Developing an Indirect ELISA for the Detection of African Swine Fever Virus Antibodies Using a Tag-Free p15 Protein Antigen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sera and Plasmid
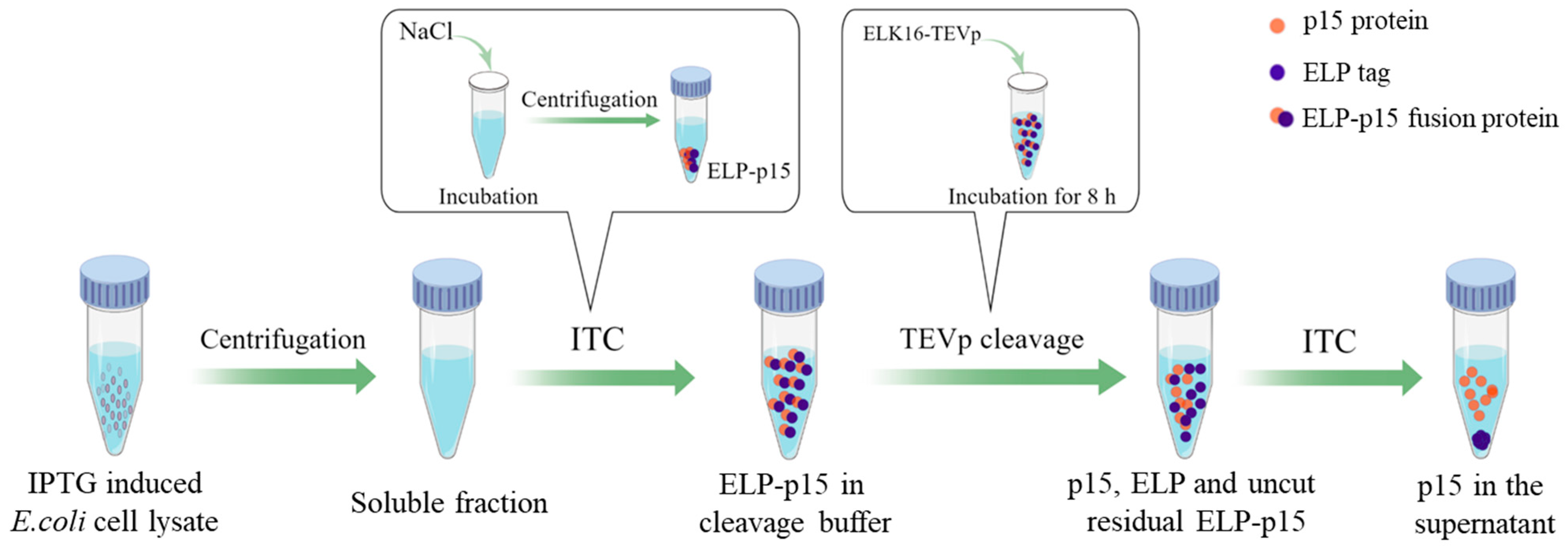
2.2. Preparation of Tag-Free p15 Protein Antigen
2.2.1. Construction of Recombinant Expression Vector pELP-p15
2.2.2. Protein Expression and Purification
2.2.3. p15 Protein Recovery via Protease Cleavage
2.3. Establishment of Indirect ELISA (iELISA) Method
2.3.1. Checkerboard Titration
2.3.2. Optimization of Reaction Conditions
2.3.3. Determination of Cut-Off Value
2.3.4. Specificity and Sensitivity Tests
2.3.5. Reproducibility Test
2.3.6. Comparison with the Commercial Kit
3. Results
3.1. p15 Protein Antigen Preparation
3.1.1. Expression of ELP-p15 Fusion Protein
3.1.2. Purification of ELP-p15 Fusion Protein
3.1.3. Recovery and Identification of Tag-Free p15 Protein
3.2. p15-iELISA Method Establishment and Evaluation
3.2.1. Working Conditions for p15-iELISA
3.2.2. Cut-Off Value Determination
3.2.3. Specificity Test
3.2.4. Sensitivity Test
3.2.5. Reproducibility Test
3.2.6. Comparison with the Commercial Kit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Clemmons, E.A.; Alfson, K.J.; Dutton, J.W., 3rd. Transboundary Animal Diseases, an Overview of 17 Diseases with Potential for Global Spread and Serious Consequences. Animals 2021, 11, 2039. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef]
- Jia, N.; Ou, Y.; Pejsak, Z.; Zhang, Y.; Zhang, J. Roles of African Swine Fever Virus Structural Proteins in Viral Infection. J. Vet. Res. 2017, 61, 135–143. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef]
- Franzoni, G.; Graham, S.P.; Giudici, S.D.; Bonelli, P.; Pilo, G.; Anfossi, A.G.; Pittau, M.; Nicolussi, P.S.; Laddomada, A.; Oggiano, A. Characterization of the interaction of African swine fever virus with monocytes and derived macrophage subsets. Vet. Microbiol. 2017, 198, 88–98. [Google Scholar] [CrossRef]
- Dixon, L.K.; Abrams, C.C.; Bowick, G.; Goatley, L.C.; Kay-Jackson, P.C.; Chapman, D.; Liverani, E.; Nix, R.; Silk, R.; Zhang, F. African swine fever virus proteins involved in evading host defence systems. Vet. Immunol. Immunopathol. 2004, 100, 117–134. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Kang, L.; Huang, L.; Zhou, S.; Hu, L.; Zheng, J.; Li, C.; Zhang, X.; He, X.; et al. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1beta and type I IFN production. PLoS Pathog. 2021, 17, e1009733. [Google Scholar] [CrossRef]
- Zheng, X.; Nie, S.; Feng, W.H. Regulation of antiviral immune response by African swine fever virus (ASFV). Virol. Sin. 2022, 37, 157–167. [Google Scholar] [CrossRef]
- Sun, M.; Yu, S.; Ge, H.; Wang, T.; Li, Y.; Zhou, P.; Pan, L.; Han, Y.; Yang, Y.; Sun, Y.; et al. The A137R Protein of African Swine Fever Virus Inhibits Type I Interferon Production via the Autophagy-Mediated Lysosomal Degradation of TBK1. J. Virol. 2022, 96, e0195721. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Li, L.; Qiao, S.; Li, G.; Tong, W.; Dong, S.; Liu, J.; Guo, Z.; Zheng, H.; Zhao, R.; Tong, G.; et al. The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects. Viruses 2022, 15, 50. [Google Scholar] [CrossRef]
- Li, L.; Qiao, S.; Liu, J.; Zhou, Y.; Tong, W.; Dong, S.; Liu, C.; Jiang, Y.; Guo, Z.; Zheng, H.; et al. A highly efficient indirect ELISA and monoclonal antibody established against African swine fever virus pK205R. Front. Immunol. 2022, 13, 1103166. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, K.; Liu, H.; Yin, Y.; Zhao, J.; Long, F.; Lu, W.; Si, H. Development of a multiplex qRT-PCR assay for detection of African swine fever virus, classical swine fever virus and porcine reproductive and respiratory syndrome virus. J. Vet. Sci. 2021, 22, e87. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Y.; Accensi, F.; Ganges, L.; Rodriguez, F.; Shan, H.; Stahl, K.; Qiu, H.J.; Belak, S. Pre-Clinical Evaluation of a Real-Time PCR Assay on a Portable Instrument as a Possible Field Diagnostic Tool: Experiences from the Testing of Clinical Samples for African and Classical Swine Fever Viruses. Transbound. Emerg. Dis. 2017, 64, e31–e35. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Oura, C.A.; Edwards, L.; Batten, C.A. Virological diagnosis of African swine fever--comparative study of available tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, D.; Li, L.; Wan, B.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Song, J.; Zhu, Z.; et al. Development of an indirect ELISA for the identification of African swine fever virus wild-type strains and CD2v-deleted strains. Front. Vet. Sci. 2022, 9, 1006895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Q.; Sun, Q.; Zhang, Y.; Li, Y.; Ma, X.; Guan, Z.; Zhang, J.; Li, Z.; Liu, K.; et al. Detection of African swine fever virus antibodies in serum using a pB602L protein-based indirect ELISA. Front. Vet. Sci. 2022, 9, 971841. [Google Scholar] [CrossRef]
- Zhong, K.; Zhu, M.; Yuan, Q.; Deng, Z.; Feng, S.; Liu, D.; Yuan, X. Development of an Indirect ELISA to Detect African Swine Fever Virus pp62 Protein-Specific Antibodies. Front. Vet. Sci. 2021, 8, 798559. [Google Scholar] [CrossRef]
- Suarez, C.; Salas, M.L.; Rodriguez, J.M. African swine fever virus polyprotein pp62 is essential for viral core development. J. Virol. 2010, 84, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef]
- Banki, M.R.; Feng, L.; Wood, D.W. Simple bioseparations using self-cleaving elastin-like polypeptide tags. Nat. Methods 2005, 2, 659–661. [Google Scholar] [CrossRef]
- Waugh, D.S. An overview of enzymatic reagents for the removal of affinity tags. Protein Expr. Purif. 2011, 80, 283–293. [Google Scholar] [CrossRef]
- Kapust, R.B.; Tozser, J.; Copeland, T.D.; Waugh, D.S. The P1′ specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 2002, 294, 949–955. [Google Scholar] [CrossRef]
- Xia, W.; Lu, H.; Li, Y.; Cao, J.; Zhou, X.; Zhang, X.; Xia, X.; Sun, H. Purification of chicken IgY by binding capture using elastin-like polypeptide-tagged immunoglobulin-binding domain of streptococcal protein G. Vet. Immunol. Immunopathol. 2017, 192, 13–19. [Google Scholar] [CrossRef]
- Li, G.Y.; Xiao, Z.Z.; Lu, H.P.; Li, Y.Y.; Zhou, X.H.; Tan, X.; Zhang, X.Y.; Xia, X.L.; Sun, H.C. A simple method for recombinant protein purification using self-assembling peptide-tagged tobacco etch virus protease. Protein Expr. Purif. 2016, 128, 86–92. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Yang, Y.; Gong, W.; Huang, K.; Zhang, Y.; Yang, Y.; Sun, X.; Ren, W.; Zhang, Q.; et al. Rapid and visual detection of African swine fever virus antibody by using fluorescent immunochromatography test strip. Talanta 2020, 219, 121284. [Google Scholar] [CrossRef] [PubMed]
- Simon-Mateo, C.; Andres, G.; Almazan, F.; Vinuela, E. Proteolytic processing in African swine fever virus: Evidence for a new structural polyprotein, pp62. J. Virol. 1997, 71, 5799–5804. [Google Scholar] [CrossRef]
- Gallardo, C.; Blanco, E.; Rodriguez, J.M.; Carrascosa, A.L.; Sanchez-Vizcaino, J.M. Antigenic properties and diagnostic potential of African swine fever virus protein pp62 expressed in insect cells. J. Clin. Microbiol. 2006, 44, 950–956. [Google Scholar] [CrossRef]
- Liao, C.M.; Huang, C.; Hsuan, S.L.; Chen, Z.W.; Lee, W.C.; Liu, C.I.; Winton, J.R.; Chien, M.S. Immunogenicity and efficacy of three recombinant subunit Pasteurella multocida toxin vaccines against progressive atrophic rhinitis in pigs. Vaccine 2006, 24, 27–35. [Google Scholar] [CrossRef]
- Madera, R.; Gong, W.; Wang, L.; Burakova, Y.; Lleellish, K.; Galliher-Beckley, A.; Nietfeld, J.; Henningson, J.; Jia, K.; Li, P.; et al. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet. Res. 2016, 12, 197. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Liu, Y.; Wang, M.; Zhang, L.; Han, L.; Chu, X.; Ding, G.; Li, Y.; Hou, Y.; et al. Indirect ELISA Using Multi-Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs. Viruses 2022, 14, 2660. [Google Scholar] [CrossRef]



| Serum Dilution | Concentrations of p15 Protein (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 16 | 8 | 4 | 2 | 1 | 0.5 | ||
| 1:100 | P | 1.716 ± 0.017 | 1.578 ± 0.016 | 1.531 ± 0.026 | 1.517 ± 0.019 | 1.519 ± 0.020 | 1.426 ± 0.034 |
| N | 0.116 ± 0.005 | 0.098 ± 0.002 | 0.102 ± 0.003 | 0.098 ± 0.003 | 0.093 ± 0.004 | 0.115 ± 0.002 | |
| P/N | 14.8 | 16.1 | 15.0 | 15.5 | 16.3 | 12.4 | |
| 1:200 | P | 1.587 ± 0.022 | 1.524 ± 0.016 | 1.488 ± 0.019 | 1.41 ± 0.017 | 1.316 ± 0.016 | 1.227 ± 0.020 |
| N | 0.097 ± 0.003 | 0.098 ± 0.002 | 0.102 ± 0.005 | 0.071 ± 0.001 | 0.07 ± 0.002 | 0.07 ± 0.002 | |
| P/N | 16.4 | 15.6 | 14.6 | 19.9 | 18.8 | 17.5 | |
| 1:400 | P | 1.458 ± 0.029 | 1.407 ± 0.032 | 1.346 ± 0.031 | 1.256 ± 0.013 | 1.175 ± 0.024 | 0.851 ± 0.014 |
| N | 0.088 ± 0.004 | 0.083 ± 0.003 | 0.081 ± 0.002 | 0.078 ± 0.002 | 0.077 ± 0.003 | 0.079 ± 0.001 | |
| P/N | 16.6 | 17.0 | 16.6 | 16.1 | 15.3 | 10.8 | |
| Samples | Intra-Assay | Inter-Assay | ||
|---|---|---|---|---|
| OD450 (Mean ± SD) | CV | OD450 (Mean ± SD) | CV | |
| 1 | 0.835 ± 0.018 | 2.16% | 0.852 ± 0.023 | 2.70% |
| 2 | 1.215 ± 0.035 | 2.88% | 1.261 ± 0.051 | 4.04% |
| 3 | 0.654 ± 0.011 | 1.68% | 0.679 ± 0.020 | 2.95% |
| 4 | 0.111 ± 0.002 | 1.80% | 0.099 ± 0.001 | 1.01% |
| 5 | 0.689 ± 0.013 | 1.89% | 0.658 ± 0.021 | 3.19% |
| 6 | 0.945 ± 0.022 | 2.33% | 1.017 ± 0.036 | 3.54% |
| Methods | Commercial Kit | Coincidence Rate | |||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| p15-iELISA | Positive | 12 | 0 | 12 | 100% |
| Negative | 0 | 102 | 102 | ||
| Total | 12 | 102 | 114 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Lu, H.; Zhu, D.; Xie, J.; Sun, F.; Xu, Y.; Zhang, H.; Wu, Z.; Xia, W.; Zhu, S. Developing an Indirect ELISA for the Detection of African Swine Fever Virus Antibodies Using a Tag-Free p15 Protein Antigen. Viruses 2023, 15, 1939. https://doi.org/10.3390/v15091939
Wu Z, Lu H, Zhu D, Xie J, Sun F, Xu Y, Zhang H, Wu Z, Xia W, Zhu S. Developing an Indirect ELISA for the Detection of African Swine Fever Virus Antibodies Using a Tag-Free p15 Protein Antigen. Viruses. 2023; 15(9):1939. https://doi.org/10.3390/v15091939
Chicago/Turabian StyleWu, Zhi, Huipeng Lu, Dewei Zhu, Jun Xie, Fan Sun, Yan Xu, Hua Zhang, Zhijun Wu, Wenlong Xia, and Shanyuan Zhu. 2023. "Developing an Indirect ELISA for the Detection of African Swine Fever Virus Antibodies Using a Tag-Free p15 Protein Antigen" Viruses 15, no. 9: 1939. https://doi.org/10.3390/v15091939
APA StyleWu, Z., Lu, H., Zhu, D., Xie, J., Sun, F., Xu, Y., Zhang, H., Wu, Z., Xia, W., & Zhu, S. (2023). Developing an Indirect ELISA for the Detection of African Swine Fever Virus Antibodies Using a Tag-Free p15 Protein Antigen. Viruses, 15(9), 1939. https://doi.org/10.3390/v15091939





