following COVID-19. BMC Gastroenterol. 2021, 21, 370. [Google Scholar] [CrossRef]
- Li, Y.; Regan, J.; Fajnzylber, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Atyeo, C.; Fischinger, S.; Gillespie, E.; et al. Liver Fibrosis Index FIB-4 Is Associated with Mortality in COVID-19. Hepatol. Commun. 2021, 5, 434. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, Biochemical and Immune Biomarker Abnormalities Associated with Severe Illness and Mortality in Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, L.X.; Feng, R.E. Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 496–502. [Google Scholar] [CrossRef]
- Sivaloganathan, H.; Ladikou, E.E.; Chevassut, T. COVID-19 Mortality in Patients on Anticoagulants and Antiplatelet Agents. Br. J. Haematol. 2020, 190, e192–e195. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of Venous Thromboembolism in Patients with Severe Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Sutandyo, N.; Kurniawati, S.A.; Jayusman, A.M.; Syafiyah, A.H.; Pranata, R.; Hanafi, A.R. Repurposing FIB-4 Index as a Predictor of Mortality in Patients with Hematological Malignancies and COVID-19. PLoS ONE 2021, 16, e0257775. [Google Scholar] [CrossRef] [PubMed]
- Kabbaha, S.; Al-Azzam, S.; Karasneh, R.A.; Khassawneh, B.Y.; Al-Mistarehi, A.-H.; Lattyak, W.J.; Aldiab, M.; Hasan, S.S.; Conway, B.R.; Aldeyab, M.A. Predictors of Invasive Mechanical Ventilation in Hospitalized COVID-19 Patients: A Retrospective Study from Jordan. Expert Rev. Respir. Med. 2022, 16, 945–952. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans with SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef]
- Li, W.; Lin, F.; Dai, M.; Chen, L.; Han, D.; Cui, Y.; Pan, P. Early Predictors for Mechanical Ventilation in COVID-19 Patients. Ther. Adv. Respir. Dis. 2020, 14, 1753466620963017. [Google Scholar] [CrossRef]
- Payán-Pernía, S.; Gómez Pérez, L.; Remacha Sevilla, Á.F.; Sierra Gil, J.; Novelli Canales, S. Absolute Lymphocytes, Ferritin, C-Reactive Protein, and Lactate Dehydrogenase Predict Early Invasive Ventilation in Patients with COVID-19. Lab. Med. 2021, 52, 141–145. [Google Scholar] [CrossRef]
- Mihai, N.; Lazar, M.; Tiliscan, C.; Barbu, E.C.; Chitu, C.E.; Stratan, L.; Ganea, O.A.; Arama, S.S.; Ion, D.A.; Arama, V. Predictors of Liver Injury in Hospitalized Patients with SARS-CoV-2 Infection. Medicina 2022, 58, 1714. [Google Scholar] [CrossRef]
- Luo, M.; Ballester, M.P.; Soffientini, U.; Jalan, R.; Mehta, G. SARS-CoV-2 Infection and Liver Involvement. Hepatol. Int. 2022, 16, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Uzum, Y.; Turkkan, E.; Uzum, Y.; Turkkan, E. Predictivity of CRP, Albumin, and CRP to Albumin Ratio on the Development of Intensive Care Requirement, Mortality, and Disease Severity in COVID-19. Cureus 2023, 15, e33600. [Google Scholar] [CrossRef] [PubMed]
| Continuous Variables | Survived (n = 6540) Median (IQR) | Non-Survived (n = 904) Median (IQR) | p Value | Non-Ventilated (n = 7022) Median (IQR) | Ventilated (n = 422) Median (IQR) | p Value |
| Age, yrs. | 63 (46–74) | 78 (70–85) | <0.001 | 65 (48–76) | 69 (61–76) | <0.001 |
| BMI, kg/m2 (n = 6550) | 27.12 (23.8–30.8) | 27.03 (23.8–31.1) | 0.376 | 26.99 (23.67–30.67) | 28.81 (25.47–32.77) | <0.001 |
| O2 saturation (%) (n = 7371) | 93 (89–96) | 87 (80–90) | <0.001 | 92 (88–96) | 85 (78–89) | <0.001 |
| ALT (IU/L) | 29 (19–46) | 31 (20.23–50.13) | 0.004 | 28 (19–46) | 35.8 (23.7–53.8) | <0.001 |
| AST (IU/L) | 38 (27–55) | 52 (35–78) | <0.001 | 38 (27–56) | 58 (39.625–78.8) | <0.001 |
| GGT (IU/L) (n = 4891) | 39 (22–74) | 46 (26–84.95) | <0.001 | 39 (22–75) | 52 (29.8–99) | <0.001 |
| LDH (IU/L) (n = 5835) | 321 (238–444) | 441 (323.5–597.5) | <0.001 | 325 (240–448) | 489 (380–655) | <0.001 |
| PLT (103/μL) | 195 (147–258) | 177 (132–236) | <0.001 | 194 (145–257) | 176 (137–227) | <0.001 |
| APRI | 0.5 (0.32–0.82) | 0.74 (0.45–1.27) | <0.001 | 0.51 (0.32–0.85) | 0.79 (0.52–1.25) | <0.001 |
| FIB-4 | 2.15(1.2–3.63) | 4.02 (2.62–6.63) | <0.001 | 2.26 (1.27–3.86) | 3.61 (2.42–5.19) | <0.001 |
| AST/ALT ratio | 1.3 (1–1.77) | 1.72 (1.26–2.3) | <0.001 | 1.33 (1–1.83) | 1.61 (1.23–2.08) | <0.001 |
| Categorical Variables | Survived (n = 6540) n (%) | Non-Survived (n = 904) n (%) | p Value | Non-Ventilated (n = 7022) n (%) | Ventilated (n = 422) n (%) | p Value |
| Sex (male) | 3458 (52.87) | 463 (51.21) | 0.368 | 3674 (52.32) | 247 (58.53) | 0.015 |
| Malignancy | 429 (6.56) | 103 (11.39) | <0.001 | 504 (7.18) | 28 (6.64) | 0.747 |
| Hypertension | 3219 (49.22) | 614 (67.92) | <0.001 | 3564 (50.75) | 269 (63.74) | <0.001 |
| Diabetes | 1277 (19.52) | 320 (35.4) | <0.001 | 1457 (20.75) | 140 (33.18) | <0.001 |
| Ischemic heart disease | 787 (12.03 | 254 (28.1) | <0.001 | 963 (13.71) | 78 (18.48) | 0.008 |
| COPD | 279 (4.27) | 79 (8.7) | <0.001 | 337 (4.8) | 21 (4.98) | 0.962 |
| Variable | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| ALT (IU/L) per 10 units change | 1.02 (0.999–1.041) | 0.056 | 1.033 (1.003–1.063) | 0.026 |
| AST (IU/L) per 10 units change | 1.109 (1.092–1.126) | <0.001 | 1.099 (1.075–1.124) | <0.001 |
| GGT (IU/L) per 10 units change | 1.011 (1.001–1.021) | 0.022 | 1.015 (1.001–1.028) | 0.031 |
| LDH (IU/L) per 10 units change | 1.031 (1.027–1.035) | <0.001 | 1.027 (1.021–1.033) | <0.001 |
| PLT (103/μL) per 10 units | 0.979 (0.972–0.987) | <0.001 | 0.976 (0.966–0.986) | <0.001 |
| APRI per 1 unit change | 1.13 (1.085–1.178) | <0.001 | 1.152 (1.095–1.213) | <0.001 |
| FIB-4 per 1 unit change | 1.07 (1.058–1.083) | <0.001 | 1.038 (1.023–1.053) | <0.001 |
| AST/ALT ratio per 1 unit change | 1.778 (1.64–1.928) | <0.001 | 1.552 (1.393–1.731) | <0.001 |
| Variable | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| ALT (IU/L) per 10 units change | 1.05 (1.023–1.077) | <0.001 | 1.009 (0.972–1.045) | 0.615 |
| AST (IU/L) per 10 units change | 1.103 (1.082–1.124) | <0.001 | 1.071 (1.043–1.098) | <0.001 |
| GGT (IU/L) per 10 units change | 1.013 (0.999–1.026) | 0.065 | 1.014 (0.993–1.03) | 0.126 |
| LDH (IU/L) per 10 units change | 1.038 (1.033–1.043) | <0.001 | 1.035 (1.035–1.026) | <0.001 |
| PLT (103/μL) per 10 units | 0.982 (0.971–0.993) | 0.002 | 0.974 (0.961–0.987) | <0.001 |
| APRI per 1 unit change | 1.11 (1.056–1.163) | <0.001 | 1.092 (1.028–1.153) | 0.002 |
| FIB-4 per 1 unit change | 1.035 (1.02–1.049) | <0.001 | 1.021 (1.002–1.039) | 0.024 |
| AST/ALT ratio per 1 unit change | 1.402 (1.268–1.547) | <0.001 | 1.461 (1.285–1.658) | <0.001 |
| Variable | AUC | 95% CI | Optimal Cut-Off Point | Sensitivity at Optimal Cut-Off Point | FPR at Optimal Cut-Off |
|---|---|---|---|---|---|
| FIB-4 | 0.733 | 0.718–0.749 | 2.764 | 0.7289823 | 0.3681957 |
| LDH (IU/L) (n = 5835) | 0.681 | 0.659–0.702 | 359.5 | 0.680315 | 0.4094231 |
| AST/ALT ratio | 0.654 | 0.634–0.673 | 1.566 | 0.5951327 | 0.3457187 |
| AST (IU/L) | 0.649 | 0.629–0.668 | 46.05 | 0.5873894 | 0.3498471 |
| APRI | 0.641 | 0.622–0.66 | 0.616 | 0.6073009 | 0.3818043 |
| PLT (103/μL) | 0.563 | 0.543–0.583 | 170.5 | 0.4734513 | 0.3718654 |
| GGT (IU/L) (n = 4891) | 0.55 | 0.526–0.574 | 44.4 | 0.5234114 | 0.4411833 |
| ALT (IU/L) | 0.529 | 0.509–0.549 | 29.15 | 0.5365044 | 0.4824159 |
| Variable | AUC | 95% CI | Optimal Cut-Off Point | Sensitivity at Optimal Cut-Off Point | FPR at Optimal Cut-Off |
|---|---|---|---|---|---|
| LDH (IU/L) (n = 5835) | 0.753 | 0.727–0.778 | 449.5 | 0.6389776 | 0.2480985 |
| AST (IU/L) | 0.69 | 0.666–0.715 | 46.15 | 0.6635071 | 0.3614355 |
| FIB-4 | 0.673 | 0.65–0.695 | 2.544 | 0.7322275 | 0.4400456 |
| APRI | 0.663 | 0.638–0.687 | 0.616 | 0.6658768 | 0.3937625 |
| AST/ALT ratio | 0.609 | 0.582–0.639 | 1.23 | 0.7535545 | 0.7535545 |
| ALT (IU/L) | 0.591 | 0.564–0.617 | 27.65 | 0.6729858 | 0.5206494 |
| GGT (IU/L) (n = 4891) | 0.59 | 0.558–0.622 | 40.65 | 0.6425993 | 0.479844 |
| PLT (103/μL) | 0.558 | 0.531–0.585 | 193.5 | 0.6184834 | 0.4998576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudka, K.; Jaroszewicz, J.; Zarębska-Michaluk, D.; Rogalska, M.; Czupryna, P.; Rorat, M.; Kozielewicz, D.; Maciukajć, J.; Kiciak, S.; Krępa, M.; et al. Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study. Viruses 2024, 16, 1530. https://doi.org/10.3390/v16101530
Żmudka K, Jaroszewicz J, Zarębska-Michaluk D, Rogalska M, Czupryna P, Rorat M, Kozielewicz D, Maciukajć J, Kiciak S, Krępa M, et al. Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study. Viruses. 2024; 16(10):1530. https://doi.org/10.3390/v16101530
Chicago/Turabian StyleŻmudka, Karol, Jerzy Jaroszewicz, Dorota Zarębska-Michaluk, Magdalena Rogalska, Piotr Czupryna, Marta Rorat, Dorota Kozielewicz, Jadwiga Maciukajć, Sławomir Kiciak, Magdalena Krępa, and et al. 2024. "Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study" Viruses 16, no. 10: 1530. https://doi.org/10.3390/v16101530
APA StyleŻmudka, K., Jaroszewicz, J., Zarębska-Michaluk, D., Rogalska, M., Czupryna, P., Rorat, M., Kozielewicz, D., Maciukajć, J., Kiciak, S., Krępa, M., Dutkiewicz, E., Stojko, M., Spychał, A., Ciechanowski, P., Bolewska, B., Podlasin, R., & Flisiak, R. (2024). Association between Liver Damage and Disease Progression Markers with Mortality Risk and Mechanical Ventilation in Hospitalized COVID-19 Patients: A Nationwide Retrospective SARSTer Study. Viruses, 16(10), 1530. https://doi.org/10.3390/v16101530








