Distinguishing Genetic Drift from Selection in Papillomavirus Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Genome Data
2.2. Phylogenetic and Cluster Analysis
2.3. Enumeration of Sequence Motifs
2.4. Statistical Analyses
3. Results
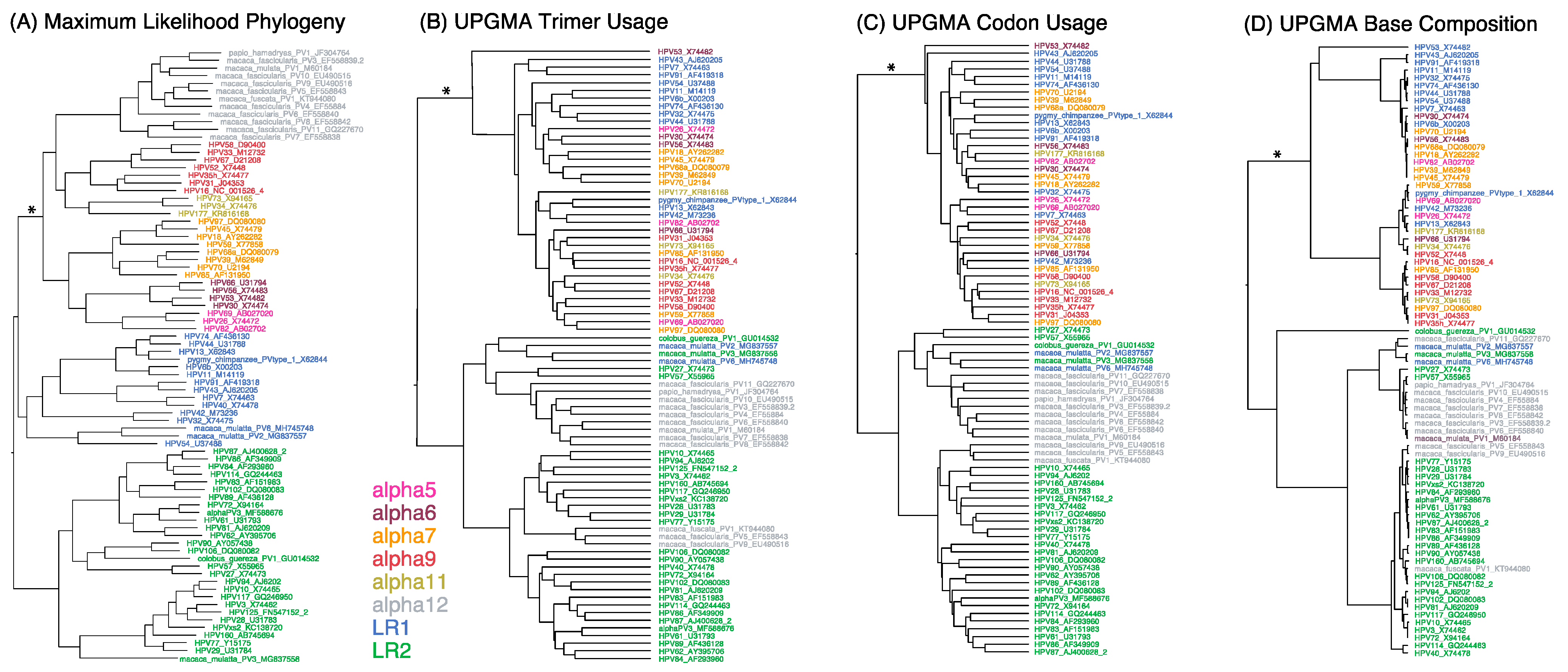
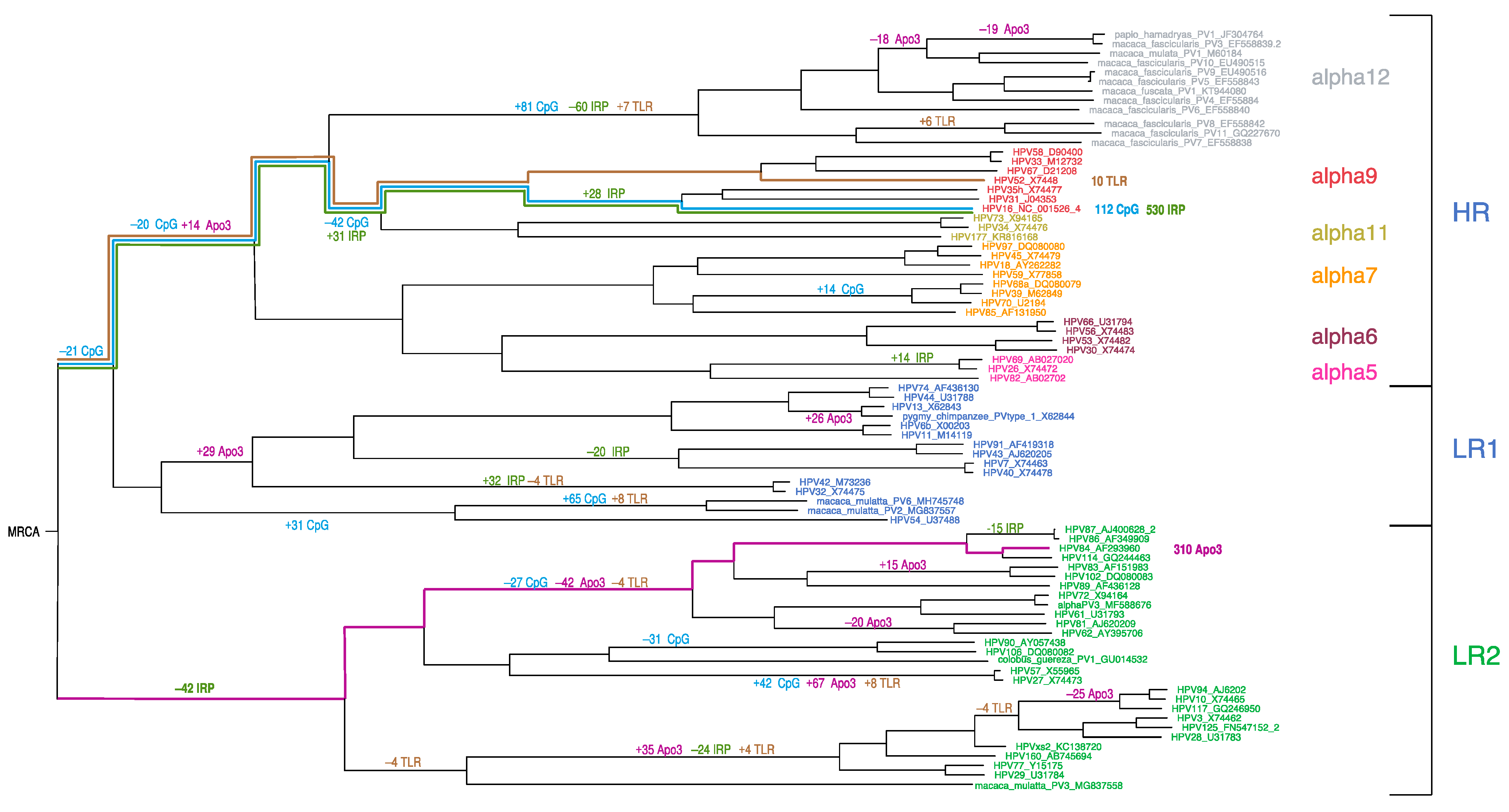
3.1. Topological Comparisons
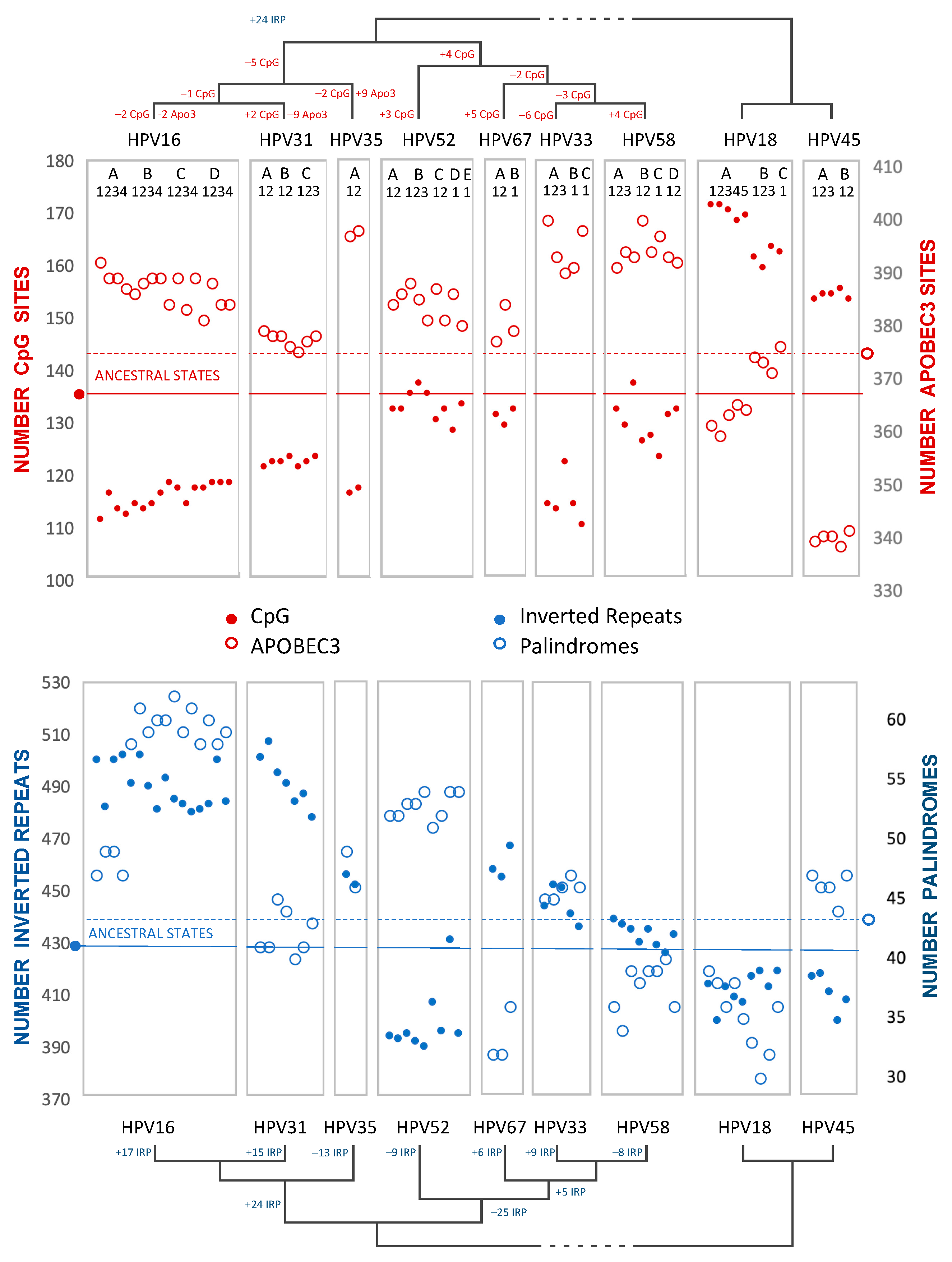
3.2. Sliding Window Analyses
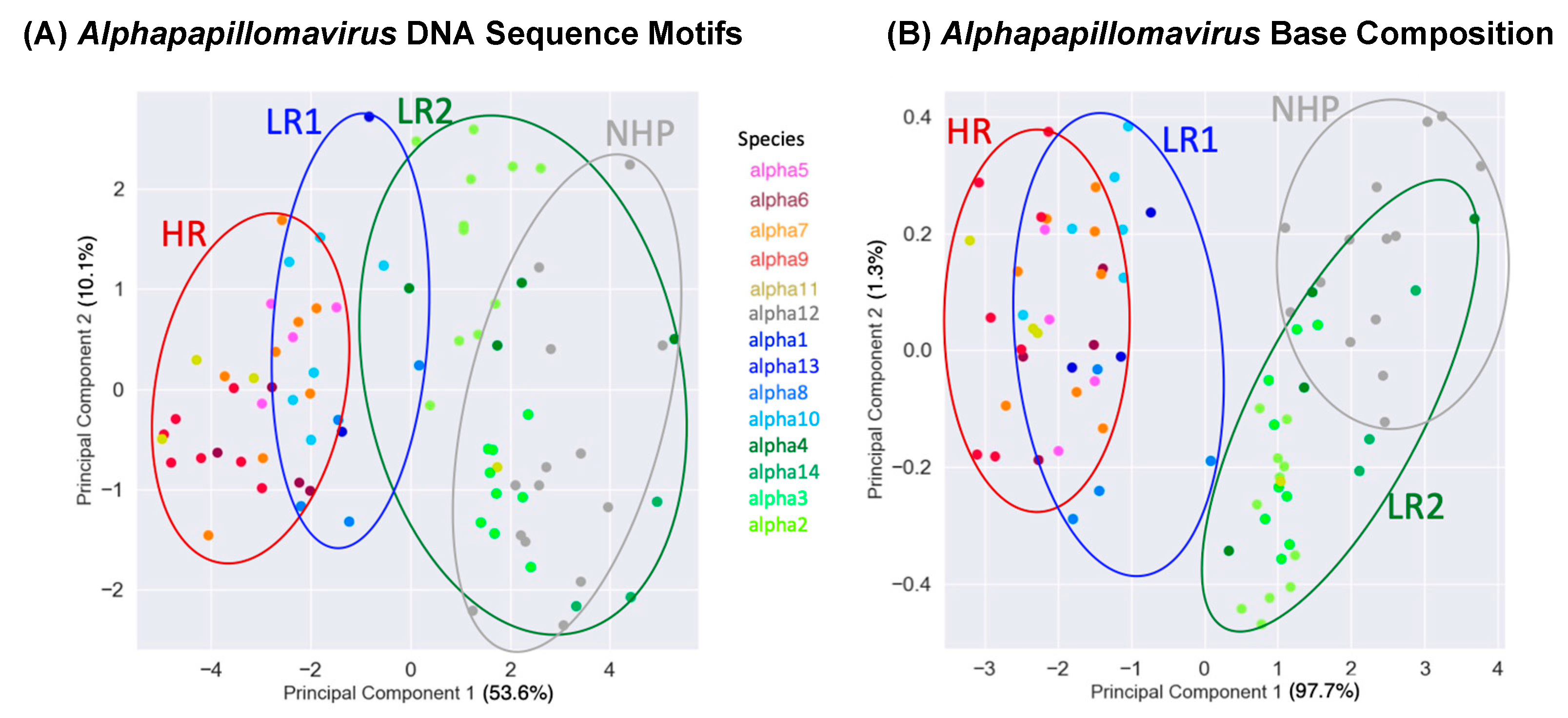
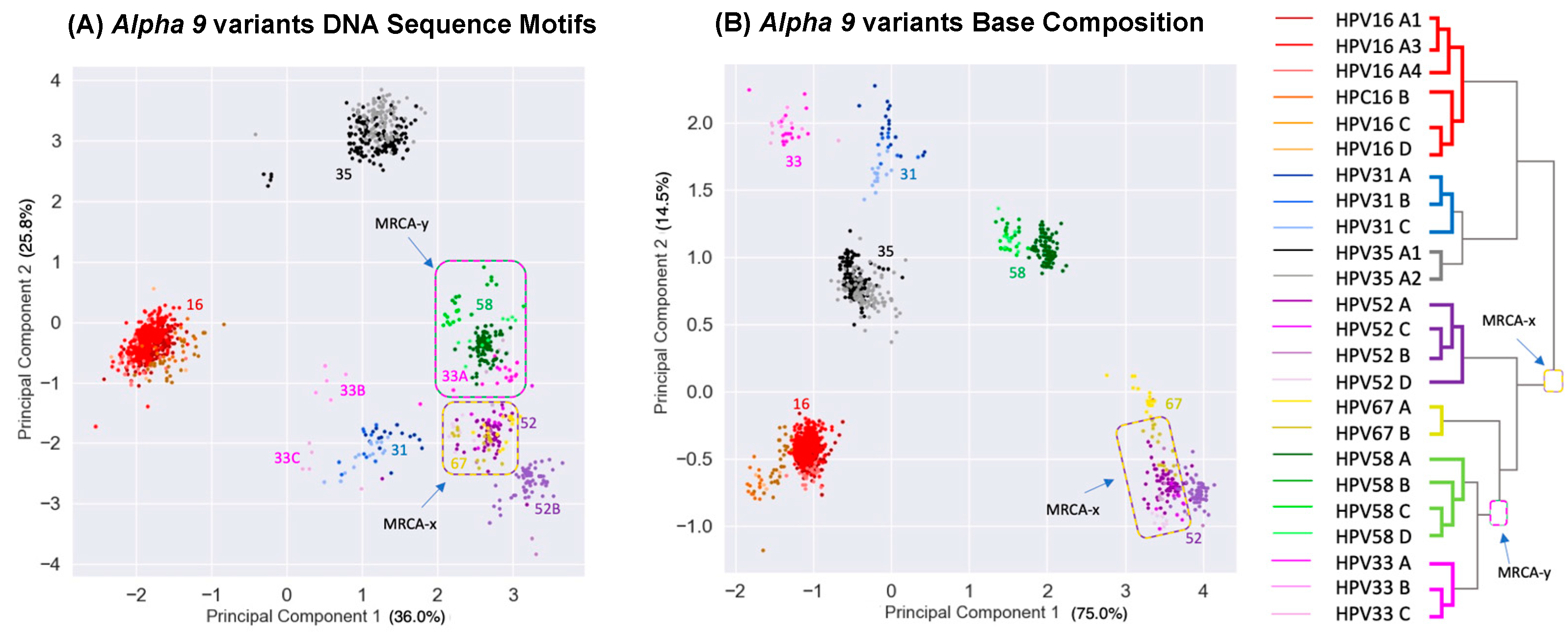
3.3. Principal Component Analysis
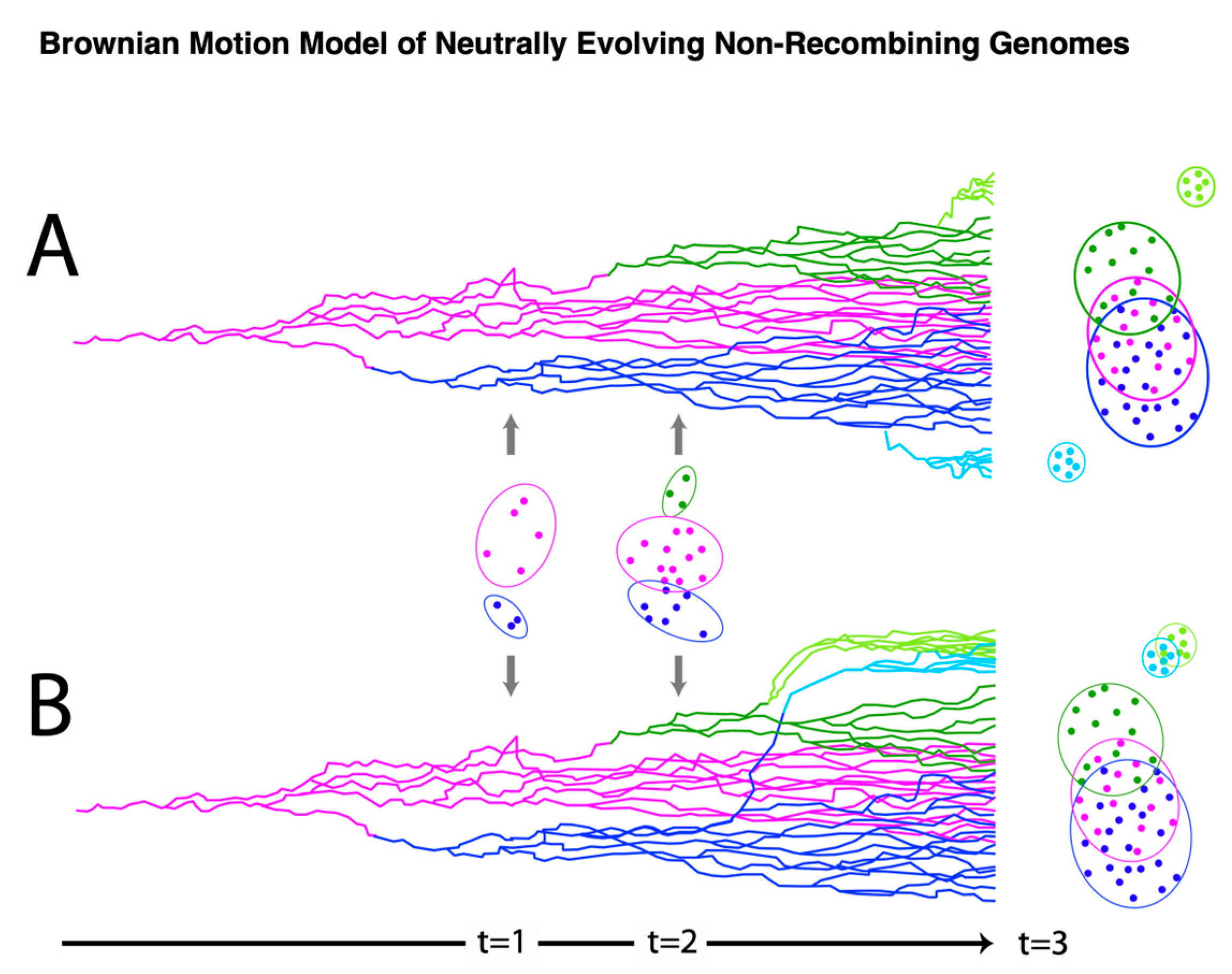
3.4. Ancestral State Reconstruction
3.5. Phylogenetic Independent Contrasts
3.6. Codon-Based Selection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kraberger, S.; Austin, C.; Farkas, K.; Desvignes, T.; Postlethwait, J.H.; Fontenele, R.S.; Schmidlin, K.; Bradley, R.W.; Warzybok, P.; Van Doorslaer, K.; et al. Discovery of novel fish papillomaviruses: From the Antarctic to the commercial fish market. Virology 2022, 565, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.G.; Harding, E.F.; Yan, G.J.H.; Selechnik, D.; Ducatez, S.; DeVore, J.L.; Zhou, J.; Sarma, R.R.; Lee, Y.P.; Richardson, M.F.; et al. Discovery of Novel Viruses Associated With the Invasive Cane Toad (Rhinella marina) in Its Native and Introduced Ranges. Front. Microbiol. 2021, 12, 733631. [Google Scholar] [CrossRef] [PubMed]
- Truchado, D.A.; Williams, R.A.J.; Benitez, L. Natural history of avian papillomaviruses. Virus Res. 2018, 252, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Arch. Virol. 2020, 165, 2737–2748. [Google Scholar] [CrossRef]
- Muhr, L.S.A.; Eklund, C.; Dillner, J. Towards quality and order in human papillomavirus research. Virology 2018, 519, 74–76. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). In Human Papillomavirus and Related Diseases in the World. 2021. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 26 June 2023).
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Rodriguez, A.C.; Chen, Z.; Wacholder, S.; Herrero, R.; Hildesheim, A.; Desalle, R.; Befano, B.; Yu, K.; Safaeian, M.; et al. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res. 2010, 70, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Jeong, D.H.; Kim, Y.N.; Jung, E.J.; Lee, K.B.; Sung, M.S.; Kim, K.T. Persistent HPV-16 infection leads to recurrence of high-grade cervical intraepithelial neoplasia. Medicine 2018, 97, e13606. [Google Scholar] [CrossRef]
- Wei, F.; Xia, N.; Ocampo, R.; Goodman, M.T.; Hessol, N.A.; Grinsztejn, B.; Ortiz, A.P.; Zhao, F.; Kojic, E.M.; Kaul, R.; et al. Age-specific prevalence of anal and cervical HPV infection and high-grade lesions in 11 177 women by HIV status: A collaborative pooled analysis of 26 studies. J. Infect. Dis. 2022, 227, 488–497. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Schiffman, M.; Herrero, R.; DeSalle, R.; Hildesheim, A.; Wacholder, S.; Cecilia Rodriguez, A.; Bratti, M.C.; Sherman, M.E.; Morales, J.; Guillen, D.; et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005, 337, 76–84. [Google Scholar] [CrossRef]
- Burk, R.D.; Chen, Z.; Van Doorslaer, K. Human Papillomaviruses: Genetic Basis of Carcinogenicity. Public Health Genom. 2009, 12, 281–290. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; Desalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS ONE 2011, 6, e20183. [Google Scholar] [CrossRef]
- Clifford, G.M.; Howell-Jones, R.; Franceschi, S. Judging the carcinogenicity of human papillomavirus types by single/multiple infection ratio in cervical cancer. Int. J. Cancer 2011, 129, 1792–1794. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Chan, P.K.S.; et al. Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology 2018, 516, 86–101. [Google Scholar] [CrossRef]
- Fitch, W.M. Rate of change of concomitantly variable codons. J. Mol. Evol. 1971, 1, 84–96. [Google Scholar] [CrossRef]
- Suzuki, Y. New methods for detecting positive selection at single amino acid sites. J. Mol. Evol. 2004, 59, 11–19. [Google Scholar] [CrossRef]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef]
- King, K.M.; Rajadhyaksha, E.V.; Tobey, I.G.; Van Doorslaer, K. Synonymous nucleotide changes drive papillomavirus evolution. Tumour Virus Res. 2022, 14, 200248. [Google Scholar] [CrossRef]
- Zhu, B.; Xiao, Y.; Yeager, M.; Clifford, G.; Wentzensen, N.; Cullen, M.; Boland, J.F.; Bass, S.; Steinberg, M.K.; Raine-Bennett, T.; et al. Mutations in the HPV16 genome induced by APOBEC3 are associated with viral clearance. Nat. Commun. 2020, 11, 886. [Google Scholar] [CrossRef]
- Mirabello, L.; Yeager, M.; Yu, K.; Clifford, G.M.; Xiao, Y.; Zhu, B.; Cullen, M.; Boland, J.F.; Wentzensen, N.; Nelson, C.W.; et al. HPV16 E7 Genetic Conservation Is Critical to Carcinogenesis. Cell 2017, 170, 1164–1174.e6. [Google Scholar] [CrossRef]
- Shackelton, L.A.; Parrish, C.R.; Holmes, E.C. Evolutionary basis of codon usage and nucleotide composition bias in vertebrate DNA viruses. J. Mol. Evol. 2006, 62, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Harari, A.; Schiffman, M.; Clifford, G.M.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Raine-Bennett, T.; Steinberg, M.; et al. Phylogenomic Analysis of Human Papillomavirus Type 31 and Cervical Carcinogenesis: A Study of 2093 Viral Genomes. Viruses 2021, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, Z.; Wentzensen, N.; Zhang, X.; Yu, K.; Yang, Q.; Mitchell, J.; et al. HPV16 Sublineage Associations With Histology-Specific Cancer Risk Using HPV Whole-Genome Sequences in 3200 Women. JNCI J. Natl. Cancer Inst. 2016, 108, djw100. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Gage, J.C.; Clifford, G.M.; Demarco, M.; Cheung, L.C.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, X.; et al. Association of HPV35 with cervical carcinogenesis among women of African ancestry: Evidence of viral-host interaction with implications for disease intervention. Int. J. Cancer 2020, 147, 2677–2686. [Google Scholar] [CrossRef]
- Bee, K.J.; Gradissimo, A.; Chen, Z.; Harari, A.; Schiffman, M.; Raine-Bennett, T.; Castle, P.E.; Clarke, M.; Wentzensen, N.; Burk, R.D. Genetic and Epigenetic Variations of HPV52 in Cervical Precancer. Int. J. Mol. Sci. 2021, 22, 6463. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, M.B.; Castle, P.E.; Herrero, R.; Schiffman, M.; Sherman, M.E.; Wacholder, S.; Rodriguez, A.C.; Hutchinson, M.L.; Bratti, M.C.; Hildesheim, A.; et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006, 66, 10112–10119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berumen, J.; Ordonez, R.M.; Lazcano, E.; Salmeron, J.; Galvan, S.C.; Estrada, R.A.; Yunes, E.; Garcia-Carranca, A.; Gonzalez-Lira, G.; Madrigal-de la Campa, A. Asian-American variants of human papillomavirus 16 and risk for cervical cancer: A case-control study. J. Natl. Cancer Inst. 2001, 93, 1325–1330. [Google Scholar] [CrossRef]
- Hildesheim, A.; Herrero, R.; Castle, P.E.; Wacholder, S.; Bratti, M.C.; Sherman, M.E.; Lorincz, A.T.; Burk, R.D.; Morales, J.; Rodriguez, A.C.; et al. HPV co-factors related to the development of cervical cancer: Results from a population-based study in Costa Rica. Br. J. Cancer 2001, 84, 1219–1226. [Google Scholar] [CrossRef]
- Xi, L.F.; Koutsky, L.A.; Hildesheim, A.; Galloway, D.A.; Wheeler, C.M.; Winer, R.L.; Ho, J.; Kiviat, N.B. Risk for high-grade cervical intraepithelial neoplasia associated with variants of human papillomavirus types 16 and 18. Cancer Epidemiol. Biomark. Prev. 2007, 16, 4–10. [Google Scholar] [CrossRef]
- Chen, Z.; Utro, F.; Platt, D.; DeSalle, R.; Parida, L.; Chan, P.K.S.; Burk, R.D. K-Mer Analyses Reveal Different Evolutionary Histories of Alpha, Beta, and Gamma Papillomaviruses. Int. J. Mol. Sci. 2021, 22, 9657. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Badal, V.; Calleja-Macias, I.E.; Kalantari, M.; Chuang, L.S.; Li, B.F.; Bernard, H.U. The human papillomavirus-18 genome is efficiently targeted by cellular DNA methylation. Virology 2004, 324, 483–492. [Google Scholar] [CrossRef]
- Mirabello, L.; Sun, C.; Ghosh, A.; Rodriguez, A.C.; Schiffman, M.; Wentzensen, N.; Hildesheim, A.; Herrero, R.; Wacholder, S.; Lorincz, A.; et al. Methylation of human papillomavirus type 16 genome and risk of cervical precancer in a Costa Rican population. J. Natl. Cancer Inst. 2012, 104, 556–565. [Google Scholar] [CrossRef]
- Ekanayake Weeramange, C.; Tang, K.D.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Punyadeera, C. DNA Methylation Changes in Human Papillomavirus-Driven Head and Neck Cancers. Cells 2020, 9, 1359. [Google Scholar] [CrossRef]
- Wentzensen, N.; Sun, C.; Ghosh, A.; Kinney, W.; Mirabello, L.; Wacholder, S.; Shaber, R.; LaMere, B.; Clarke, M.; Lorincz, A.T.; et al. Methylation of HPV18, HPV31, and HPV45 genomes and cervical intraepithelial neoplasia grade 3. J. Natl. Cancer Inst. 2012, 104, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.; Samal, J.; Kandpal, M.; Vasaikar, S.; Biswas, B.; Gomes, J.; Vivekanandan, P. CpG dinucleotide frequencies reveal the role of host methylation capabilities in parvovirus evolution. J. Virol. 2013, 87, 13816–13824. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Jiang, M.; Shen, Z.; Hulbert, A.; Zhou, X.H.; Lin, Y.Y.; Kiviat, N.B.; Koutsky, L.A. Inverse association between methylation of human papillomavirus type 16 DNA and risk of cervical intraepithelial neoplasia grades 2 or 3. PLoS ONE 2011, 6, e23897. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Macaluso, M.; Alvarez, R.D.; Chen, M.; Badiga, S.; Edberg, J.C.; Partridge, E.E.; Johanning, G.L. A higher degree of methylation of the HPV 16 E6 gene is associated with a lower likelihood of being diagnosed with cervical intraepithelial neoplasia. Cancer 2011, 117, 957–963. [Google Scholar] [CrossRef]
- Clarke, M.A.; Wentzensen, N.; Mirabello, L.; Ghosh, A.; Wacholder, S.; Harari, A.; Lorincz, A.; Schiffman, M.; Burk, R.D. Human papillomavirus DNA methylation as a potential biomarker for cervical cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2125–2137. [Google Scholar] [CrossRef]
- Feng, C.; Dong, J.; Chang, W.; Cui, M.; Xu, T. The Progress of Methylation Regulation in Gene Expression of Cervical Cancer. Int. J. Genom. 2018, 2018, 8260652. [Google Scholar] [CrossRef]
- Vartanian, J.P.; Guetard, D.; Henry, M.; Wain-Hobson, S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science 2008, 320, 230–233. [Google Scholar] [CrossRef]
- Warren, C.J.; Westrich, J.A.; Doorslaer, K.V.; Pyeon, D. Roles of APOBEC3A and APOBEC3B in Human Papillomavirus Infection and Disease Progression. Viruses 2017, 9, 233. [Google Scholar] [CrossRef]
- Poulain, F.; Lejeune, N.; Willemart, K.; Gillet, N.A. Footprint of the host restriction factors APOBEC3 on the genome of human viruses. PLoS Pathog. 2020, 16, e1008718. [Google Scholar] [CrossRef]
- Xu, W.K.; Byun, H.; Dudley, J.P. The Role of APOBECs in Viral Replication. Microorganisms 2020, 8, 1899. [Google Scholar] [CrossRef]
- Burgers, W.A.; Blanchon, L.; Pradhan, S.; de Launoit, Y.; Kouzarides, T.; Fuks, F. Viral oncoproteins target the DNA methyltransferases. Oncogene 2007, 26, 1650–1655. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Tsang, W.P.; Tsang, T.Y.; Co, N.N.; Yau, P.L.; Kwok, T.T. HPV-16 E6 upregulation of DNMT1 through repression of tumor suppressor p53. Oncol. Rep. 2010, 24, 1599–1604. [Google Scholar] [CrossRef]
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol. Lett. 2018, 15, 11–22. [Google Scholar] [CrossRef]
- Wallace, N.A.; Munger, K. The curious case of APOBEC3 activation by cancer-associated human papillomaviruses. PLoS Pathog. 2018, 14, e1006717. [Google Scholar] [CrossRef]
- Zhe, X.; Xin, H.; Pan, Z.; Jin, F.; Zheng, W.; Li, H.; Li, D.; Cao, D.; Li, Y.; Zhang, C.; et al. Genetic variations in E6, E7 and the long control region of human papillomavirus type 16 among patients with cervical lesions in Xinjiang, China. Cancer Cell Int. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Wong, B.H.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Cytosine deamination and selection of CpG suppressed clones are the two major independent biological forces that shape codon usage bias in coronaviruses. Virology 2007, 369, 431–442. [Google Scholar] [CrossRef]
- Van Doorslaer, K. Evolution of the Papillomaviridae. Virology 2013, 445, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qiu, Y.; Xing, C.; Zhou, J.H.; Yang, W.H.; Wang, Q.; Li, J.Y.; Han, X.; Zhang, Y.Z.; Ge, X.Y. Detection and genome characterization of two novel papillomaviruses and a novel polyomavirus in tree shrew (Tupaia belangeri chinensis) in China. Virol. J. 2019, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Latsuzbaia, A.; Wienecke-Baldacchino, A.; Tapp, J.; Arbyn, M.; Karabegovic, I.; Chen, Z.; Fischer, M.; Muhlschlegel, F.; Weyers, S.; Pesch, P.; et al. Characterization and Diversity of 243 Complete Human Papillomavirus Genomes in Cervical Swabs Using Next Generation Sequencing. Viruses 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Boland, J.F.; Torres-Gonzalez, E.; Albanez, A.; Zhou, W.; Steinberg, M.K.; Diaw, L.; Mitchell, J.; Roberson, D.; Cullen, M.; et al. The D2 and D3 Sublineages of Human Papilloma Virus 16-Positive Cervical Cancer in Guatemala Differ in Integration Rate and Age of Diagnosis. Cancer Res. 2020, 80, 3803–3809. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Du, H.; Ou, Z. Mutation Profiles, Glycosylation Site Distribution and Codon Usage Bias of Human Papillomavirus Type 16. Viruses 2021, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Tluckova, K.; Marusic, M.; Tothova, P.; Bauer, L.; Sket, P.; Plavec, J.; Viglasky, V. Human papillomavirus G-quadruplexes. Biochemistry 2013, 52, 7207–7216. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. One-electron oxidation of DNA and inflammation processes. Nat. Chem. Biol. 2006, 2, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M.; Wu, T.; Weeratna, R.; Efler, S.M.; Love-Homan, L.; Yang, L.; Yi, A.K.; Short, D.; Davis, H.L. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. USA 1998, 95, 12631–12636. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87. [Google Scholar] [CrossRef]
- Hasan, U.A.; Zannetti, C.; Parroche, P.; Goutagny, N.; Malfroy, M.; Roblot, G.; Carreira, C.; Hussain, I.; Muller, M.; Taylor-Papadimitriou, J.; et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef]
- Pohar, J.; Yamamoto, C.; Fukui, R.; Cajnko, M.M.; Miyake, K.; Jerala, R.; Bencina, M. Selectivity of Human TLR9 for Double CpG Motifs and Implications for the Recognition of Genomic DNA. J. Immunol. 2017, 198, 2093–2104. [Google Scholar] [CrossRef]
- Cannella, F.; Pierangeli, A.; Scagnolari, C.; Cacciotti, G.; Tranquilli, G.; Stentella, P.; Recine, N.; Antonelli, G. TLR9 is expressed in human papillomavirus-positive cervical cells and is overexpressed in persistent infections. Immunobiology 2015, 220, 363–368. [Google Scholar] [CrossRef]
- King, K.; Larsen, B.B.; Gryseels, S.; Richet, C.; Kraberger, S.; Jackson, R.; Worobey, M.; Harrison, J.S.; Varsani, A.; Van Doorslaer, K. Coevolutionary Analysis Implicates Toll-Like Receptor 9 in Papillomavirus Restriction. mBio 2022, 13, e0005422. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Waltke, M.; Angeletti, P.C. Evolutionary variation of papillomavirus E2 protein and E2 binding sites. Virol. J. 2011, 8, 379. [Google Scholar] [CrossRef]
- Newhouse, C.D.; Silverstein, S.J. Orientation of a novel DNA binding site affects human papillomavirus-mediated transcription and replication. J. Virol. 2001, 75, 1722–1735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, R.; Kim, C.; Sei, E.; Foukakis, T.; Crosetto, N.; Chan, L.K.; Srinivasan, M.; Zhang, H.; Meric-Bernstam, F.; Navin, N. Nanogrid single-nucleus RNA sequencing reveals phenotypic diversity in breast cancer. Nat. Commun. 2017, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Ganapathiraju, M.K.; Subramanian, S.; Chaparala, S.; Karunakaran, K.B. A reference catalog of DNA palindromes in the human genome and their variations in 1000 Genomes. Hum. Genome Var. 2020, 7, 40. [Google Scholar] [CrossRef]
- Swofford, D. PAUP* (* Phylogenetic Analysis Using PAUP). 2021. Available online: https://paup.phylosolutions.com (accessed on 26 June 2023).
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef]
- Monit, C.; Goldstein, R.A.; Towers, G.; Hue, S. Positive Selection Analysis of Overlapping Reading Frames Is Invalid. AIDS Res. Hum. Retroviruses 2015, 31, 947. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package); Department of Genome Sciences, University of Washington: Seattle, WA, 2013. [Google Scholar]
- Rambaut, A. FigTree; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2016. [Google Scholar]
- Maddison, W.P.; Maddison, D.R.M. Mesquite: A Modular System for Evolutionary Analysis; 2021. Available online: https://www.mesquiteproject.org/ (accessed on 26 June 2023).
- Kosakovsky Pond, S.L.; Poon, A.F.Y.; Velazquez, R.; Weaver, S.; Hepler, N.L.; Murrell, B.; Shank, S.D.; Magalis, B.R.; Bouvier, D.; Nekrutenko, A.; et al. HyPhy 2.5-A Customizable Platform for Evolutionary Hypothesis Testing Using Phylogenies. Mol. Biol. Evol. 2020, 37, 295–299. [Google Scholar] [CrossRef]
- Weaver, S.; Shank, S.D.; Spielman, S.J.; Li, M.; Muse, S.V.; Kosakovsky Pond, S.L. Datamonkey 2.0: A Modern Web Application for Characterizing Selective and Other Evolutionary Processes. Mol. Biol. Evol. 2018, 35, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, D.; Giaro, K. Comparing Phylogenetic Trees by Matching Nodes Using the Transfer Distance Between Partitions. J. Comput. Biol. 2017, 24, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, X.; Gao, Y.; Zhu, J.; Liu, S.; Gao, G.; Gao, P. Molecular Mechanism of RNA Recognition by Zinc-Finger Antiviral Protein. Cell Rep. 2020, 30, 46–52 e44. [Google Scholar] [CrossRef]
- Blanquart, F.; Wymant, C.; Cornelissen, M.; Gall, A.; Bakker, M.; Bezemer, D.; Hall, M.; Hillebregt, M.; Ong, S.H.; Albert, J.; et al. Viral genetic variation accounts for a third of variability in HIV-1 set-point viral load in Europe. PLoS Biol. 2017, 15, e2001855. [Google Scholar] [CrossRef]
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford Series in Ecology and Evolution; Oxford University Press: Oxford, UK; New York, NY, USA, 1991; 239p. [Google Scholar]
- Harmon, L. Phylogenetic Comparative Methods: Learning from Trees; 2018. Available online: https://lukejharmon.github.io/pcm/ (accessed on 26 June 2023).
- O’Meara, B.C.; Beaulieu, J.M. Modelling Stabilizing Selection: The Attraction of Ornstein–Uhlenbeck Models. In Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 381–393. [Google Scholar]
- Edwards, A.W.F.; Cavalli-Sforza, L.L. Reconstruction of evolutionary trees. In Phenetic and Phylogenetic Classification; Heywood, W.H., McNeill, J., Eds.; Systematics Association: London, UK, 1964; pp. 67–76. [Google Scholar]
- Felsenstein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Einstein, A.; Fürth, R.; Cowper, A.D. Investigations on the Theory of the Brownian Movement; Dutton and Company: New York, NY, USA, 1919; 124p. [Google Scholar]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer: Sunderland, MA, USA, 1998; 980p. [Google Scholar]
- Pagel, M.; Cunningham, C. The Maximum Likelihood Approach to Reconstructing Ancestral Character States of Discrete Characters on Phylogenies. Syst. Biol. 1999, 48, 612–622. [Google Scholar] [CrossRef]
- Rudner, R.; Karkas, J.D.; Chargaff, E. Separation of B. subtilis DNA into complementary strands. 3. Direct analysis. Proc. Natl. Acad. Sci. USA 1968, 60, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Murrell, B.; Smith, M.D.; Kosakovsky Pond, S.L.; Scheffler, K. RELAX: Detecting relaxed selection in a phylogenetic framework. Mol. Biol. Evol. 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, N. Directional mutation pressure and neutral molecular evolution. Proc. Natl. Acad. Sci. USA 1988, 85, 2653–2657. [Google Scholar] [CrossRef]
- Jermiin, L.S.; Graur, D.; Lowe, R.M.; Crozier, R.H. Analysis of directional mutation pressure and nucleotide content in mitochondrial cytochrome b genes. J. Mol. Evol. 1994, 39, 160–173. [Google Scholar] [CrossRef]
- Elde, N.C.; Chen, Z.; DeSalle, R.; Schiffman, M.; Herrero, R.; Wood, C.E.; Ruiz, J.C.; Clifford, G.M.; Chan, P.K.S.; Burk, R.D. Niche adaptation and viral transmission of human papillomaviruses from archaic hominins to modern humans. PLOS Pathog. 2018, 14, e1007352. [Google Scholar] [CrossRef]
- Felsenstein, J. The evolutionary advantage of recombination. Genetics 1974, 78, 737–756. [Google Scholar] [CrossRef]
- Kypr, J.; Mrázek, J.; Reich, J. Nucleotide composition bias and CpG dinucleotide content in the genomes of HIV and HTLV. Biochim. Biophys. Acta (BBA) -Gene Struct. Expr. 1989, 1009, 280–282. [Google Scholar] [CrossRef]
- Khrustalev, V.V.; Barkovsky, E.V. Unusual nucleotide content of Rubella virus genome as a consequence of biased RNA-editing: Comparison with Alphaviruses. Int. J. Bioinform. Res. Appl. 2011, 7, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U. Regulation of the transcription of genital human papillomaviruses by CDP, nucleosomes and nuclear-matrix attachment regions. Papillomavirus Rep. 2000, 11, 73–80. [Google Scholar]
- Stunkel, W.; Huang, Z.; Tan, S.H.; O’Connor, M.J.; Bernard, H.U. Nuclear matrix attachment regions of human papillomavirus type 16 repress or activate the E6 promoter, depending on the physical state of the viral DNA. J. Virol. 2000, 74, 2489–2501. [Google Scholar] [CrossRef]
- Tan, S.H.; Bartsch, D.; Schwarz, E.; Bernard, H.U. Nuclear matrix attachment regions of human papillomavirus type 16 point toward conservation of these genomic elements in all genital papillomaviruses. J. Virol. 1998, 72, 3610–3622. [Google Scholar] [CrossRef]
- Swanton, C.; McGranahan, N.; Starrett, G.J.; Harris, R.S. APOBEC Enzymes: Mutagenic Fuel for Cancer Evolution and Heterogeneity. Cancer Discov. 2015, 5, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends Biochem. Sci. 2016, 41, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Hakata, Y.; Miyazawa, M. Deaminase-Independent Mode of Antiretroviral Action in Human and Mouse APOBEC3 Proteins. Microorganisms 2020, 8, 1976. [Google Scholar] [CrossRef]
- Mourier, T.; Sadykov, M.; Carr, M.J.; Gonzalez, G.; Hall, W.W.; Pain, A. Host-directed editing of the SARS-CoV-2 genome. Biochem. Biophys. Res. Commun. 2021, 538, 35–39. [Google Scholar] [CrossRef]
- Shapiro, M.; Krug, L.T.; MacCarthy, T. Mutational pressure by host APOBEC3s more strongly affects genes expressed early in the lytic phase of herpes simplex virus-1 (HSV-1) and human polyomavirus (HPyV) infection. PLoS Pathog. 2021, 17, e1009560. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Sharma, S.; Patnaik, S.K.; Kemer, Z.; Baysal, B.E. Transient overexpression of exogenous APOBEC3A causes C-to-U RNA editing of thousands of genes. RNA Biol. 2017, 14, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Furano, A.V. Breaking bad: The mutagenic effect of DNA repair. DNA Repair. 2015, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Berget, S.M.; Moore, C.; Sharp, P.A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc. Natl. Acad. Sci. USA 1977, 74, 3171–3175. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Air, G.M.; Barrell, B.G.; Brown, N.L.; Coulson, A.R.; Fiddes, C.A.; Hutchison, C.A.; Slocombe, P.M.; Smith, M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature 1977, 265, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewska, A.; Tukendorf, A.; Semczuk, M. [Frequency of HPV infection and level of glutathione in serum of women with cervix dysplasia]. Med. Dosw. Mikrobiol. 1995, 47, 213–218. [Google Scholar]
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006, 4, e180. [Google Scholar] [CrossRef]
- Plotkin, J.B.; Robins, H.; Levine, A.J. Tissue-specific codon usage and the expression of human genes. Proc. Natl. Acad. Sci. USA 2004, 101, 12588–12591. [Google Scholar] [CrossRef]
- Ladoukakis, E.D.; Eyre-Walker, A. The excess of small inverted repeats in prokaryotes. J. Mol. Evol. 2008, 67, 291–300. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Faraonio, R.; Buonaguro, L.; Annunziata, C.; Starita, N.; Cerasuolo, A.; Pezzuto, F.; Tornesello, A.L.; Buonaguro, F.M. The Role of microRNAs, Long Non-coding RNAs, and Circular RNAs in Cervical Cancer. Front. Oncol. 2020, 10, 150. [Google Scholar] [CrossRef]
- Bai, L.; Wei, L.; Wang, J.; Li, X.; He, P. Extended effects of human papillomavirus 16 E6-specific short hairpin RNA on cervical carcinoma cells. Int. J. Gynecol. Cancer 2006, 16, 718–729. [Google Scholar] [CrossRef]
- Wertheim, J.O.; Kosakovsky Pond, S.L. Purifying selection can obscure the ancient age of viral lineages. Mol. Biol. Evol. 2011, 28, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Bravo, I.G.; Félez-Sánchez, M. Papillomaviruses. Evol. Med. Public Health 2015, 2015, 32–51. [Google Scholar] [CrossRef]
- Kapralov, M.V.; Filatov, D.A. Molecular adaptation during adaptive radiation in the Hawaiian endemic genus Schiedea. PLoS ONE 2006, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Gobeil, S.M.C.; Janowska, K.; McDowell, S.; Mansouri, K.; Parks, R.; Stalls, V.; Kopp, M.F.; Manne, K.; Li, D.; Wiehe, K.; et al. Effect of natural mutations of SARS-CoV-2 on spike structure, conformation, and antigenicity. Science 2021, 373, eabi6226. [Google Scholar] [CrossRef] [PubMed]
- Kustin, T.; Stern, A.; Yeager, M. Biased Mutation and Selection in RNA Viruses. Mol. Biol. Evol. 2021, 38, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Mooers, A.O.; Holmes, E.C. The evolution of base composition and phylogenetic inference. Trends Ecol. Evol. 2000, 15, 365–369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burk, R.D.; Mirabello, L.; DeSalle, R. Distinguishing Genetic Drift from Selection in Papillomavirus Evolution. Viruses 2023, 15, 1631. https://doi.org/10.3390/v15081631
Burk RD, Mirabello L, DeSalle R. Distinguishing Genetic Drift from Selection in Papillomavirus Evolution. Viruses. 2023; 15(8):1631. https://doi.org/10.3390/v15081631
Chicago/Turabian StyleBurk, Robert D., Lisa Mirabello, and Robert DeSalle. 2023. "Distinguishing Genetic Drift from Selection in Papillomavirus Evolution" Viruses 15, no. 8: 1631. https://doi.org/10.3390/v15081631
APA StyleBurk, R. D., Mirabello, L., & DeSalle, R. (2023). Distinguishing Genetic Drift from Selection in Papillomavirus Evolution. Viruses, 15(8), 1631. https://doi.org/10.3390/v15081631





