Viruses of Apple Are Seedborne but Likely Not Vertically Transmitted
Abstract
1. Introduction
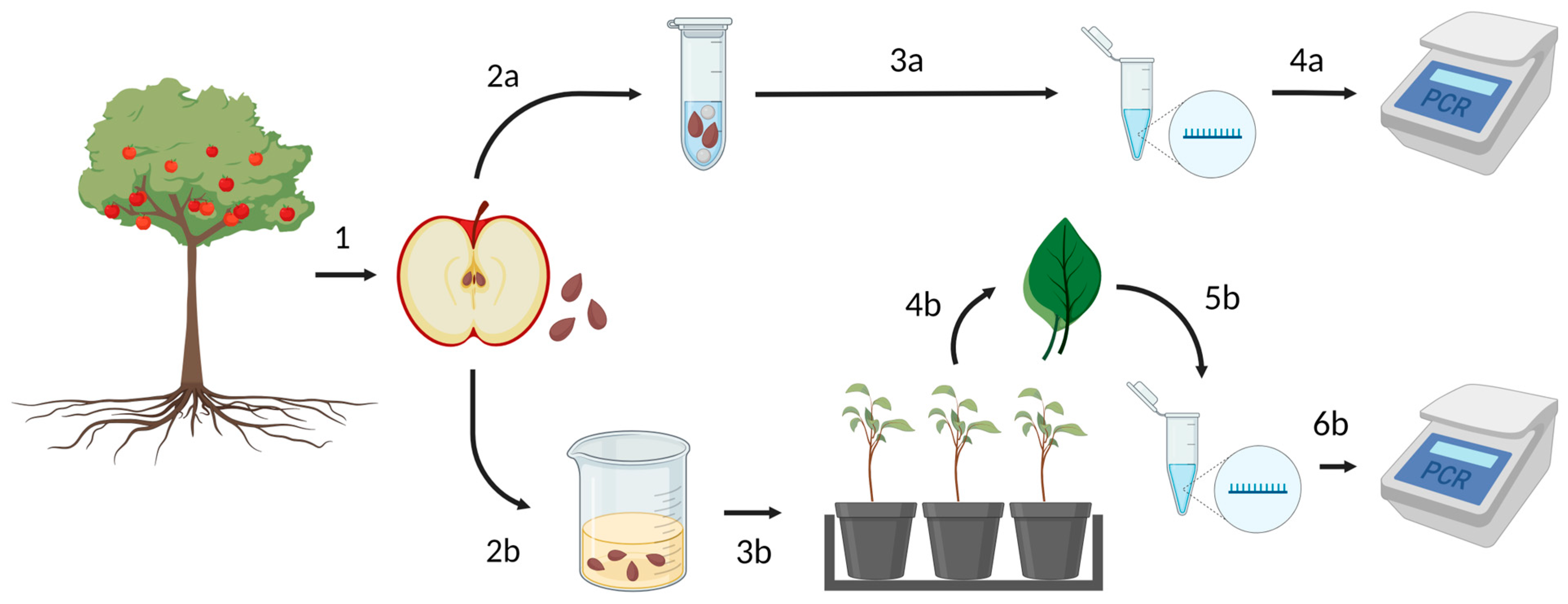
2. Materials and Methods
2.1. Experimental Apple Orchard Establishment and Fruit Collection
2.2. Apple Seed Collection and Germination
2.3. Virus Detection in Apple Trees
2.4. Virus Detection in Apple Seeds
2.5. ASPV Detection in Apple Fruits
2.6. Virus Detection in Apple Seedlings
3. Results
3.1. Detection of Viruses and Viroids in Maternal Apple Trees by Multiplex PCR-Based Amplicon Sequencing
3.2. Evaluation of Seed Transmission of ACLSV, ASGV, and ASPV in Malus domestica
3.3. Evaluation of Seedborne Nature of ACLSV, AGCaV, ARWV2, ASGV, ASPV, and CCGaV in Malus domestica
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT: Crops and Livestock Products. Available online: http://www.fao.org/faostat/en (accessed on 7 August 2023).
- Hadidi, A.; Barba, M. Economic impact of pome and stone fruit viruses and viroids. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St. Paul, MN, USA, 2011; pp. 1–7. [Google Scholar]
- CABI. CABI Compendium. Available online: https://www.cabidigitallibrary.org/journal/cabicompendium (accessed on 9 August 2023).
- Xiao, H.; Hao, W.; Storoschuk, G.; MacDonald, J.L.; Sanfaçon, H. Characterizing the virome of apple orchards affected by rapid decline in the Okanagan and Similkameen Valleys of British Columbia (Canada). Pathogens 2022, 11, 1231. [Google Scholar] [CrossRef]
- Liu, H.; Wu, L.; Nikolaeva, E.; Peter, K.; Liu, Z.; Mollov, D.; Cao, M.; Li, R. Characterization of a new apple luteovirus identified by high-throughput sequencing. Virol. J. 2018, 15, 85. [Google Scholar] [CrossRef]
- Rott, M.E.; Kesanakurti, P.; Berwarth, C.; Rast, H.; Boyes, I.; Phelan, J.; Jelkmann, W. Discovery of negative-sense RNA viruses in trees infected with apple rubbery wood disease by next-generation sequencing. Plant Dis. 2018, 102, 1254–1263. [Google Scholar] [CrossRef]
- Navarro, B.; Minutolo, M.; De Stradis, A.; Palmisano, F.; Alioto, D.; Di Serio, F. The first phlebo-like virus infecting plants: A case study on the adaptation of negative-stranded RNA viruses to new hosts. Mol. Plant Pathol. 2018, 19, 1075–1089. [Google Scholar] [CrossRef]
- Navarro, B.; Zicca, S.; Minutolo, M.; Saponari, M.; Alioto, D.; Di Serio, F. A negative-stranded RNA virus infecting citrus trees: The second member of a new genus within the order Bunyavirales. Front. Microbiol. 2018, 9, 2340. [Google Scholar] [CrossRef]
- Costa, L.C.; Hu, X.; Malapi-Wight, M.; Foster, J.A.; McFarland, C.; Hurtado-Gonzales, O.P. Identification of a novel robigovirus and a Prunus-infecting tepovirus in Pyrus communis and their transmissibility on Malus spp. Eur. J. Plant Pathol. 2022, 162, 275–288. [Google Scholar] [CrossRef]
- Messmer, A.; Sanderson, D.; Braun, G.; Serra, P.; Flores, R.; James, D. Molecular and phylogenetic identification of unique isolates of hammerhead viroid-like RNA from ‘Pacific Gala’ apple (Malus domestica) in Canada. Can. J. Plant Pathol. 2017, 39, 342–353. [Google Scholar] [CrossRef]
- Serra, P.; Messmer, A.; Sanderson, D.; James, D.; Flores, R. Apple hammerhead viroid-like RNA is a bona fide viroid: Autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res. 2018, 249, 8–15. [Google Scholar] [CrossRef]
- Wright, A.A.; Cross, A.R.; Harper, S.J. A bushel of viruses: Identification of seventeen novel putative viruses by RNA-seq in six apple trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef]
- Fulton, J.P. Dual transmission of tobacco ringspot virus and tomato ringspot virus by Xiphinema americanum. Phytopathology 1967, 57, 535–537. [Google Scholar]
- McGuire, J.M. Retention of tobacco ringspot virus by Xiphinema americanum. Phytopathology 1973, 63, 324–326. [Google Scholar] [CrossRef]
- Hadidi, A.; Hansen, A.J.; Parish, C.L.; Yang, X. Scar skin and dapple apple viroids are seed-borne and persistent in infected apple trees. Res. Virol. 1991, 142, 289–296. [Google Scholar] [CrossRef]
- Khan, A.; Korban, S.S. Breeding and genetics of disease resistance in temperate fruit trees: Challenges and new opportunities. Theor. Appl. Genet. 2022, 135, 3961–3985. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, United States Department of Agriculture. What Plant Material Requires a Permit? Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/import-information/permits/plants-and-plant-products-permits/plants-for-planting/ct_permit_plantmaterials (accessed on 12 September 2023).
- European and Mediterranean Plant Protection Organization. Pathogen-Tested Material of Malus, Pyrus and Cydonia. EPPO Bull. 1999, 29, 239–252. [Google Scholar] [CrossRef]
- Fazio, G. Genetics, breeding, and genomics of apple rootstocks. In The Apple Genome; Korban, S.S., Ed.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2021; pp. 105–130. [Google Scholar] [CrossRef]
- Volk, G.M.; Richards, C.M.; Reilley, A.A.; Henk, A.D.; Forsline, P.L.; Aldwinckle, H.S. Ex situ conservation of vegetatively propagated species: Development of a seed-based core collection for Malus sieversii. J. Am. Soc. Hortic. Sci. 2005, 130, 203–210. [Google Scholar] [CrossRef]
- Li, L.; Wen, L.; Wang, G.; Lyu, Y.; Yang, Z.; Yang, X.; Li, Q.; Hong, N. Seed transmission of three viruses in two pear rootstock species Pyrus betulifolia and P. calleryana. Viruses 2022, 14, 599. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://soilseries.sc.egov.usda.gov (accessed on 22 August 2023).
- Costa, L.C.; Atha, B.; Hu, X.; Lamour, K.; Yang, Y.; O’Connell, M.; McFarland, C.; Foster, J.A.; Hurtado-Gonzales, O.P. High-throughput detection of a large set of viruses and viroids of pome and stone fruit trees by multiplex PCR-based amplicon sequencing. Front. Plant Sci. 2022, 13, 1072768. [Google Scholar] [CrossRef]
- Menzel, W.; Jelkmann, W.; Maiss, E. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J. Virol. Methods 2002, 99, 81–92. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Wunderlich, L.; Nouri, M.T.; Golino, D.; Al Rwahnih, M. Incidence and detection of negative-stranded RNA viruses infecting apple and pear trees in California. J. Phytopathol. 2022, 170, 15–20. [Google Scholar] [CrossRef]
- Beaver-Kanuya, E.; Harper, S.J. Development of RT-qPCR assays for the detection of three latent viruses of pome. J. Virol. Methods 2020, 278, 113836. [Google Scholar] [CrossRef]
- Fuchs, M.; Kahlke, C.; Donahue, D.; Wallis, A.; Basedow, M. Distribution of viruses in New York apple orchards. N. Y. Fruit Q. 2018, 26, 5–9. [Google Scholar]
- Hull, R. Plant to plant movement. In Plant Virology, 5th ed.; Hull, R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 669–751. [Google Scholar] [CrossRef]
- Johansen, E.; Edwards, M.C.; Hampton, R.O. Seed transmission of viruses: Current perspectives. Annu. Rev. Phytopathol. 1994, 32, 363–386. [Google Scholar] [CrossRef]
- Mathur, S.B.; Haware, M.P.; Hampton, R.O. Identification, significance and transmission of seed borne pathogens. In World Crops: Cool Season Food Legumes; Summerfield, R.J., Ed.; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1988; Volume 5, pp. 351–365. [Google Scholar] [CrossRef]
- Agarwal, J.B.; Sinclair, V.K. Principles of Seed Pathology, 2nd ed.; Agarwal, J.B., Sinclair, V.K., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Broadbent, L. The epidemiology of tomato mosaic. Ann. Appl. Biol. 1965, 56, 177–205. [Google Scholar] [CrossRef]
- Allarangaye, M.D.; Traoré, O.; Traoré, E.V.S.; Millogo, R.J.; Konaté, G. Evidence of non-transmission of rice yellow mottle virus through seeds of wild host species. J. Plant Pathol. 2006, 88, 309–315. Available online: http://www.jstor.org/stable/41998336 (accessed on 30 August 2023).
- Hanssen, I.M.; Mumford, R.; Blystad, D.-R.; Cortez, I.; Hasiów-Jaroszewska, B.; Hristova, D.; Pagán, I.; Pereira, A.-M.; Peters, J.; Pospieszny, H.; et al. Seed transmission of pepino mosaic virus in tomato. Eur. J. Plant Pathol. 2010, 126, 145–152. [Google Scholar] [CrossRef]
- Ling, K.-S. Pepino mosaic virus on tomato seed: Virus location and mechanical transmission. Plant Dis. 2008, 92, 1701–1705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, H.-J.; Qin, B.-X.; Chen, H.-Y.; Peng, B.; Cai, J.-H.; Gu, Q.-S. The rate of seed contamination and transmission of cucumber green mottle mosaic virus in watermelon and melon. Sci. Agric. Sin. 2011, 44, 1527–1532. [Google Scholar] [CrossRef]
- Demski, J.W. Tobacco mosaic virus is seedborne in pimento peppers. Plant Dis. 1981, 63, 723–724. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed transmission of tobamoviruses: Aspects of global disease distribution. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; Intech: London, UK, 2017; pp. 233–260. [Google Scholar] [CrossRef]
- Isogai, M.; Yoshikoshi, M.; Seki, K.; Masuko-Suzuki, H.; Watanabe, M.; Matsuo, K.; Yaegashi, H. Seed transmission of raspberry bushy dwarf virus is blocked in Nicotiana benthamiana plants by preventing virus entry into the embryo from the infected embryo sac and endosperm. Arch. Virol. 2023, 168, 138. [Google Scholar] [CrossRef]
| Family | Genus | Virus or Viroid | Abbreviation |
|---|---|---|---|
| Avsunviroidae | Pelamoviroid | Apple hammerhead viroid | AHVd |
| Betaflexiviridae | Capillovirus | Apple stem grooving virus | ASGV |
| Foveavirus | Apple green crinkle-associated virus | AGCaV | |
| Apple stem pitting virus | ASPV | ||
| Tepovirus | Prunus virus T | PrVT | |
| Trichovirus | Apple chlorotic leaf spot virus | ACLSV | |
| Bromoviridae | Ilarvirus | Apple mosaic virus | ApMV |
| Tombusviridae | Luteovirus | Apple luteovirus 1 | ALV1 |
| Partitiviridae | Alphapartitivirus | Pyrus pyrifolia partitivirus 2 | PpPV2 |
| Phenuiviridae | Coguvirus | Citrus concave gum-associated virus | CCGaV |
| Citrus virus A | CiVA | ||
| Rubodvirus | Apple rubbery wood virus 1 | ARWV1 | |
| Apple rubbery wood virus 2 | ARWV2 | ||
| Pospiviroidae | Apscaviroid | Apple dimple fruit viroid | ADFVd |
| Apple fruit crinkle viroid | AFCVd | ||
| Apple scar skin viroid | ASSVd | ||
| Pear blister canker viroid | PBCVd | ||
| Secoviridae | Nepovirus | Tobacco ringspot virus | TRSV |
| Tomato ringspot virus | ToRSV |
| Target | Type | Oligo Name | Sequence (5′–3′) | Target Region | Reference |
|---|---|---|---|---|---|
| RT-PCR | |||||
| ACLSV | Primer | ACLSV-F | TTCATGGAAAGACAGGGGCAA | Coat protein | [24] |
| Primer | ACLSV-R | AAGTCTACAGGCTATTTATTATAAGTCTAA | |||
| ASGV | Primer | ASGV-F | GCCACTTCTAGGCAGAACTCTTTGAA | Coat protein | [24] |
| Primer | ASGV-R | AACCCCTTTTTGTCCTTCAGTACGAA | |||
| ASPV | Primer | ASPV-F | ATGTCTGGAACCTCATGCTGCAA | Coat protein | [24] |
| Primer | ASPV-R | TTGGGATCAACTTTACTAAAAAGCATAA | |||
| nad5 | Primer | nad5-F | GATGCTTCTTGGGGCTTCTTGTT | nad5 mRNA | [24] |
| Primer | nad5-R | CTCCAGTCACCAACATTGGCATAA | |||
| RT-qPCR | |||||
| AGCaV | Primer | AGCaV-RT-F | GCVAAYGCCACAAGCAAA | Coat protein | [23] |
| Primer | AGCaV-RT-R | GYCCAAYYTTTCCCCCAGTGA | |||
| Probe | AGCaV-RT-P | CAAGCTGTYAACTTTGGY | |||
| ARWV2 | Primer | ARWV2-RT-F | GAGATTCCAAGCTATTTCATAAGCATAA | RdRP (Segment L) | [25] |
| Primer | ARWV2-RT-R | TGATAAAAGGCACACAAGTTGCA | |||
| Probe | ARWV2-RT-P | ATCCCCAGTGCTGAAA | |||
| ASPV | Primer | ASPV-RT-F | GAGGAGGTTGGCACGATGATC | Coat protein | [26] |
| Primer | ASPV-RT-R | GCAGCATGAGGTTCCAGACAT | |||
| Probe | ASPV-RT-P | AACTGAAGGGTGCACTTTGAGGCA | |||
| CCGaV | Primer | CCGaV-RT-F | GCCTTTCTTCGTACATGTGATGAG | RdRP (RNA1) | [25] |
| Primer | CCGaV-RT-R | GGCAAACTGTATGGGAAAGCTAA | |||
| Probe | CCGaV-RT-P | TGTGGAAATGCAAGAGA | |||
| Virus or Viroid | Trees Infected | % Trees Infected |
|---|---|---|
| Apple stem grooving virus | 175 | 100 |
| Apple chlorotic leaf spot virus | 136 | 77.7 |
| Apple stem pitting virus | 125 | 71.4 |
| Citrus concave gum-associated virus | 106 | 60.6 |
| Apple hammerhead viroid | 104 | 59.4 |
| Apple green crinkle-associated virus | 56 | 32.0 |
| Apple rubbery wood virus 2 | 25 | 14.3 |
| Tobacco ringspot virus | 3 | 1.7 |
| Tomato ringspot virus | 1 | 0.6 |
| Seeds | Seedlings | |||||
|---|---|---|---|---|---|---|
| Virus | N a | Positive b | Incidence (%) | N a | Positive b | Incidence (%) |
| ACLSV | 41 | 38 | 92.7 | 1121 | 0 | 0 |
| ASGV | 49 | 47 | 95.9 | 1412 | 0 | 0 |
| ASPV | 38 | 35 | 92.1 | 1035 | 0 | 0 |
| AGCaV | 20 | 8 | 40.0 | nt | na | na |
| ARWV2 | 5 | 1 | 20.0 | nt | na | na |
| CCGaV | 32 | 27 | 84.4 | nt | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunsch, A.; Hoff, B.; Sazo, M.M.; van Zoeren, J.; Lamour, K.H.; Hurtado-Gonzales, O.P.; Fuchs, M. Viruses of Apple Are Seedborne but Likely Not Vertically Transmitted. Viruses 2024, 16, 95. https://doi.org/10.3390/v16010095
Wunsch A, Hoff B, Sazo MM, van Zoeren J, Lamour KH, Hurtado-Gonzales OP, Fuchs M. Viruses of Apple Are Seedborne but Likely Not Vertically Transmitted. Viruses. 2024; 16(1):95. https://doi.org/10.3390/v16010095
Chicago/Turabian StyleWunsch, Anna, Bailey Hoff, Mario Miranda Sazo, Janet van Zoeren, Kurt H. Lamour, Oscar P. Hurtado-Gonzales, and Marc Fuchs. 2024. "Viruses of Apple Are Seedborne but Likely Not Vertically Transmitted" Viruses 16, no. 1: 95. https://doi.org/10.3390/v16010095
APA StyleWunsch, A., Hoff, B., Sazo, M. M., van Zoeren, J., Lamour, K. H., Hurtado-Gonzales, O. P., & Fuchs, M. (2024). Viruses of Apple Are Seedborne but Likely Not Vertically Transmitted. Viruses, 16(1), 95. https://doi.org/10.3390/v16010095






