Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Search Results
3.2. Reports of Emergent INSTI-Associated DRMs
3.3. Phenotypic Impact of DTG-Selected INSTI-Associated DRMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society–USA Panel. JAMA 2022, 329, 63. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Available online: https://www.who.int/publications-detail-redirect/9789240031593 (accessed on 17 August 2022).
- Rhee, S.-Y.; Grant, P.M.; Tzou, P.L.; Barrow, G.; Harrigan, P.R.; Ioannidis, J.P.A.; Shafer, R.W. A Systematic Review of the Genetic Mechanisms of Dolutegravir Resistance. J. Antimicrob. Chemother. 2019, 74, 3135–3149. [Google Scholar] [CrossRef] [PubMed]
- Tzou, P.L.; Rhee, S.-Y.; Descamps, D.; Clutter, D.S.; Hare, B.; Mor, O.; Grude, M.; Parkin, N.; Jordan, M.R.; Bertagnolio, S.; et al. Integrase Strand Transfer Inhibitor (INSTI)-Resistance Mutations for the Surveillance of Transmitted HIV-1 Drug Resistance. J. Antimicrob. Chemother. 2020, 75, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rhee, S.-Y.; Taylor, J.; Shafer, R.W. Comparison of the Precision and Sensitivity of the Antivirogram and PhenoSense HIV Drug Susceptibility Assays. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 38, 439. [Google Scholar] [CrossRef] [PubMed]
- Eron, J.J.; Clotet, B.; Durant, J.; Katlama, C.; Kumar, P.; Lazzarin, A.; Poizot-Martin, I.; Richmond, G.; Soriano, V.; Ait-Khaled, M.; et al. Safety and Efficacy of Dolutegravir in Treatment-Experienced Subjects with Raltegravir-Resistant HIV Type 1 Infection: 24-Week Results of the VIKING Study. J. Infect. Dis. 2013, 207, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Maggiolo, F.; Penco, G.; Wright, D.; Mills, A.; Grossberg, R.; Molina, J.-M.; Chas, J.; Durant, J.; Moreno, S.; et al. Dolutegravir in Antiretroviral-Experienced Patients with Raltegravir- and/or Elvitegravir-Resistant HIV-1: 24-Week Results of the Phase III VIKING-3 Study. J. Infect. Dis. 2014, 210, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.D.; Wrin, T.; Grant, R.M.; Martin, J.N.; Segal, M.R.; Petropoulos, C.J.; Deeks, S.G. Evolution of Phenotypic Drug Susceptibility and Viral Replication Capacity during Long-Term Virologic Failure of Protease Inhibitor Therapy in Human Immunodeficiency Virus-Infected Adults. J. Virol. 2002, 76, 11104–11112. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus Raltegravir in Antiretroviral-Experienced, Integrase-Inhibitor-Naive Adults with HIV: Week 48 Results from the Randomised, Double-Blind, Non-Inferiority SAILING Study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef]
- Lepik, K.J.; Harrigan, P.R.; Yip, B.; Wang, L.; Robbins, M.A.; Zhang, W.W.; Toy, J.; Akagi, L.; Lima, V.D.; Guillemi, S.; et al. Emergent Drug Resistance with Integrase Strand Transfer Inhibitor-Based Regimens. AIDS 2017, 31, 1425–1434. [Google Scholar] [CrossRef]
- Oldenbuettel, C.; Wolf, E.; Ritter, A.; Noe, S.; Heldwein, S.; Pascucci, R.; Wiese, C.; Krosigk, A.V.; Jaegel-Guedes, E.; Jaeger, H.; et al. Dolutegravir Monotherapy as Treatment De-Escalation in HIV-Infected Adults with Virological Control: DoluMono Cohort Results. Antivir. Ther. 2017, 22, 169–172. [Google Scholar] [CrossRef]
- Wijting, I.; Rokx, C.; Boucher, C.; van Kampen, J.; Pas, S.; de Vries-Sluijs, T.; Schurink, C.; Bax, H.; Derksen, M.; Andrinopoulou, E.-R.; et al. Dolutegravir as Maintenance Monotherapy for HIV (DOMONO): A Phase 2, Randomised Non-Inferiority Trial. Lancet HIV 2017, 4, e547–e554. [Google Scholar] [CrossRef]
- Blanco, J.L.; Rojas, J.; Paredes, R.; Negredo, E.; Mallolas, J.; Casadella, M.; Clotet, B.; Gatell, J.M.; de Lazzari, E.; Martinez, E.; et al. Dolutegravir-Based Maintenance Monotherapy versus Dual Therapy with Lamivudine: A Planned 24 Week Analysis of the DOLAM Randomized Clinical Trial. J. Antimicrob. Chemother. 2018, 73, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; Baptista, T.; Diogo, I.; Aleixo, M.J.; Marques, N.; Mansinho, K.; Gomes, P. Two Cases of Dolutegravir Failure with R263K Mutation. AIDS 2018, 32, 2639–2640. [Google Scholar] [CrossRef]
- Cochrane, S.; Daniel, J.; Forsyth, S.; Smit, E. First Reported Case of Integrase (R263K, G163R) and Reverse Transcriptase (M184V)-Transmitted Drug Resistance from a Drug-Naive Patient Failing Triumeq. AIDS 2018, 32, 1905. [Google Scholar] [CrossRef]
- Fulcher, J.A.; Du, Y.; Zhang, T.; Sun, R.; Landovitz, R.J. Emergence of Integrase Resistance Mutations During Initial Therapy Containing Dolutegravir. Clin. Infect. Dis. 2018, 67, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Chueca, N.; D’Avolio, A.; Zarzalejos, J.M.; Garcia, F. Virological Failure in HIV to Triple Therapy with Dolutegravir-Based Firstline Treatment: Rare but Possible. Open Forum Infect. Dis. 2019, 6, ofy332. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, B.O.; Zheng, L.; Stefanescu, A.; Nyaku, A.; Bezins, B.; Wallis, C.L.; Godfrey, C.; Sax, P.E.; Acosta, E.; Haas, D.; et al. ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)–Infected Participants with HIV-1 RNA <500000 Copies/mL. Clin. Infect. Dis. 2018, 66, 1689–1697. [Google Scholar] [CrossRef]
- Ahmed, N.; Flavell, S.; Ferns, B.; Frampton, D.; Edwards, S.G.; Miller, R.F.; Grant, P.; Nastouli, E.; Gupta, R.K. Development of the R263K Mutation to Dolutegravir in an HIV-1 Subtype D Virus Harboring 3 Class-Drug Resistance. Open Forum Infect. Dis. 2019, 6, ofy329. [Google Scholar] [CrossRef] [PubMed]
- Hocqueloux, L.; Raffi, F.; Prazuck, T.; Bernard, L.; Sunder, S.; Esnault, J.-L.; Rey, D.; Le Moal, G.; Roncato-Saberan, M.; André, M.; et al. Dolutegravir Monotherapy versus Dolutegravir/Abacavir/Lamivudine for Virologically Suppressed People Living With Chronic Human Immunodeficiency Virus Infection: The Randomized Noninferiority MONotherapy of TiviCAY Trial. Clin. Infect. Dis. 2019, 69, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Lübke, N.; Jensen, B.; Hüttig, F.; Feldt, T.; Walker, A.; Thielen, A.; Däumer, M.; Obermeier, M.; Kaiser, R.; Knops, E.; et al. Failure of Dolutegravir First-Line ART with Selection of Virus Carrying R263K and G118R. N. Engl. J. Med. 2019, 381, 887–889. [Google Scholar] [CrossRef]
- Mahomed, K.; Wallis, C.L.; Dunn, L.; Maharaj, S.; Maartens, G.; Meintjes, G. Case Report: Emergence of Dolutegravir Resistance in a Patient on Second-Line Antiretroviral Therapy. S. Afr. J. HIV Med. 2020, 21, 1062. [Google Scholar] [CrossRef] [PubMed]
- Frange, P.; Blanche, S.; Veber, F.; Avettand-Fenoel, V. Dolutegravir in the Long Term in Children and Adolescents: Frequent Virological Failure but Rare Acquired Genotypic Resistance. HIV Med. 2021, 22, 958–964. [Google Scholar] [CrossRef]
- Seatla, K.K.; Maruapula, D.; Choga, W.T.; Ntsipe, T.; Mathiba, N.; Mogwele, M.; Kapanda, M.; Nkomo, B.; Ramaabya, D.; Makhema, J.; et al. HIV-1 Subtype C Drug Resistance Mutations in Heavily Treated Patients Failing Integrase Strand Transfer Inhibitor-Based Regimens in Botswana. Viruses 2021, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Turkova, A.; White, E.; Mujuru, H.A.; Kekitiinwa, A.R.; Kityo, C.M.; Violari, A.; Lugemwa, A.; Cressey, T.R.; Musoke, P.; Variava, E.; et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N. Engl. J. Med. 2021, 385, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Botha, J.C.; Steegen, K.; Edoo, M.; Nel, J.; van Zyl, G.U. Low-Level Viraemia despite Emergence of Dolutegravir-Resistant Variants. S. Afr. J. HIV Med. 2022, 23, 1398. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.; Delgado, E.; Benito, S.; Moreno-Lorenzo, M.; Thomson, M.M.; the Spanish Group for the Study of Antiretroviral Drug Resistance. Factors Associated with HIV-1 Resistance to Integrase Strand Transfer Inhibitors in Spain: Implications for Dolutegravir-Containing Regimens. Front. Microbiol. 2022, 13, 1051096. [Google Scholar] [CrossRef] [PubMed]
- Mandikiyana Chirimuta, L.A.; Pascoe, M.J.; Lowe, S. Emergent Dolutegravir Resistance in Integrase-Naïve, Treatment Experienced Patients from Zimbabwe. S. Afr. J. HIV Med. 2022, 23, 1435. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Musaazi, J.; Kityo, C.; Walimbwa, S.; Hoppe, A.; Balyegisawa, A.; Asienzo, J.; Kaimal, A.; Mirembe, G.; Lugemwa, A.; et al. Efficacy and Safety of Dolutegravir or Darunavir in Combination with Lamivudine plus Either Zidovudine or Tenofovir for Second-Line Treatment of HIV Infection (NADIA): Week 96 Results from a Prospective, Multicentre, Open-Label, Factorial, Randomised, Non-Inferiority Trial. Lancet HIV 2022, 9, e381–e393. [Google Scholar] [CrossRef]
- Revollo, B.; Viñuela, L.; de la Mora, L.; García, F.; Noguera-Julián, M.; Parera, M.; Paredes, R.; Llibre, J.M. Integrase Resistance Emergence with Dolutegravir/Lamivudine with Prior HIV-1 Suppression. J. Antimicrob. Chemother. 2022, 77, 1738–1740. [Google Scholar] [CrossRef]
- Schramm, B.; Temfack, E.; Descamps, D.; Nicholas, S.; Peytavin, G.; Bitilinyu-Bangoh, J.E.; Storto, A.; Lê, M.P.; Abdi, B.; Ousley, J.; et al. Viral Suppression and HIV-1 Drug Resistance 1 Year after Pragmatic Transitioning to Dolutegravir First-Line Therapy in Malawi: A Prospective Cohort Study. Lancet HIV 2022, 9, e544–e553. [Google Scholar] [CrossRef]
- Underwood, M.; Horton, J.; Nangle, K.; Hopking, J.; Smith, K.; Aboud, M.; Wynne, B.; Sievers, J.; Stewart, E.L.; Wang, R. Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study. Antimicrob. Agents Chemother. 2022, 66, e0164321. [Google Scholar] [CrossRef] [PubMed]
- van Kampen, J.J.A.; Pham, H.T.; Yoo, S.; Overmars, R.J.; Lungu, C.; Mahmud, R.; Schurink, C.A.M.; van Boheemen, S.; Gruters, R.A.; Fraaij, P.L.A.; et al. HIV-1 Resistance against Dolutegravir Fluctuates Rapidly alongside Erratic Treatment Adherence: A Case Report. J. Glob. Antimicrob. Resist. 2022, 31, 323–327. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, J.J.; Chipungu, C.; Nkhoma, L.; Kanise, H.; Hosseinipour, M.C.; Sagno, J.B.; Simon, K.; Cox, C.; Hoffman, R.; Steegen, K.; et al. Dolutegravir Resistance in Malawi’s National HIV Treatment Program. Open Forum Infect. Dis. 2022, 9, ofac148. [Google Scholar] [CrossRef] [PubMed]
- Vavro, C.; Ruel, T.; Wiznia, A.; Montañez, N.; Nangle, K.; Horton, J.; Buchanan, A.M.; Stewart, E.L.; Palumbo, P. Emergence of Resistance in HIV-1 Integrase with Dolutegravir Treatment in a Pediatric Population from the IMPAACT P1093 Study. Antimicrob. Agents Chemother. 2022, 66, e0164521. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Kida, I.M.; Maina, U.A.; Ibrahim, A.H.; Mshelia, J.; Wisso, H.; Adamu, A.; Onyemata, J.E.; Edun, M.; Yusuph, H.; et al. Limited Emergence of Resistance to Integrase Strand Transfer Inhibitors (INSTIs) in ART-Experienced Participants Failing Dolutegravir-Based Antiretroviral Therapy: A Cross-Sectional Analysis of a Northeast Nigerian Cohort. J. Antimicrob. Chemother. 2023, 78, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Armenia, D.; Santoro, M.M.; Charpentier, C.; Bertoli, A.; Forbici, F.; Calvez, V.; Descamps, D.; Ceccherini-Silberstein, F.; Marcelin, A.-G.; Flandre, P.; et al. Evaluation of Integrase Resistance in Individuals Who Failed a Regimen Containing Dolutegravir in French and Italian Clinical Settings. J. Antimicrob. Chemother. 2023, 78, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Ambrose, A.; Kanitkar, T.; Flores, K.; Simoes, P.; Hart, J.; Hunter, A.; Akodu, J.; Barber, T.J. Real World Use of Dolutegravir Two Drug Regimens. AIDS 2023, 37, 785. [Google Scholar] [CrossRef]
- Chinula, L.; Ziemba, L.; Brummel, S.; McCarthy, K.; Coletti, A.; Krotje, C.; Johnston, B.; Knowles, K.; Moyo, S.; Stranix-Chibanda, L.; et al. Efficacy and Safety of Three Antiretroviral Therapy Regimens Started in Pregnancy up to 50 Weeks Post Partum: A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet HIV 2023, 10, e363–e374. [Google Scholar] [CrossRef]
- Diaz, R.S.; Hunter, J.R.; Camargo, M.; Dias, D.; Galinskas, J.; Nassar, I.; De Lima, I.B.; Caldeira, D.B.; Sucupira, M.C.; Schechter, M. Dolutegravir-Associated Resistance Mutations after First-Line Treatment Failure in Brazil. BMC Infect. Dis. 2023, 23, 347. [Google Scholar] [CrossRef]
- Kamori, D.; Barabona, G.; Rugemalila, J.; Maokola, W.; Masoud, S.S.; Mizinduko, M.; Sabasaba, A.; Ruhago, G.; Sambu, V.; Mushi, J.; et al. Emerging Integrase Strand Transfer Inhibitor Drug Resistance Mutations among Children and Adults on ART in Tanzania: Findings from a National Representative HIV Drug Resistance Survey. J. Antimicrob. Chemother. 2023, 78, 779–787. [Google Scholar] [CrossRef]
- Khamadi, S.A.; Bahemana, E.; Dear, N.; Mavere, C.; George, F.; Kapene, R.; Papianus, G.; Willoughby, W.; Chambers, J.; Ganesan, K.; et al. Factors Associated With Viral Suppression and Drug Resistance in Children and Adolescents Living With HIV in Care and Treatment Programs in Southern Tanzania. J. Pediatr. Infect. Dis. Soc. 2023, 12, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Malinga, S.; Khan, A.; Archary, M. Breaking the Unbreakable: A Paediatric Case of Dolutegravir Resistance from KwaZulu-Natal. S. Afr. J. HIV Med. 2023, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Palmier, E.; De Miguel, R.; Montejano, R.; Busca, C.; Micán, R.; Ramos, L.; Cadiñanos, J.; Serrano, L.; Bernardino, J.I.; Pérez-Valero, I.; et al. Three-Year Efficacy of Switching to Dolutegravir plus Lamivudine: A Real-World Study. HIV Med. 2023, 24, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.E.; Hluhanich, R.M.; Goodman, D.D.; Andreatta, K.N.; Margot, N.A.; Ye, L.; Niedziela-Majka, A.; Barnes, T.L.; Novikov, N.; Chen, X.; et al. Impact of Primary Elvitegravir Resistance-Associated Mutations in HIV-1 Integrase on Drug Susceptibility and Viral Replication Fitness. Antimicrob. Agents Chemother. 2013, 57, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.E.; Ram, R.R.; White, K.L.; Miller, M.D.; Callebaut, C. Pre-Existing HIV-1 Integrase Polymorphisms Do Not Impact Treatment Response to Elvitegravir-Containing Fixed-Dose Combination Regimens in Treatment-Naive Patients; HIV Glasgow: Glasgow, UK, 2016. [Google Scholar]
- Andreatta, K.N.; Chang, S.; Martin, R.; Willkom, M.; White, K. Integrase Inhibitor Resistance Selections Initiated with Drug Resistant HIV-1. In Proceedings of the Conference on Retroviruses and Oppotunistic Infections, Boston, MA, USA, 4–7 March 2018. [Google Scholar]
- George, J.M.; Kuriakose, S.S.; Dee, N.; Stoll, P.; Lalani, T.; Dewar, R.; Khan, M.A.; Rehman, M.T.; Grossman, Z.; Maldarelli, F.; et al. Rapid Development of High-Level Resistance to Dolutegravir With Emergence of T97A Mutation in 2 Treatment-Experienced Individuals With Baseline Partial Sensitivity to Dolutegravir. Open Forum Infect. Dis. 2018, 5, ofy221. [Google Scholar] [CrossRef] [PubMed]
- Hardy, I.; Brenner, B.; Quashie, P.; Thomas, R.; Petropoulos, C.; Huang, W.; Moisi, D.; Wainberg, M.A.; Roger, M. Evolution of a Novel Pathway Leading to Dolutegravir Resistance in a Patient Harbouring N155H and Multiclass Drug Resistance. J. Antimicrob. Chemother. 2015, 70, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Margot, N.A.; Ram, R.R.; White, K.L.; Abram, M.E.; Callebaut, C. Antiviral Activity of HIV-1 Integrase Strand-Transfer Inhibitors against Mutants with Integrase Resistance-Associated Mutations and Their Frequency in Treatment-Naïve Individuals. J. Med. Virol. 2019, 91, 2188–2194. [Google Scholar] [CrossRef]
- Marzinke, M.A.; Grinsztejn, B.; Fogel, J.M.; Piwowar-Manning, E.; Li, M.; Weng, L.; McCauley, M.; Cummings, V.; Ahmed, S.; Haines, C.D.; et al. Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. J. Infect. Dis. 2021, 224, 1581–1592. [Google Scholar] [CrossRef]
- Mesplède, T.; Quashie, P.K.; Osman, N.; Han, Y.; Singhroy, D.N.; Lie, Y.; Petropoulos, C.J.; Huang, W.; Wainberg, M.A. Viral Fitness Cost Prevents HIV-1 from Evading Dolutegravir Drug Pressure. Retrovirology 2013, 10, 22. [Google Scholar] [CrossRef]
- Mesplede, T.; Quashie, P.K.; Hassounah, S.; Osman, N.; Han, Y.; Liang, J.; Singhroy, D.N.; Wainberg, M.A. The R263K Substitution in HIV-1 Subtype C Is More Deleterious for Integrase Enzymatic Function and Viral Replication than in Subtype B. AIDS 2015, 29, 1459–1466. [Google Scholar] [CrossRef]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Jaeger, H.; Orrell, C.; Nagimova, F.; Bredeek, F.; García Deltoro, M.; Swindells, S.; Andrade-Villanueva, J.F.; et al. Long-Acting Cabotegravir and Rilpivirine Dosed Every 2 Months in Adults with HIV-1 Infection (ATLAS-2M), 48-Week Results: A Randomised, Multicentre, Open-Label, Phase 3b, Non-Inferiority Study. Lancet 2021, 396, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Puertas, M.C.; Ploumidis, G.; Ploumidis, M.; Fumero, E.; Clotet, B.; Walworth, C.M.; Petropoulos, C.J.; Martinez-Picado, J. Pan-Resistant HIV-1 Emergence in the Era of Integrase Strand-Transfer Inhibitors: A Case Report. Lancet Microbe 2020, 1, e130–e135. [Google Scholar] [CrossRef]
- Quashie, P.K.; Oliviera, M.; Veres, T.; Osman, N.; Han, Y.-S.; Hassounah, S.; Lie, Y.; Huang, W.; Mesplède, T.; Wainberg, M.A. Differential Effects of the G118R, H51Y, and E138K Resistance Substitutions in Different Subtypes of HIV Integrase. J. Virol. 2015, 89, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Overton, E.T.; Orkin, C.; Swindells, S.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Girard, P.-M.; Oka, S.; Andrade-Villanueva, J.F.; et al. Long-Acting Injectable Cabotegravir + Rilpivirine for HIV Maintenance Therapy: Week 48 Pooled Analysis of Phase 3 ATLAS and FLAIR Trials. J. Acquir. Immune Defic. Syndr. 1999 2020, 85, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Fornabaio, C.; Malena, M.; Galli, L.; Poli, A.; Menozzi, M.; Zazzi, M.; White, K.L.; Castagna, A.; PRESTIGIO Study Group. Susceptibility to HIV-1 Integrase Strand Transfer Inhibitors (INSTIs) in Highly Treatment-Experienced Patients Who Failed an INSTI-Based Regimen. Int. J. Antimicrob. Agents 2020, 56, 106027. [Google Scholar] [CrossRef] [PubMed]
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 7086–7097. [Google Scholar] [CrossRef]
- Underwood, M.R.; Johns, B.A.; Sato, A.; Martin, J.N.; Deeks, S.G.; Fujiwara, T. The Activity of the Integrase Inhibitor Dolutegravir against HIV-1 Variants Isolated from Raltegravir-Treated Adults. J. Acquir. Immune Defic. Syndr. 1999 2012, 61, 297–301. [Google Scholar] [CrossRef]
- van Wyk, J.; Orkin, C.; Rubio, R.; Bogner, J.; Baker, D.; Khuong-Josses, M.-A.; Parks, D.; Angelis, K.; Kahl, L.P.; Matthews, J.; et al. Brief Report: Durable Suppression and Low Rate of Virologic Failure 3 Years After Switch to Dolutegravir + Rilpivirine 2-Drug Regimen: 148-Week Results From the SWORD-1 and SWORD-2 Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 1999 2020, 85, 325–330. [Google Scholar] [CrossRef]
- Varghese, V.; Pinsky, B.A.; Smith, D.S.; Klein, D.; Shafer, R.W. Q148N, a Novel Integrase Inhibitor Resistance Mutation Associated with Low-Level Reduction in Elvitegravir Susceptibility. AIDS Res. Hum. Retroviruses 2016, 32, 702–704. [Google Scholar] [CrossRef]
- Underwood, M. Euro Resistance Wk: Resistance Post Week 48 in ART-Experienced, Integrase Inhibitor-Naive Subjects With Dolutegravir (DTG) vs. Raltegravir (RAL) in SAILING (ING111762). In Proceedings of the 13th European HIV & Hepatitis Workshop, Barcelona, Spain, 3–5 June 2015; Available online: https://www.natap.org/2015/HIV/061715_02.htm (accessed on 18 July 2023).
- Posada, D.; Crandall, K.A. Selecting Models of Nucleotide Substitution: An Application to Human Immunodeficiency Virus 1 (HIV-1). Mol. Biol. Evol. 2001, 18, 897–906. [Google Scholar] [CrossRef]
- Anstett, K.; Mesplede, T.; Oliveira, M.; Cutillas, V.; Wainberg, M.A. Dolutegravir Resistance Mutation R263K Cannot Coexist in Combination with Many Classical Integrase Inhibitor Resistance Substitutions. J. Virol. 2015, 89, 4681–4684. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.A.; Cleyle, J.; Yoo, S.; Forrest, M.; Krullaars, Z.; Pham, H.T.; Mesplède, T. The G118R plus R263K Combination of Integrase Mutations Associated with Dolutegravir-Based Treatment Failure Reduces HIV-1 Replicative Capacity and Integration. Antimicrob. Agents Chemother. 2023, 67, e01386-22. [Google Scholar] [CrossRef] [PubMed]
- Anstett, K.; Cutillas, V.; Fusco, R.; Mesplède, T.; Wainberg, M.A. Polymorphic Substitution E157Q in HIV-1 Integrase Increases R263K-Mediated Dolutegravir Resistance and Decreases DNA Binding Activity. J. Antimicrob. Chemother. 2016, 71, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.A.; Panpradist, N.; Beck, I.A.; Lutz, B.; Lai, J.; Kanthula, R.M.; Kantor, R.; Tripathi, A.; Saravanan, S.; MacLeod, I.J.; et al. Current Status of Point-of-Care Testing for Human Immunodeficiency Virus Drug Resistance. J. Infect. Dis. 2017, 216, S824–S828. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-Y.; Parkin, N.; Harrigan, P.R.; Holmes, S.; Shafer, R.W. Genotypic Correlates of Resistance to the HIV-1 Strand Transfer Integrase Inhibitor Cabotegravir. Antivir. Res. 2022, 208, 105427. [Google Scholar] [CrossRef] [PubMed]
- Malet, I.; Subra, F.; Charpentier, C.; Collin, G.; Descamps, D.; Calvez, V.; Marcelin, A.-G.; Delelis, O. Mutations Located Outside the Integrase Gene Can Confer Resistance to HIV-1 Integrase Strand Transfer Inhibitors. mBio 2017, 8, e00922-17. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kubota, M.; Shigemi, U.; Ode, H.; Yokomaku, Y.; Kirby, K.A.; Sarafianos, S.G.; Iwatani, Y. Specific Mutations in the HIV-1 G-Tract of the 3′-Polypurine Tract Cause Resistance to Integrase Strand Transfer Inhibitors. J. Antimicrob. Chemother. 2022, 77, 574–577. [Google Scholar] [CrossRef]
- Richetta, C.; Subra, F.; Malet, I.; Leh, H.; Charpentier, C.; Corona, A.; Collin, G.; Descamps, D.; Deprez, E.; Parissi, V.; et al. Mutations in the 3′-PPT Lead to HIV-1 Replication without Integration. J. Virol. 2022, 96, e00676-22. [Google Scholar] [CrossRef]
- Wijting, I.E.A.; Lungu, C.; Rijnders, B.J.A.; van der Ende, M.E.; Pham, H.T.; Mesplede, T.; Pas, S.D.; Voermans, J.J.C.; Schuurman, R.; van de Vijver, D.A.M.C.; et al. HIV-1 Resistance Dynamics in Patients With Virologic Failure to Dolutegravir Maintenance Monotherapy. J. Infect. Dis. 2018, 218, 688–697. [Google Scholar] [CrossRef]
- Malet, I.; Delelis, O.; Nguyen, T.; Leducq, V.; Abdi, B.; Morand-Joubert, L.; Calvez, V.; Marcelin, A.-G. Variability of the HIV-1 3′ Polypurine Tract (3′PPT) Region and Implication in Integrase Inhibitor Resistance. J. Antimicrob. Chemother. 2019, 74, 3440–3444. [Google Scholar] [CrossRef]
- Seatla, K.K.; Maruapula, D.; Choga, W.T.; Morerinyane, O.; Lockman, S.; Novitsky, V.; Kasvosve, I.; Moyo, S.; Gaseitsiwe, S. Limited HIV-1 Subtype C Nef 3′PPT Variation in Combination Antiretroviral Therapy Naïve and Experienced People Living with HIV in Botswana. Pathogens 2021, 10, 1027. [Google Scholar] [CrossRef]
- Van Duyne, R.; Kuo, L.S.; Pham, P.; Fujii, K.; Freed, E.O. Mutations in the HIV-1 Envelope Glycoprotein Can Broadly Rescue Blocks at Multiple Steps in the Virus Replication Cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 9040–9049. [Google Scholar] [CrossRef]
- Hikichi, Y.; Van Duyne, R.; Pham, P.; Groebner, J.L.; Wiegand, A.; Mellors, J.W.; Kearney, M.F.; Freed, E.O. Mechanistic Analysis of the Broad Antiretroviral Resistance Conferred by HIV-1 Envelope Glycoprotein Mutations. mBio 2021, 12, e03134-20. [Google Scholar] [CrossRef]
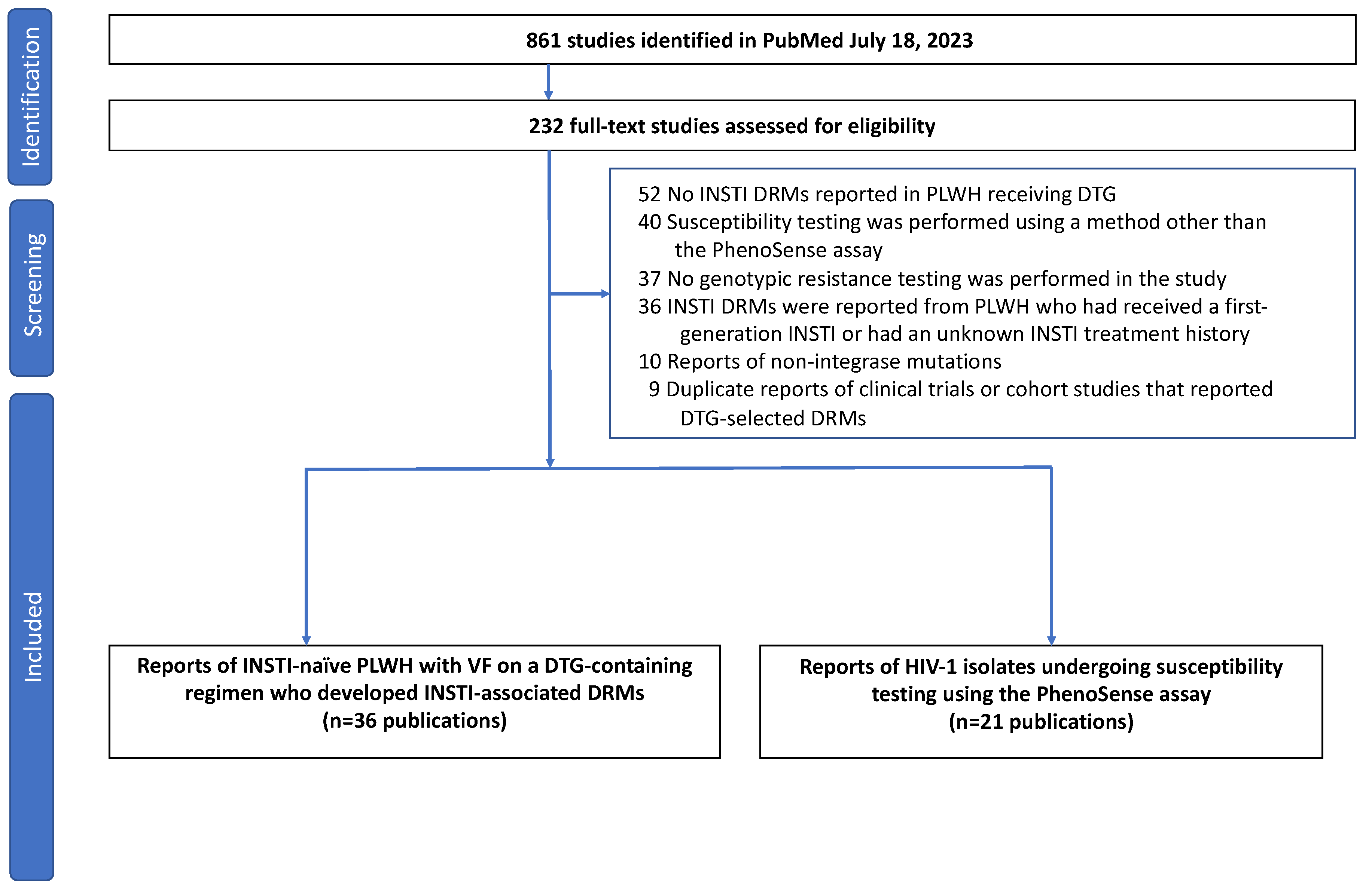
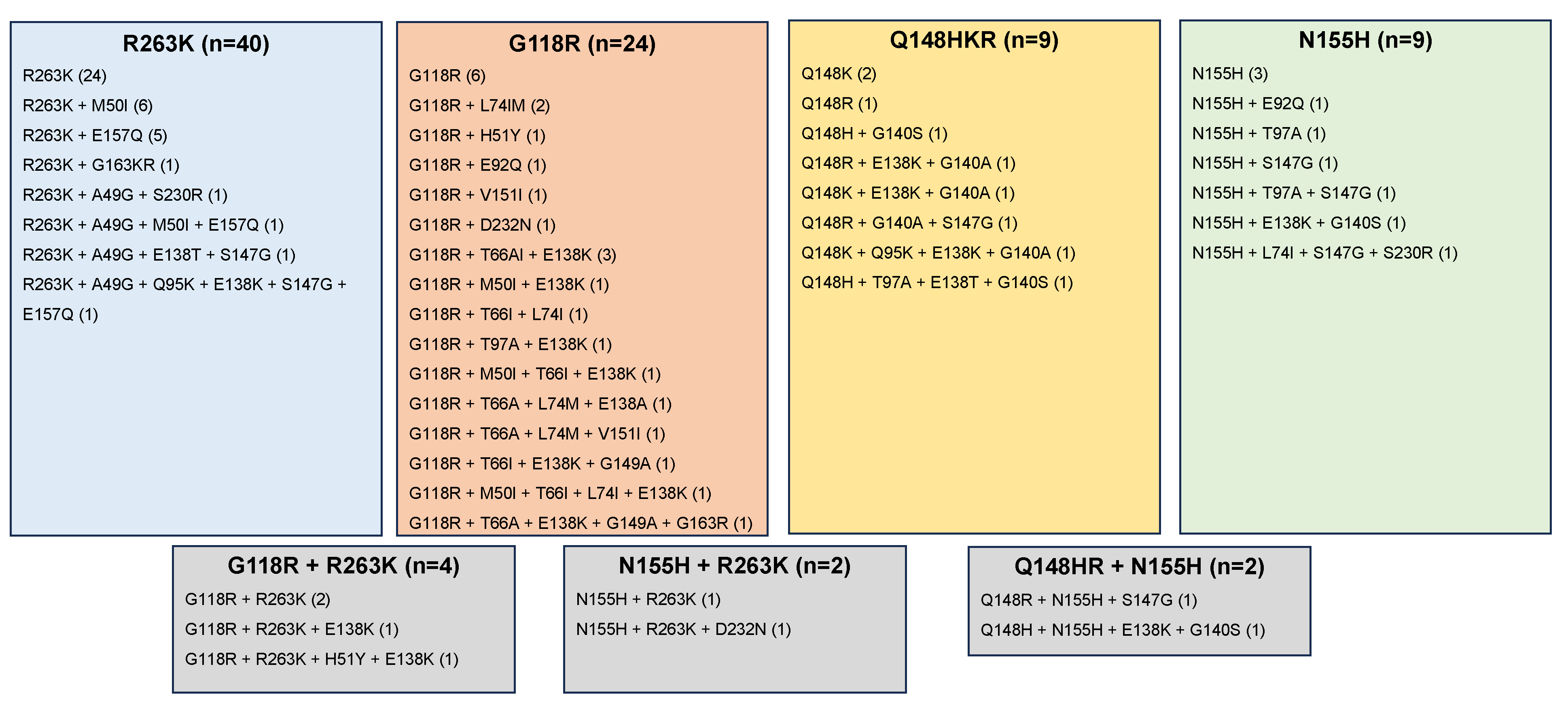
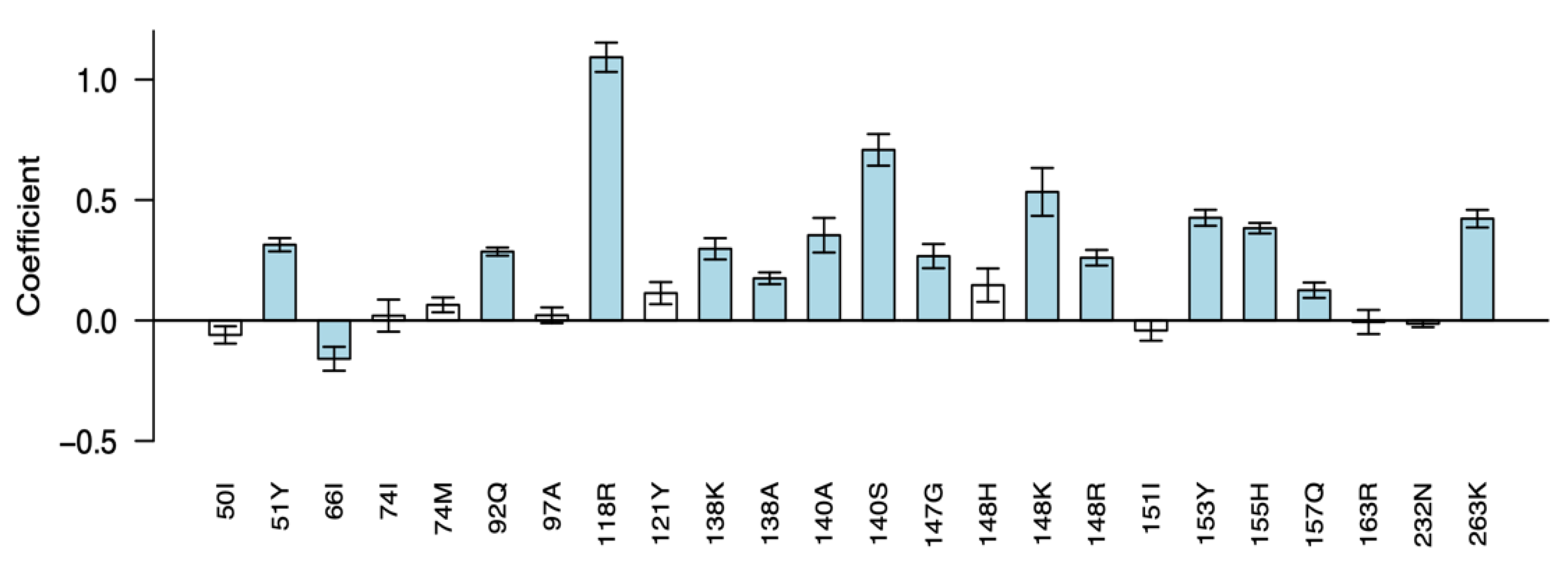
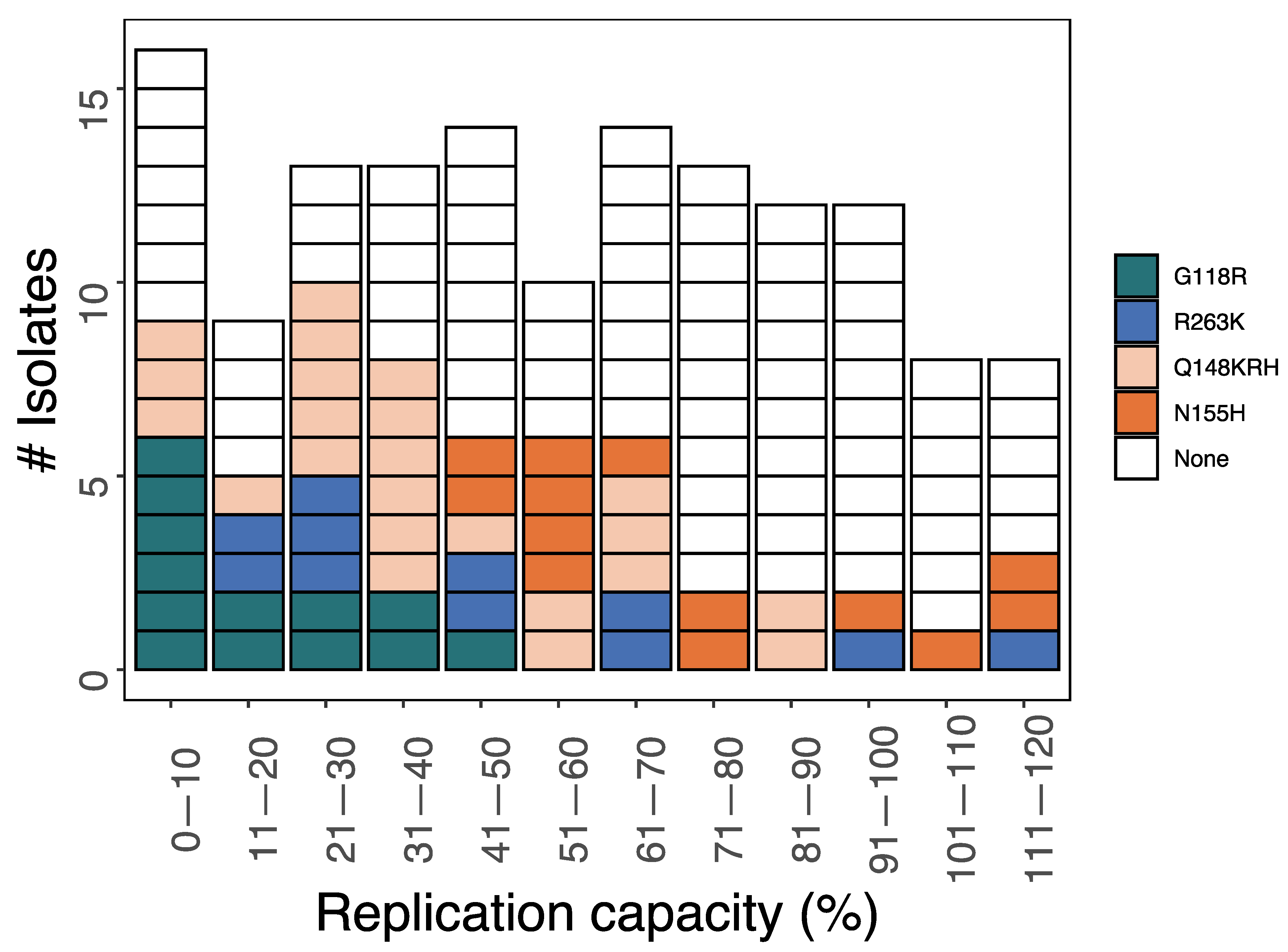
| Author (Year) 1 | Countries | Population 2 | ART Hx | VL Status | ART Regimen 3 | # with DRMs |
|---|---|---|---|---|---|---|
| Blanco (2018) [13] | Spain | Trial | Experienced | VS | DTG | 2 |
| Cahn (2013) [9] | Multinational | Trial | Experienced | Viremic | DTG + OBR | 5 |
| Chinula (2023) [39] | Multinational | Trial | Naïve | Viremic | DTG + 2 NRTIs | 1 |
| Hocqueloux (2019) [20] | France | Trial | Experienced | VS | DTG | 2 |
| Paton (2022) [29] | Sub-Saharan Africa | Trial | Experienced | Viremic | DTG + 2 NRTIs | 9 |
| Taiwo (2018) [18] | U.S. | Trial | Naïve | Viremic | DTG + 3TC | 1 |
| Turkova (2021) [25] | Multinational | Trial | Experienced | Viremic | DTG + 2 NRTIs | 4 |
| Underwood (2022) [32] | Multinational | Trial | First-line VF | Viremic | DTG + 2 NRTIs | 6 |
| Vavro (2022) [35] | U.S. | Trial | Experienced | Viremic | DTG + 2 NRTIs | 6 |
| Wijting (2017) [12] | Netherlands | Trial | Experienced | VS | DTG | 4 |
| Abdullah (2023) [36] | Nigeria | Cohort | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Khamadi (2023) [42] | Tanzania | Cohort | Experienced | Viremic | DTG + 2 NRTIs | 3 |
| Oldenbuettel (2017) [11] | Germany | Cohort | Experienced | VS | DTG | 1 |
| Bowman (2023) [38] | U.K. | Cohort | Experienced | VS | DTG + 3TC | 1 |
| Palmier (2023) [44] | Spain | Cohort | Experienced | VS | DTG + 3TC | 1 |
| Armenia (2023) [37] | Italy, France | Case Series | Naïve, Experienced | Viremic, VS | Multiple | 9 |
| Diaz (2023) [40] | Brazil | Case Series | Naïve | Viremic | DTG + 2 NRTIs | 4 |
| Frange (2021) [23] | France | Case Series | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Gil (2022) [27] | Spain | Case Series | Naïve, Experienced | Not reported | DTG + 2 NRTIs | 5 |
| Kamori (2023) [41] | Tanzania | Case Series | Experienced | Viremic | DTG + 2 NRTIs | 3 |
| Lepik (2017) [10] | Canada | Case Series | Naïve, Experienced | Viremic | DTG + 2 NRTIs | 3 |
| Schramm (2022) [31] | Malawi | Case Series | Experienced | Viremic | DTG + 2 NRTIs | 2 |
| Seatla (2021) [24] | Botswana | Case Series | Experienced | Viremic | DTG + 2 NRTIs | 3 |
| Van Oosterhout (2022) [34] | Malawi | Case Series | Naïve, Experienced | Viremic, Not reported | DTG + 2 NRTIs | 8 |
| Ahmed (2019) [19] | East Africa | Case Report(s) | Experienced | Viremic | DTG + DRV | 1 |
| Botha (2022) [26] | South Africa | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Cardoso (2018) [14] | Portugal | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 2 |
| Cochrane (2018) [15] | U.K. | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Fulcher (2018) [16] | U.S. | Case Report(s) | Naïve | Viremic | DTG + 2 NRTIs | 1 |
| Lubke (2019) [21] | Germany | Case Report(s) | Naïve | Viremic | DTG + 2 NRTIs | 1 |
| M. Chirimuta (2022) [28] | Zimbabwe | Case Report(s) | Experienced | VS, Not reported | DTG + 2 NRTIs | 2 |
| Mahomed (2020) [22] | South Africa | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Malinga (2023) [43] | South Africa | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| Pena Lopez (2018) [17] | Spain | Case Report(s) | Naïve | Viremic | DTG + 2 NRTIs | 1 |
| Revollo (2022) [30] | Spain | Case Report(s) | Experienced | VS | DTG + 3TC | 1 |
| van Kampen (2022) [33] | Netherlands | Case Report(s) | Experienced | Viremic | DTG + 2 NRTIs | 1 |
| ART History | VL Status | ART Regimen | # with DRMs |
|---|---|---|---|
| Naïve | Viremic | DTG + 2 NRTIs | 13 |
| Naïve | Viremic | DTG + 3TC | 2 |
| Experienced | Viremic | DTG + 2 NRTIs | 49 |
| Experienced | Viremic | DTG + OBR | 5 |
| Experienced | Viremic | DTG + b/DRV | 1 |
| Experienced | VS | DTG | 11 |
| Experienced | VS | DTG + 2 NRTIs | 4 |
| Experienced | VS | DTG + 3TC | 3 |
| Experienced | VS | DTG + RPV | 1 |
| Experienced | Uncertain | DTG + 2 NRTIs | 9 |
| Experienced | Uncertain | DTG + 3TC | 1 |
| DRM A | DRM B | A and B | A Alone | B Alone | Neither | Spearman Rho | p Value |
|---|---|---|---|---|---|---|---|
| Positively correlated DRMs 2 | |||||||
| G140S | Q148H | 3 | 1 | 0 | 95 | 0.86 | <0.001 |
| G140A | Q148K | 2 | 2 | 2 | 93 | 0.48 | <0.001 |
| G140A | Q148R | 2 | 2 | 3 | 92 | 0.42 | <0.001 |
| T66A | G118R | 5 | 0 | 23 | 70 | 0.37 | <0.001 |
| S147G | N155H | 4 | 4 | 9 | 82 | 0.32 | 0.001 |
| E157Q | R263K | 7 | 0 | 40 | 52 | 0.29 | 0.004 |
| S147G | Q148R | 2 | 6 | 3 | 88 | 0.27 | 0.007 |
| G118R | E138K | 8 | 17 | 8 | 62 | 0.24 | 0.02 |
| L74M | G118R | 2 | 0 | 25 | 70 | 0.23 | 0.02 |
| G140S | N155H | 2 | 2 | 11 | 84 | 0.22 | 0.03 |
| T97A | N155H | 2 | 2 | 11 | 84 | 0.22 | 0.03 |
| A49G | R263K | 4 | 0 | 43 | 52 | 0.22 | 0.03 |
| L74I | G118R | 3 | 1 | 25 | 69 | 0.21 | 0.04 |
| Negatively correlated DRMs 3 | |||||||
| G118R | R263K | 3 | 24 | 42 | 28 | −0.44 | <0.001 |
| E138K | R263K | 2 | 16 | 43 | 36 | −0.34 | <0.001 |
| Q148KRH | R263K | 0 | 11 | 46 | 41 | −0.33 | <0.001 |
| N155H | R263K | 2 | 11 | 45 | 41 | −0.25 | 0.01 |
| G118R | N155H | 0 | 29 | 13 | 57 | −0.25 | 0.01 |
| T66I | R263K | 0 | 6 | 47 | 46 | −0.24 | 0.02 |
| G118R | Q148KRH | 0 | 28 | 11 | 59 | −0.23 | 0.03 |
| Signature DRM | # Additional DRMs | # Results | Median Fold Reduced Susceptibility | IQR | Range |
|---|---|---|---|---|---|
| G118R | 0 | 2 | 18.8 | 14–23 | 9.6–28 |
| 1 | 7 | 22 | 11–29 | 7.2–30 | |
| ≥2 | 5 | 16 | 13–22 | 8.0–52 | |
| R263K | 0 | 7 | 2.0 | 1.8–2.2 | 1.5–3.3 |
| 1 | 5 | 2.1 | 1.7–4.2 | 1.3–7.0 | |
| ≥2 | 1 | 6.3 | 6.3 | 6.3 | |
| N155H | 0 | 8 | 1.4 | 1.2–1.6 | 1.1–2.1 |
| 1 | 14 | 1.7 | 1.5–2.0 | 1.1–3.5 | |
| ≥2 | 8 | 3.1 | 1.9–24 | 1.5–68 | |
| Q148H/R/K | 0 | 11 | 0.8 | 0.7–1.1 | 0.4–1.6 |
| 1 | 44 | 3.4 | 1.9–5.5 | 0.5–17 | |
| ≥2 | 27 | 8.8 | 3.5–15 | 0.6–186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, K.; Rhee, S.-Y.; Chu, C.; Avalos, A.; Ahluwalia, A.K.; Gupta, R.K.; Jordan, M.R.; Shafer, R.W. Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review. Viruses 2023, 15, 1932. https://doi.org/10.3390/v15091932
Tao K, Rhee S-Y, Chu C, Avalos A, Ahluwalia AK, Gupta RK, Jordan MR, Shafer RW. Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review. Viruses. 2023; 15(9):1932. https://doi.org/10.3390/v15091932
Chicago/Turabian StyleTao, Kaiming, Soo-Yon Rhee, Carolyn Chu, Ava Avalos, Amrit K. Ahluwalia, Ravindra K. Gupta, Michael R. Jordan, and Robert W. Shafer. 2023. "Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review" Viruses 15, no. 9: 1932. https://doi.org/10.3390/v15091932
APA StyleTao, K., Rhee, S.-Y., Chu, C., Avalos, A., Ahluwalia, A. K., Gupta, R. K., Jordan, M. R., & Shafer, R. W. (2023). Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review. Viruses, 15(9), 1932. https://doi.org/10.3390/v15091932







