Antiviral Effect of Candies Containing Persimmon-Derived Tannin against SARS-CoV-2 Delta Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tannin Preparation
2.2. Candies
2.3. Virus Preparation and Biosafety
2.4. Healthy Volunteers
2.5. Patients with COVID-19
2.6. Determination of Virus Titers Using the Plaque Assay
2.7. qPCR
2.8. Statistical Analysis
3. Results
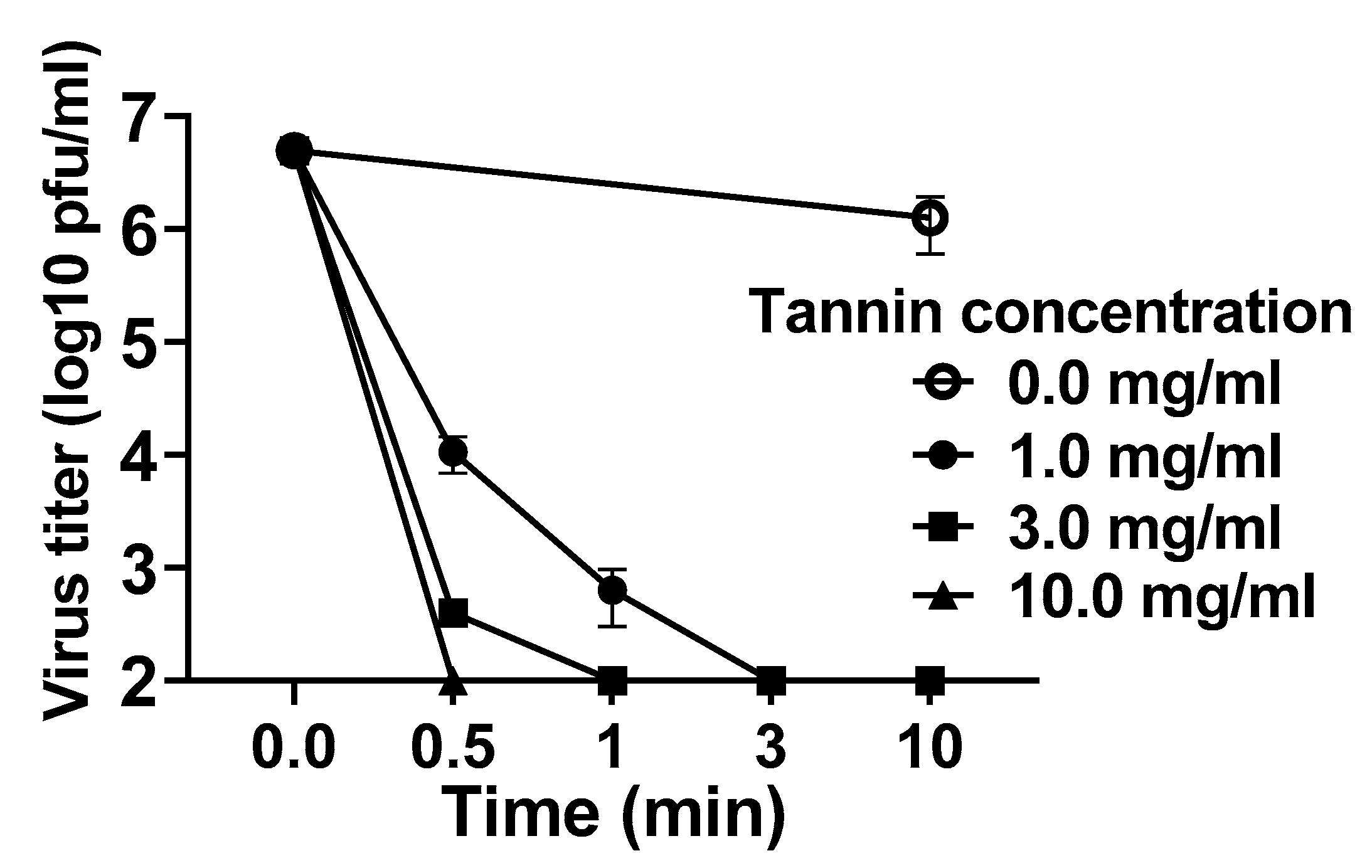
3.1. Persimmon-Derived Tannin Inactivates the Delta Variant of SARS-CoV-2 In Vitro
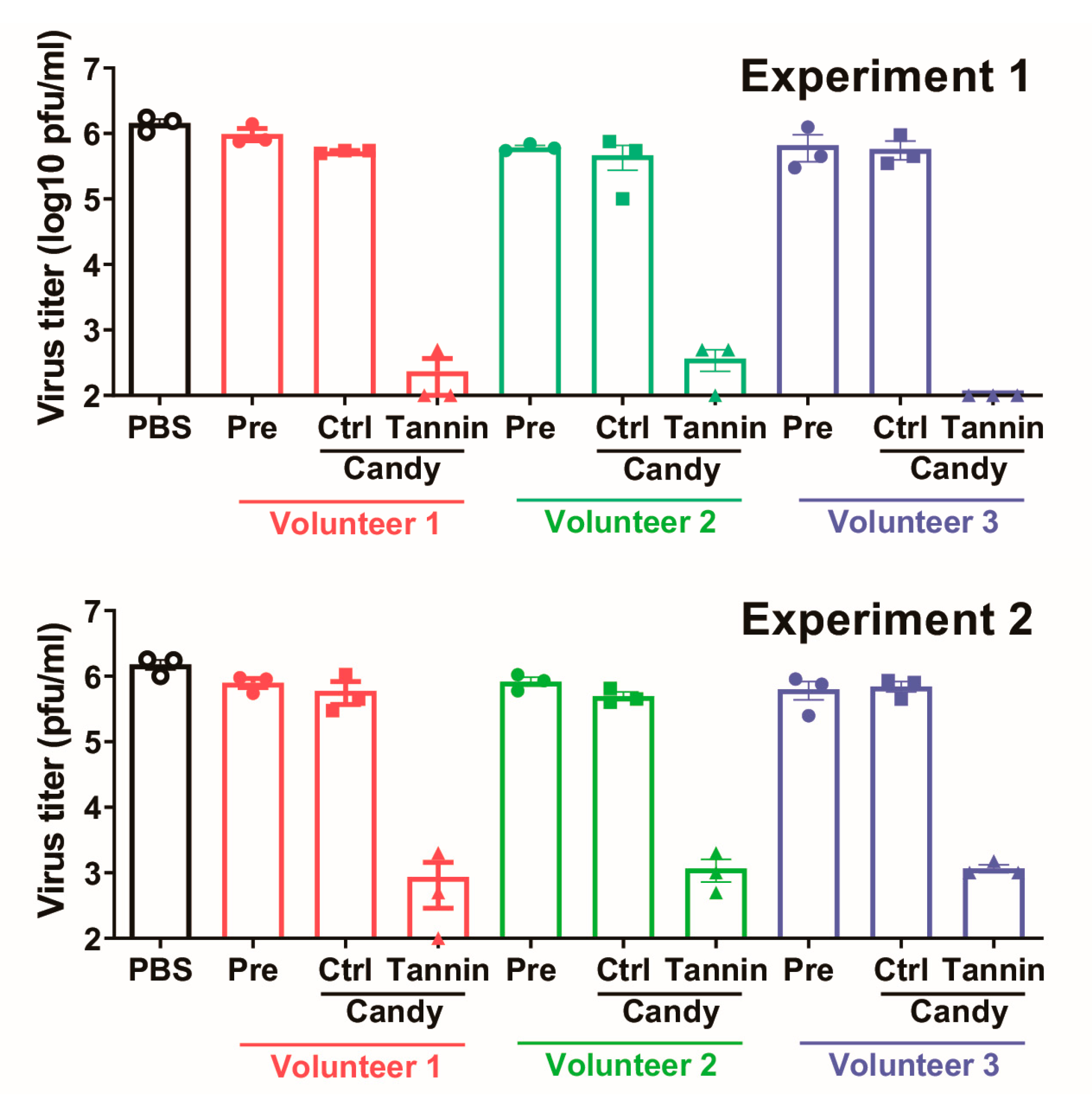
3.2. Saliva Sampled While Eating Persimmon-Derived Tannin-Containing Candy Inactivates the Delta Variant of SARS-CoV-2 In Vitro
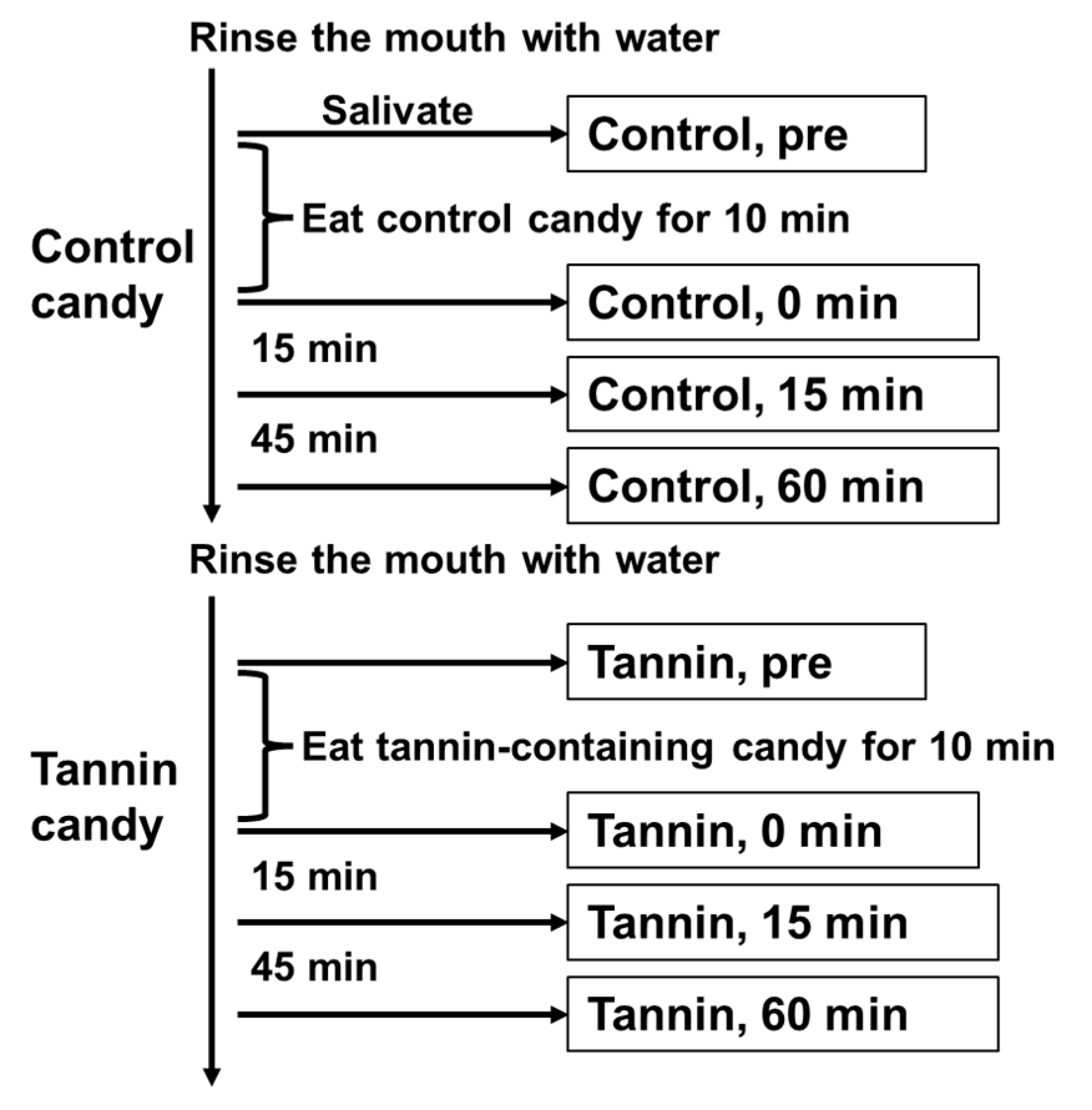
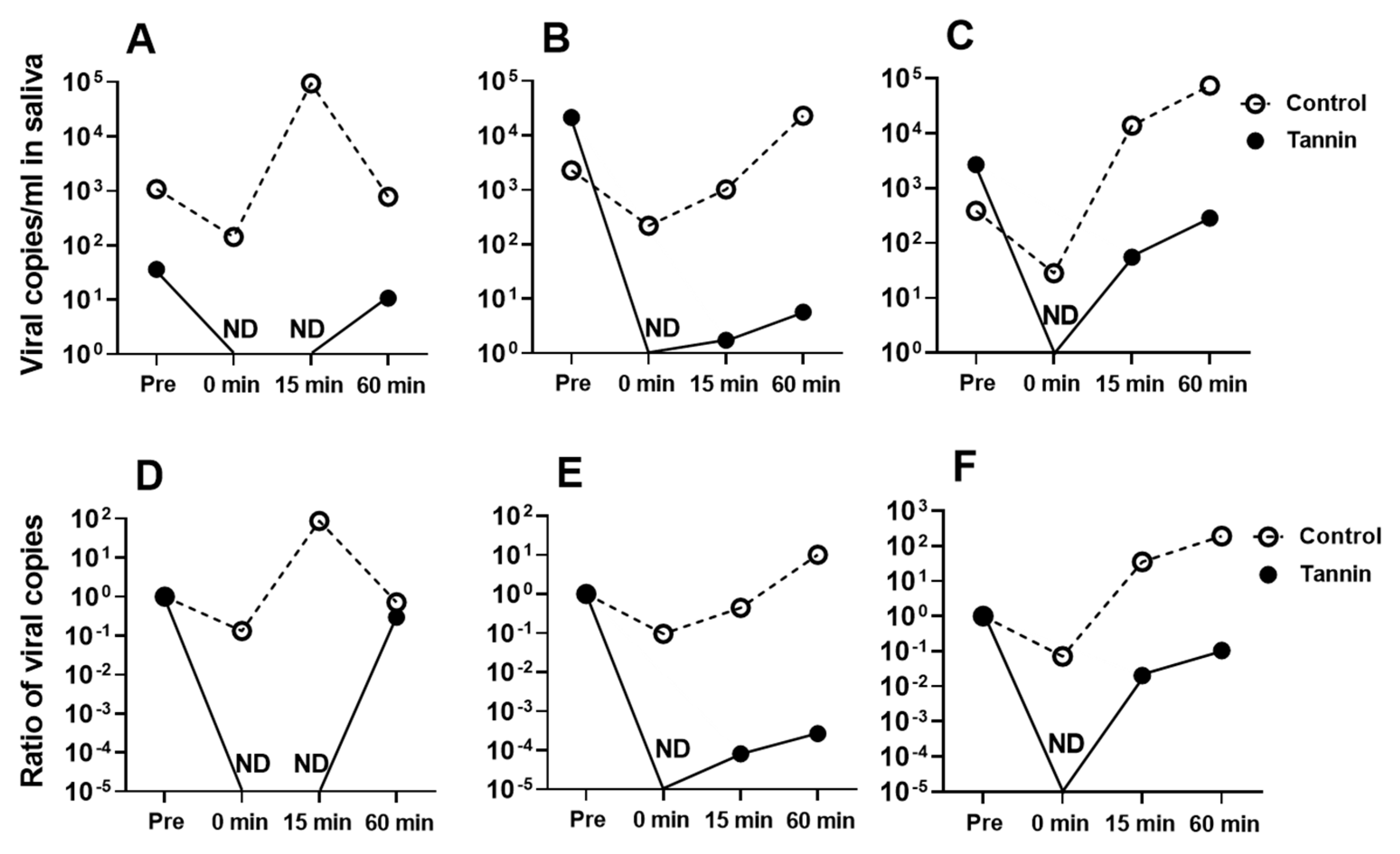
3.3. Administering Persimmon-Derived Tannin-Containing Candy to Patients with COVID-19 Caused by SARS-CoV-2 Delta Variant Can Suppress the Viral Load in Their Saliva
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minkoff, J.M.; TenOever, B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef] [Green Version]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.I.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci. Rep. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kitabatake, M.; Ouji-Sageshima, N.; Yasui, S.; Mochida, N.; Nakano, R.; Kasahara, K.; Tomoda, K.; Yano, H.; Kayano, S.I.; et al. Persimmon-derived tannin has bacteriostatic and anti-inflammatory activity in a murine model of Mycobacterium avium complex (MAC) disease. PLoS ONE 2017, 12, e0183489. [Google Scholar] [CrossRef] [Green Version]
- Ueda, K.; Kawabata, R.; Irie, T.; Nakai, Y.; Tohya, Y.; Sakaguchi, T. Inactivation of Pathogenic Viruses by Plant-Derived Tannins: Strong Effects of Extracts from Persimmon (Diospyros kaki) on a Broad Range of Viruses. PLoS ONE 2013, 8, e55343. [Google Scholar]
- Furukawa, R.; Kitabatake, M.; Ouji-Sageshima, N.; Suzuki, Y.; Nakano, A.; Matsumura, Y.; Nakano, Y.; Kasahara, K.; Kubo, K.; Kayano, S.I.; et al. Persimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model. Sci. Rep. 2021, 11, 23695. [Google Scholar] [CrossRef]
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. Can. Med. Assoc. J. 2021, 193, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, N.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; Mcneal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2021. [Google Scholar]
- Kobayashi, Y.; Arashiro, T.; Otsuka, M.; Tsuchihashi, Y.; Takahashi, T.; Arima, Y.; Ko, Y.K.; Otani, K.; Yamauchi, M.; Kamigaki, T.; et al. Replacement of SARS-CoV-2 strains with variants carrying N501Y and L452R mutations in Japan: An epidemiological surveillance assessment. West. Pac. Surveill. Response J. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.-S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023, 257, 115503. [Google Scholar] [CrossRef]
- Molino, S.; Pisarevsky, A.; Badu, S.; Wu, Q.; Mingorance, F.L.; Vega, P.; Stefanolo, J.P.; Repetti, J.; Ludueña, G.; Pepa, P.; et al. Randomized placebo-controlled trial of oral tannin supplementation on COVID-19 symptoms, gut dysbiosis and cytokine response. J. Funct. Foods 2022, 99, 105356. [Google Scholar] [CrossRef]
- Carrouel, F.; Gadea, E.; Esparcieux, A.; Dimet, J.; Langlois, M.E.; Perrier, H.; Dussart, C.; Bourgeois, D. Saliva Quantification of SARS-CoV-2 in Real-Time PCR From Asymptomatic or Mild COVID-19 Adults. Front. Microbiol. 2022, 12, 786042. [Google Scholar] [CrossRef]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Lee, F.C.; Sunshine, G.; McCord, R.; Howard-Williams, M.; Kompaniyets, L.; Dunphy, C.; Gakh, M.; Weber, R.; Sauber-Schatz, E.; et al. CDC COVID-19 Response Team, Mitigation Policy Analysis Unit; CDC Public Health Law Program. Association of State-Issued Mask Mandates and Allowing On-Premises Restaurant Dining with County-Level COVID-19 Case and Death Growth Rates—United States. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Moreno, M.V.; Mira, A.; Ausina-Marquez, V.; Ferrer, M.D. Oral antiseptics against coronavirus: In-vitro and clinical evidence. J. Hosp. Infect. 2021, 113, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.M.; Strine, M.S.; Watkins, A.E.; Boot, M.; Kalinich, C.C.; Harden, C.A.; Vogels, C.B.F.; Casanovas-Massana, A.; Moore, A.J.; Muenker, M.C.; et al. Stability of SARS-CoV-2 RNA in nonsupplemented saliva. Emerg. Infect. Dis. 2021, 27, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, R.; Kitabatake, M.; Ouji-Sageshima, N.; Tomita, D.; Kumamoto, M.; Suzuki, Y.; Nakano, A.; Nakano, R.; Matsumura, Y.; Kayano, S.-i.; et al. Antiviral Effect of Candies Containing Persimmon-Derived Tannin against SARS-CoV-2 Delta Strain. Viruses 2023, 15, 1636. https://doi.org/10.3390/v15081636
Furukawa R, Kitabatake M, Ouji-Sageshima N, Tomita D, Kumamoto M, Suzuki Y, Nakano A, Nakano R, Matsumura Y, Kayano S-i, et al. Antiviral Effect of Candies Containing Persimmon-Derived Tannin against SARS-CoV-2 Delta Strain. Viruses. 2023; 15(8):1636. https://doi.org/10.3390/v15081636
Chicago/Turabian StyleFurukawa, Ryutaro, Masahiro Kitabatake, Noriko Ouji-Sageshima, Dai Tomita, Makiko Kumamoto, Yuki Suzuki, Akiyo Nakano, Ryuichi Nakano, Yoko Matsumura, Shin-ichi Kayano, and et al. 2023. "Antiviral Effect of Candies Containing Persimmon-Derived Tannin against SARS-CoV-2 Delta Strain" Viruses 15, no. 8: 1636. https://doi.org/10.3390/v15081636
APA StyleFurukawa, R., Kitabatake, M., Ouji-Sageshima, N., Tomita, D., Kumamoto, M., Suzuki, Y., Nakano, A., Nakano, R., Matsumura, Y., Kayano, S.-i., Yano, H., Tamaki, S., & Ito, T. (2023). Antiviral Effect of Candies Containing Persimmon-Derived Tannin against SARS-CoV-2 Delta Strain. Viruses, 15(8), 1636. https://doi.org/10.3390/v15081636




