Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Patients Characteristics
3.2. Outcomes
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Therapeutics and COVID-19. Available online: https://www.who.int/teams/health-care-readiness/COVID-19/therapeutics (accessed on 16 February 2023).
- Yang, L.; Wang, Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023, 257, 115503. [Google Scholar] [CrossRef] [PubMed]
- Tomalka, J.A.; Suthar, M.S.; Deeks, S.G.; Sekaly, R.P. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat. Immunol. 2022, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Diaz, G.; Gottlieb, R.L.; Lobo, S.M.; Robinson, P.; Hunter, B.D.; Cavalcante, A.W.; Overcash, J.S.; Hanania, N.A.; Skarbnik, A.; et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial. Intensive Care Med. 2021, 47, 1258–1270. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.-M.; Preziosi, M.-P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.-P..; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, X. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. [Google Scholar] [CrossRef]
- Sun, F.; Lin, Y.; Wang, X.; Gao, Y.; Ye, S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect. Dis. 2022, 22, 1279. [Google Scholar] [CrossRef]
- Burki, T. The future of Paxlovid for COVID-19. Lancet Respir. Med. 2022, 10, e68. [Google Scholar] [CrossRef]
- Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir|Nature. Available online: https://www.nature.com/articles/s41586-022-05514-2 (accessed on 28 July 2023).
- Foisy, M.M.; Yakiwchuk, E.M.; Hughes, C.A. Induction effects of ritonavir: Implications for drug interactions. Ann. Pharmacother. 2008, 42, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Decoding molnupiravir-induced mutagenesis in SARS-CoV-2. J. Biol. Chem. 2021, 297, 100867. [Google Scholar] [CrossRef]
- Lleo, A.; Wang, G.-Q.; Gershwin, M.E.; Hirschfield, G.M. Primary biliary cholangitis. Lancet 2020, 396, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015, 50, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E.; et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 2022, 615, 134–142. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Ward, A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Ursodeoxycholic acid: A review of its pharmacological properties and therapeutic efficacy. Drugs 1984, 27, 95–131. [Google Scholar] [CrossRef]
- Hofmann, A.F. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand. J. Gastroenterol. Suppl. 1994, 204, 1–15. [Google Scholar] [CrossRef]
- Jones, R.J.; Lee, K.S.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Abecasis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Basak, G.; et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A position statement from an international expert group. Bone Marrow Transplant. 2020, 55, 485–495. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.-S. Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children. Liver Int. 1950, 43, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- John, B.V.; Bastaich, D.; Webb, G.; Brevini, T.; Moon, A.; Ferreira, R.D.; Chin, A.M.; Kaplan, D.E.; Taddei, T.H.; Serper, M.; et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J. Intern. Med. 2023, 293, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, N.; Cui, X.; Lin, Y.; Li, X. Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease. Front. Cell. Infect. Microbiol. 2023, 13, 1178590. [Google Scholar] [CrossRef] [PubMed]
- Liverpool COVID-19 Interactions. Available online: https://www.covid19-druginteractions.org/checker (accessed on 8 August 2023).
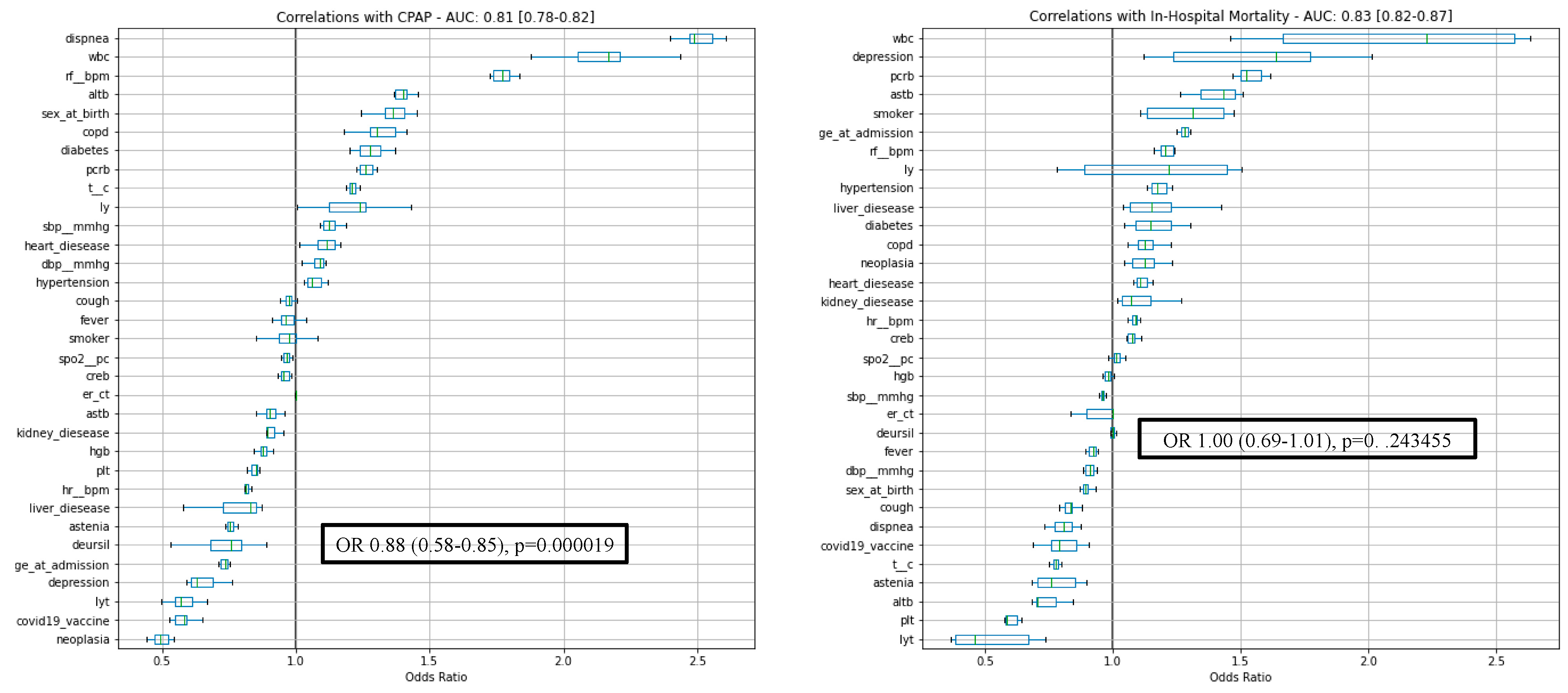
| UDCA-Taking Group (n = 57) | Control Group (n = 3790) | Total Group (n = 3847) | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age (years) (mean, IQR) | 70.0 (58.0–82.0) | 74.0 (61.0–83.0) | 74.0 (61.0–83.0) | 0.87 (0.81–0.98) | 0.000166 |
| Sex (n, %) | |||||
| Male | 31 (54.4%) | 2296 (60.6%) | 2327 (60.5%) | 0.83 (0.70–0.98) | 0.000393 |
| Female | 26 (45.6%) | 1494 (39.4%) | 1520 (39.5%) | ||
| Comorbidities (n, %) | |||||
| Arterial Hypertension | 10 (17.5%) | 1020 (26.9%) | 1030 (26.8%) | 0.36 (0.24–0.43) | 0.000000001 |
| Diabetes Mellitus type 2 | 9 (15.8%) | 520 (12.8%) | 529 (13.8%) | 0.95 (0.63–1.21) | 0.707347 |
| Chronic Obstructive Pulmonary Disease | 7 (12.3%) | 342 (9.0%) | 349 (9.1%) | 2.20 (1.39–2.73) | 0.000048 |
| Heart Disease | 15 (26.3%) | 746 (19.7%) | 761 (19.8%) | 2.43 (2.18–2.94) | 0.00000006 |
| Chronic Kidney Disease | 8 (14.0%) | 293 (7.7%) | 301 (7.8%) | 2.35 (2.10–2.06) | 0.00000049 |
| Liver disease | 8 (14.0%) | 96 (2.5%) | 104 (2.7%) | 5.23 (2.99–7.05) | 0.000001 |
| Neoplasia | 7 (12.3%) | 358 (9.4%) | 365 (9.5%) | 0.91 (0.62–1.20) | 0.239422 |
| COVID-19 vaccination (n,%) | |||||
| Vaccination | 31 (54.4%) | 1145 (30.2%) | 1176 (30.6%) | 1.39 (1.10–1.68) | 0.000329 |
| No vaccination | 26 (45.6%) | 2645 (69.8%) | 2671 69.4%) | ||
| Biochemistry (mean, range) | |||||
| AST (IU/L) | 27.0 (20.75–52.75) | 30.5 (22.0–44.38) | |||
| 26.75 (15.50–50.25) | 29.32 (18.19–51.5) | ||||
| ALT (IU/L) | |||||
| 9 (15.8%) | 983 (25.9%) | ||||
| Outcomes (n,%) | 13 (22.8%) | 806 (21.3%) | |||
| CPAP | 992 (25.8%) | 0.76 (0.58–0.85) | 0.000019 | ||
| In-hospital deaths | 819(21.3%) | 1.00 (0.69–1.01) | 0.243455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colapietro, F.; Angelotti, G.; Masetti, C.; Shiffer, D.; Pugliese, N.; De Nicola, S.; Carella, F.; Desai, A.; Ormas, M.; Calatroni, M.; et al. Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses 2023, 15, 1738. https://doi.org/10.3390/v15081738
Colapietro F, Angelotti G, Masetti C, Shiffer D, Pugliese N, De Nicola S, Carella F, Desai A, Ormas M, Calatroni M, et al. Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses. 2023; 15(8):1738. https://doi.org/10.3390/v15081738
Chicago/Turabian StyleColapietro, Francesca, Giovanni Angelotti, Chiara Masetti, Dana Shiffer, Nicola Pugliese, Stella De Nicola, Francesco Carella, Antonio Desai, Monica Ormas, Marta Calatroni, and et al. 2023. "Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients" Viruses 15, no. 8: 1738. https://doi.org/10.3390/v15081738
APA StyleColapietro, F., Angelotti, G., Masetti, C., Shiffer, D., Pugliese, N., De Nicola, S., Carella, F., Desai, A., Ormas, M., Calatroni, M., Omodei, P., Ciccarelli, M., Aliberti, S., Reggiani, F., Bartoletti, M., Cecconi, M., Lleo, A., Aghemo, A., & Voza, A. (2023). Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses, 15(8), 1738. https://doi.org/10.3390/v15081738








