Abstract
It is known that SARS-CoV-2 infection can result in gastrointestinal symptoms. For some, these symptoms may persist beyond acute infection, in what is known as ‘post-COVID syndrome’. We conducted a systematic review to examine the prevalence of persistent gastrointestinal symptoms and the incidence of new gastrointestinal illnesses following acute SARS-CoV-2 infection. We searched the scientific literature using MedLine, SCOPUS, Europe PubMed Central and medRxiv from December 2019 to July 2023. Two reviewers independently identified 45 eligible articles, which followed participants for various gastrointestinal outcomes after acute SARS-CoV-2 infection. The study quality was assessed using the Joanna Briggs Institute Critical Appraisal Tools. The weighted pooled prevalence for persistent gastrointestinal symptoms of any nature and duration was 10.8% compared with 4.9% in healthy controls. For seven studies at low risk of methodological bias, the symptom prevalence ranged from 0.2% to 24.1%, with a median follow-up time of 18 weeks. We also identified a higher risk for future illnesses such as irritable bowel syndrome, dyspepsia, hepatic and biliary disease, liver disease and autoimmune-mediated illnesses such as inflammatory bowel disease and coeliac disease in historically SARS-CoV-2-exposed individuals. Our review has shown that, from a limited pool of mostly low-quality studies, previous SARS-CoV-2 exposure may be associated with ongoing gastrointestinal symptoms and the development of functional gastrointestinal illness. Furthermore, we show the need for high-quality research to better understand the SARS-CoV-2 association with gastrointestinal illness, particularly as population exposure to enteric infections returns to pre-COVID-19-restriction levels.
1. Introduction
Since its emergence in late 2019, the COVID-19 pandemic has resulted in over 750 million infections and 6 million deaths worldwide as of July 2023 [1]. While most cases are experienced as a self-limiting respiratory tract infection, some individuals may develop a more severe or protracted illness. Post-COVID syndrome, or long-COVID, refers to symptoms that persist beyond 12 weeks from the onset of infection [2]. In the UK, self-reported health data indicate that 2.1 million people, or 3.3% of the general population, were suffering from post-COVID in January 2023 [3]. Symptoms of post-COVID syndrome appear to affect every body system, often overlapping and with a significant mental health impact [2].
It is established that SARS-CoV-2 (the virus that causes COVID-19 disease) infection can result in gastrointestinal symptoms: in 2020, a meta-analysis of 8302 patients identified diarrhoea in 12% of paediatric and 9% of adult cases [4]. Elshazli et al. also identified gastrointestinal symptoms in 20% of 25,252 patients in 2020, with anorexia, dysgeusia, diarrhoea, nausea and haematemesis being the most common [5]. In Liverpool, UK, gastrointestinal symptoms were observed in a third of hospitalised COVID-19 patients [6]. Research investigating gastrointestinal symptoms as a component of post-COVID syndrome, however, is less substantial. One review of post-COVID syndrome patients identified persistent symptoms of dysgeusia and diarrhoea at a frequency of 7% and 6%, respectively [7]. Similarly, an electronic health record study of 273,618 individuals in the USA found persistent gastrointestinal symptoms in 8.3% of participants six months after COVID-19 onset [8]. Chopra et al. also found that patients in India presenting with diarrhoea during acute COVID-19 were more likely to suffer from post-COVID syndrome symptoms such as fatigue, dyspnoea and chest discomfort [9].
When considering how SARS-CoV-2 interacts with the gastrointestinal system and how this may result in persistent post-viral symptoms, several pathophysiological mechanisms have been proposed. SARS-CoV-2 initially binds with the angiotensin-converting enzyme-2 (ACE-2) receptor, which is highly expressed by ileal enterocytes [10,11]. The binding of SARS-CoV-2 to ACE-2 receptors may disrupt angiotensin homeostasis and reduce tryptophan absorption, resulting in inflammation and alteration of the gut microbiota [12,13]. Alteration of the gut microbiome is known to occur during acute COVID-19 infection, as it does in many other viral respiratory tract infections [14,15]. This can result in the depletion of beneficial gut commensals and the proliferation of opportunistic pathogens in the gastrointestinal tract [16]. Zuo et al. found this dysbiotic state to worsen over time, even after recovery from the acute illness, in moderate to severe hospitalised COVID-19 cases [17]. Phetsouphanh et al. identified raised levels of IFN-β, IFN-λ1 and highly activated innate immune cells eight months after COVID-19 diagnosis, while other studies detected the persistence of SARS-CoV-2 viral matter in the intestinal epithelium beyond recovery [18,19,20]. Another mechanism that has been recently suggested is the formation of fibrinolysis-resistant amyloid microclots and platelet pathology in post-COVID syndrome patients [21]. Kell et al. proposed that these microclots may impair tissue perfusion and be a key determinant of post-COVID syndrome [22].
Although, to the best of the authors’ knowledge, no studies thus far have investigated the mental well-being impact of persistent gastrointestinal post-COVID symptoms, the psychosocial impact of functional gastrointestinal disorders (FGIDs) is known to be significant. The similarity between FGIDs and post-COVID gastrointestinal symptoms can be drawn in their clinical features and poorly understood causal mechanisms, both likely including the intestinal microbiota and gut–brain axis [23]. Around half of irritable bowel syndrome (IBS) patients are thought to have concurrent mental health conditions, and symptom-specific anxiety can impair functions of daily life in affected patients [24]. The development of low mood and anxiety states has also been observed in post-COVID syndrome [25].
Given the potential for a substantial physical and mental disease burden of both post-COVID syndrome and gastrointestinal illness and the lack of clinical guidelines on managing post-COVID gastrointestinal sequelae, we conducted a systematic review aiming to summarise the current evidence in two areas. Firstly, to provide an overview of the prevalence of persistent gastrointestinal symptoms following acute SARS-CoV-2 infection, and secondly, to estimate the incidence of newly diagnosed gastrointestinal illness following recent SARS-CoV-2 infection (not including gastrointestinal complications of acute COVID-19).
2. Methods
2.1. Search Strategy
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards for systematic reviews and registered with the International Register of Systematic Reviews (PROSPERO reference CRD42022315792, available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022315792, accessed on 23 July 2023).
We conducted a systematic search of the literature using OVID MedLine, SCOPUS and Europe PubMed Central from1 December 2019 to 3 July 2023. We also searched pre-publication and the other literature indexed in medRxiv. Search terms were constructed around three themes: COVID-19, gastrointestinal symptoms or illness and observational study designs. We did not include terms such as ‘obesity’ due to the volume of unsuitable studies returned. Our search terms were reviewed by all authors and a health science librarian. Our full search strategy is shown in Table 1. Exact searches used for each database are provided in the supplementary files.

Table 1.
Overview of search strategy, including limits.
2.2. Inclusion/Exclusion Criteria
Studies were included in the review if they followed up with participants for ongoing gastrointestinal symptoms or new gastrointestinal illnesses beyond acute COVID-19 infection. We used the following inclusion criteria: (1) follow-up of gastrointestinal symptoms and the development of new gastrointestinal illness from 4 weeks after COVID-19 diagnosis or onset, as per the National Institute for Health and Care Excellence (NICE) UK case definition for acute COVID-19 infection [2]; and (2) observational studies, including cohort, case-control and cross-sectional studies. We excluded studies that (1) were conducted before December 2019; (2) were case reports, opinions, commentaries and interventional studies; (3) included unconfirmed COVID-19 or other SARS-like illnesses; (4) involved animals; and (5) did not meet the inclusion criteria. Pre-print articles were considered acceptable if they met the inclusion criteria.
We anticipated studies reporting persistent symptom prevalence would involve participants reporting a gastrointestinal symptom during acute infection and its persistence beyond four weeks from diagnosis or beyond the resolution of other COVID-19 symptoms. While symptom persistence beyond twelve weeks may warrant a diagnosis of post-COVID syndrome, this diagnostic term was not used earlier in the pandemic and would not detect participants with post-viral symptoms lasting for between four and twelve weeks. Due to the nature of the review topic, we imposed no restrictions on participant age or comorbidity, and studies were not required to include a control or comparator group for inclusion. Two reviewers (MJH and NMV) independently screened the citations, and any discrepancies were resolved by consulting a third reviewer (DH).
2.3. Data Extraction
- The following data were extracted into a spreadsheet for manual review.
- Study details: publication date, journal, authors, year, location, study design and setting (i.e., community or hospital) and funding source.
- Population characteristics including age, number of cases and controls, case definition, illness severity, vaccination status, SARS-CoV-2 variant and diagnostic criteria.
- Acute COVID-19 symptoms that relate to the gastrointestinal system.
- Point prevalence of persistent gastrointestinal symptoms after acute COVID-19, and the timepoint and method for which these symptoms were reported. Post-COVID symptoms reported would be persistent in nature, i.e., participants with a short-lived, unrelated episode of acute gastroenteritis at follow-up would not be captured.
- Incidence of new gastrointestinal illness presenting after recovery from acute COVID-19.
2.4. Risk of Bias and Quality Assessment
Studies eligible for inclusion were quality assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools for observational studies [26]. Quality and risk of bias were assessed by two reviewers independently (MJH and NMV). The JBI Critical Appraisal Tools assess the methodological quality and risk of bias of each study, appraising aspects such as exposure and outcome measurement, controlling for confounding variables, loss of participants to follow-up and appropriateness of statistical analysis. The strength of methodological quality was graded by the percentage of positive answers, with studies scoring ≥ 70% considered high quality, studies scoring 50–69% considered moderate quality and studies scoring ≤ 49% considered low quality [27,28].
2.5. Data Analysis
We conducted a narrative synthesis of the studies to summarise the characteristics of each study, method of diagnosis and gastrointestinal-specific outcome measurements. We then conducted a descriptive analysis of all studies and reported the point prevalence for each symptom and the time at which this was captured. Where possible, persistent symptoms were grouped into one of four categories: diarrhoea (including loose stools and liquid stools); nausea and vomiting (including feeling and being sick); taste and smell disorders (including ageusia, dysgeusia, anosmia and altered taste and smell); and abdominal pain (including stomach ache/pain). We estimated the pooled prevalence for each symptom category across all studies by calculating the weighted average of the number of symptomatic cases divided by the total cohort size. We calculated all pooled prevalence estimates under the Freeman–Tukey double arcsine transformation and reported the 95% confidence intervals obtained under a random-effects model. Funnel plots were used to help identify heterogeneity and bias.
Change in point prevalence was visualised against time from acute COVID-19 resolution. Where participants reported more than one symptom per category (i.e., nausea, vomiting or haematemesis), the highest reported prevalence was used in the analysis. Overall pooled prevalence for any gastrointestinal complaint was only calculated from studies which reported this specifically and not calculated from the raw data to avoid double counting of participants reporting multiple symptoms. We also calculated the median symptom prevalence across all studies and for studies deemed to be at low risk of methodological bias, including interquartile ranges and median follow-up times.
For studies reporting the prevalence of gastrointestinal symptoms in both COVID-19 cases and controls, we calculated the odds ratio of having persistent gastrointestinal symptoms in a COVID-19 cohort vs. healthy controls for each symptom in each study. We calculated the I2 statistic to assess heterogeneity between studies and conducted both common and random effects meta-analyses to estimate overall odds ratios and 95% confidence intervals using the ‘metabin’ function in R package ‘meta’, version 6.0-0 in R version 4.1.0 [29].
3. Results
The initial search after duplicate removal identified 2549 potentially relevant studies; of these, 45 were eligible for inclusion in the review (Figure 1). Each reported the point prevalence of persistent gastrointestinal symptoms at specific timepoints, up to a maximum of 18 months from SARS-CoV-2 infection. The studies were conducted from early 2020 to mid-2023 in twenty-eight different countries across Europe, the Americas, Africa and Asia (Table 2 and Table 3). Forty-one studies were sourced from peer-review journals [8,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Four studies were pre-print and were awaiting peer review at the time of writing [70,71,72,73].
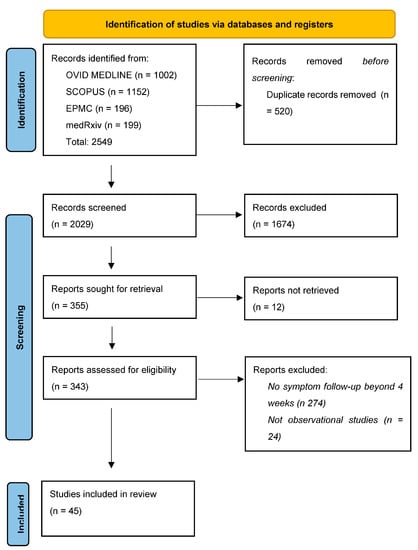
Figure 1.
PRISMA flowchart detailing search results.

Table 2.
Descriptive summary of studies reporting the point prevalence of persistent gastrointestinal symptoms after acute COVID-19.

Table 3.
Descriptive summary of studies reporting the incidence of new gastrointestinal illness after acute SARS-CoV-2 infection.
3.1. Study Characteristics and Outcome Measurement
Most studies (n = 35) followed up participants from COVID-19 diagnosis or acute illness to recovery (Table 2 and Table 3) and assessed for post-viral symptoms thereafter. Four studies followed up patients for a different clinical reason, such as nutritional status and endoscopy, and assessed post-COVID symptoms as a secondary outcome. The remaining six studies were cross-sectional and reported the point prevalence of a persistent symptom in participants who had historically tested positive for SARS-CoV-2 [30,31,32,55,63,70]. Three of these studies had a maximum possible duration between diagnosis and inclusion in the study of 16 months, although, for most participants, this was less than six months [30,31,32]. One cross-sectional study did not specify the study period or time to follow up [70]. Prevalence studies were generally conducted from early 2020 through to 2023, whereas studies reporting incidence were conducted from early 2022 to 2023.
Studies varied in how they defined COVID-19 cases. Twenty-eight studies included only participants with a positive reverse transcription polymerase chain reaction test (RT-PCR) for SARS-CoV-2 [32,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57,58,60,61,62,65,67,68,69,71,72,73]. Seven studies included participants who tested positive via an unspecified diagnostic method [30,33,56,59,64,66,70]. Two database studies included participants with an ICD-10 code for confirmed COVID-19 in their electronic health record (EHR) [8,60]. Three studies included patients with COVID-19 pneumonia diagnosed through a computerised tomography (CT) scan in addition to those diagnosed via RT-PCR [34,35,36]. Two included those diagnosed with clinical symptoms in addition to those diagnosed via RT-PCR and CT scan, but this formed a small percentage of the overall cohort (4% and 16%) [31,37]. One study included participants with COVID-19 illness diagnosed based on clinical history and examination by a physician, in addition to those with laboratory-confirmed SARS-CoV-2 infection [38]. Two studies included participants with positive anti-SARS-CoV-2 antibodies on serological testing, with one also including participants diagnosed via RT-PCR [39,40]. One study included participants reporting either a positive RT-PCR or SARS-CoV-2 rapid antigen test.
Only one study reported SARS-CoV-2 vaccination status; this is reported as a descriptive statistic, however, and is not a covariate used in the analysis [56]. It is likely the vast majority of participants included in other studies were not vaccinated due to the timing of studies in relation to vaccine rollout globally. The studies also varied in their clinical settings and ascertainment of gastrointestinal symptoms. Fourteen studies were conducted on patients who were hospitalised with COVID-19, discharged and followed up in the community [33,34,35,41,42,43,44,45,57,60,62,63,67,71], twenty-one studies involved both hospitalised and community-managed cases [8,30,32,36,37,40,46,47,48,49,50,55,59,61,64,65,66,68,69,70,72], seven studies involved community cases that did not require hospital admission [38,39,51,52,53,56,58] and three studies did not specify the setting of recruitment [31,54,73].
Most studies (n = 27) measured gastrointestinal outcomes using a survey or questionnaire administered via telephone or electronically [30,31,32,36,37,38,39,40,45,46,47,49,50,51,52,53,54,55,56,57,58,59,61,63,65,70,71]. Seven studies used EHRs to identify gastrointestinal endpoints based on clinical codes [8,60,66,67,69,72,73]. Eight studies involved an in-person clinical assessment by a healthcare professional [33,34,35,41,43,44,64,68]. One study involved both a questionnaire and clinical assessment with a healthcare professional [48]. Two studies measured outcomes through both clinical assessment and endoscopy [42,62].
Symptom Prevalence
Overall, the average prevalence of persistent post-COVID gastrointestinal symptoms of any nature and duration was 10.8% when weighted by cohort size (n = 111,198 across seven studies). For two studies reporting the total prevalence of persistent gastrointestinal symptoms of any nature and duration in healthy controls, the weighted average prevalence was 4.9% (n = 106,710). The median prevalence for all symptoms across all studies reporting prevalence data was 5.2% (IQR 9.3; 1.2–10.5, n = 36 studies). For seven studies reporting prevalence and deemed to be at low risk of methodological bias, prevalence estimates ranged from 0.2% to 24.1%, with a median follow-up time of 18 weeks.
Figure 2 shows the prevalence of persistent gastrointestinal symptoms over time in adults. We distinguished between studies that report point prevalence at a specific time point and studies that reported prevalence with minimum symptom duration (i.e., diarrhoea at 12 weeks vs. diarrhoea for at least 12 weeks). For studies reporting symptom prevalence at multiple timepoints, we selected the latest timepoint to avoid double counting of participants. We calculated pooled symptom prevalence for studies reporting diarrhoea, nausea and vomiting, taste and smell disorders and abdominal pain, persisting for between one and 18 months (Table 4).
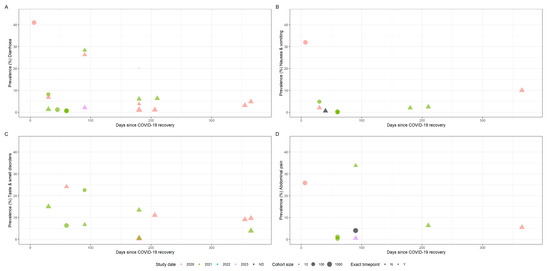
Figure 2.
Time plots showing symptom prevalence against time since acute SARS-CoV-2 infection in adults (A: diarrhoea, B: nausea & vomiting, C: taste & smell disorders, D: abdominal pain). Size of points indicates cohort size. Triangles indicate point prevalence at that exact timepoint, whereas circles indicate studies reporting the lower bound of symptom duration. Colour indicates the study end date by year, whereas black indicates no study end date was specified.

Table 4.
Average pooled prevalence (with 95% confidence interval) of persistent symptoms for any duration between 1 month to 18 months.
Four studies reported the prevalence of diarrhoea, nausea and vomiting, taste and smell disorders and abdominal pain in both a SARS-CoV-2 exposed cohort and an unexposed control group. Funnel plots showed asymmetry, primarily driven by two larger studies in children, which show a protective effect for SARS-CoV-2 exposure against persistent gastrointestinal symptoms [52,55]. Another large study in adults that suggested SARS-CoV-2 to be protective against persistent nausea and diarrhoea, however, found taste and smell disorders to be strongly associated with SARS-CoV-2 exposure. Individual funnel plots for each symptom category are provided as a supplement (Supplementary Figures S1–S4). Each funnel plot shows a pooled estimate calculated under a random effects model, which we do not report due to significant inter-study heterogeneity. However, we report individual odds ratios with 95% confidence intervals for each study (Figure 3).
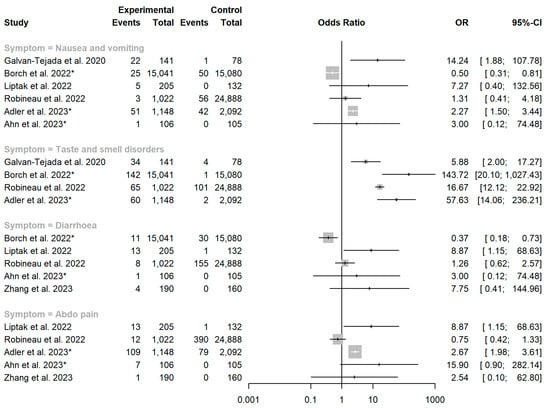
Figure 3.
Forest plot showing the association between SARS-CoV-2 exposure and persistent gastrointestinal manifestations. OR = odds ratio. Asterisk (*) denotes studies conducted in children aged <18 years. Studies included: [40,47,51,52,55,56,68].
3.2. Studies with the Highest Quality Score
Six studies in adults scored very highly for methodological quality and were deemed to be at low risk of bias (Supplementary Table S1). The majority of studies scored poorly due to the absence of a comparator group. Noviello et al. compared persistent gastrointestinal symptoms between a cohort five months after acute COVID-19 and a cohort of healthy controls using the Structured Assessment of Gastrointestinal Symptoms (SAGIS) questionnaire, a validated tool used to identify the presence of a broad range of gastrointestinal illnesses [49,74]. There was a higher prevalence of diarrhoea in the COVID-19 cohort at five months than in healthy controls (21.2% vs. 9.6%, p = 0.05). While the incidence of IBS was similar in both cohorts (adjusted risk ratio 1.07 [95% CI: 0.72–1.60]), the prevalence of diarrhoea was higher in the COVID-19 cohort (adjusted risk ratio 1.88 [95% CI: 0.99–3.54]) [49].
Liptak et al. followed up with patients with moderate to severe COVID-19 and patients with mild COVID-19 seven months after infection and compared them with test-negative controls who visited a hospital emergency department. They reported at least one gastrointestinal symptom in 19% of the moderate to severe group and 7.3% of the mild group, while only 3.0% of the controls had any gastrointestinal symptom (p ≤ 0.01). Diarrhoea and abdominal pain were more prevalent in the COVID-19 cohorts vs. test-negative controls (p < 0.05 for diarrhoea and p < 0.001 for abdominal pain) [47].
Zhang et al. investigated the relationship between gastrointestinal symptoms in acute COVID-19 and the subsequent risk of functional gastrointestinal disorders (FGIDs). They reported a higher incidence of FGIDs at six months in SARS-CoV-2 positive vs. SARS-CoV-2 negative-serology cohorts (8.9% vs. 3.12%, p = 0.025). In multivariate analysis, the development of FGIDs was shown to be associated with gastrointestinal symptoms at COVID-19 onset [68].
Robineau et al. assessed for post-COVID syndrome symptoms using a questionnaire in 25,910 participants after performing home-dried blood spot testing for anti-SARS-CoV-2 antibodies. The presence of nausea, diarrhoea and constipation was weakly associated with SARS-CoV-2 seronegativity (adjusted odds ratios: 0.68 [95% CI: 0.16–1.95] for nausea; 0.61 [95% CI: 0.26–1.27] for diarrhoea; and 0.78 [95% CI: 0.42–1.33] for constipation). Abdominal pain lasting more than two months was significantly associated with negative SARS-CoV-2 serology (adjusted OR 0.42 [95% CI: 0.21–0.74], p = 0.006). Persistent anosmia or dysgeusia was strongly associated with seropositivity (adjusted OR 8.89 [95% CI: 6.03–13.28], p < 0.0001) [40].
Chang et al. conducted a database cohort study to investigate associations between SARS-CoV-2 and subsequent risk for inflammatory bowel disease (IBD) and coeliac disease while adjusting for healthcare-seeking behaviour by using a historical control cohort and propensity-score matching. They identified a hazard ratio for incident inflammatory bowel disease and coeliac disease diagnoses of 1.78 (95%CI: 1.72–1.84) and 2.68 (95%CI: 2.51–2.85), respectively.
3.3. Studies Conducted in Specific Patient Groups
Four studies were conducted in patient cohorts. One study reported the prevalence of post-COVID symptoms in a cohort of 222 IBD patients and found that 42.3% of ulcerative colitis (UC) and 45.9% of Crohn’s disease (CD) patients had symptoms lasting for more than twelve weeks. The most common persistent symptoms were abdominal pain (~11% in CD and ~4% in UC), diarrhoea (~8% in CD and ~3% in UC) and nausea (~7% in CD and ~3% in UC). Vomiting was less common (~0–1% in both groups). Symptoms persistence beyond 12 weeks was associated with discontinuation of immunosuppressive therapy in UC patients and initial hospitalisation in CD patients [46].
Dagher et al. reported gastrointestinal symptoms in 36.9% of a cohort of cancer patients after SARS-CoV-2 infection, with a median symptom duration of seven months. Post-COVID symptoms of any nature were not found to be associated with cancer type, leucopenia, age or need for hospital admission during acute COVID-19, however [59].
Belkacemi et al. investigated persistent post-COVID symptoms in 1217 unvaccinated end-stage renal disease patients undergoing renal replacement therapy (RRT) and reported the point prevalence of diarrhoea and taste and smell disorders at six months as 6% and 2.3%, respectively (n = 216). The risk of having persisting clinical symptoms at six months was higher in patients who were hospitalised with moderate to severe disease (1.64 times) and those requiring intensive care treatment (5.03 times). Older age and longer duration of dialysis were also found to increase the risk of persistent symptoms [37]. Another study on RRT and kidney transplant patients conducted in Thailand reported the prevalence of anorexia and abdominal pain at 90.9% and 62.5%, respectively, after three months. This study also found older age to be associated with gastrointestinal manifestations of post-COVID syndrome [57].
3.4. Children
Five studies included children under 18 years old. Borch et al. reported that, in children aged 6–17 years, diarrhoea and nausea were significantly less prevalent in the SARS-CoV-2 exposed group than in the control group (OR: 0.37, 95% CI: 0.18–0.73) one month after acute COVID-19 recovery [52]. Penner et al. found that 6.5% and 2.6% of children who had been hospitalised for paediatric multisystem inflammatory syndrome (n = 46) experienced abdominal pain and diarrhoea, respectively, at six months. This study also reported raised calprotectin, a biomarker found in faeces indicating gut inflammation, in 31% of children at six weeks and 7% at six months [43].
At 24 weeks post-COVID, Sedik et al. reported abdominal pain in 2% of children (n = 105) and that most children recovered quickly without significant sequelae [65]. A cross-sectional study conducted by Adler et al., however, identified a statistically significant higher prevalence of abdominal pain in children with a history of COVID-19 compared with those without (9.5% vs. 3.8%, p < 0.001) [55]. Both studies found persistent symptoms to be more prevalent in children aged over 11 years. Ahn et al. conducted a case-control study in younger children (median age 3, IQR 1.0–9.0) and identified abdominal pain as the most commonly reported post-COVID symptom persisting for more than two months [56].
3.5. Incidence of Gastrointestinal Illness
Eleven studies reported the incidence of new gastrointestinal illness following SARS-CoV-2 infection. This included various illnesses encompassing functional disorders, motility disorders, hepatic and biliary disorders, autoimmune-mediated illness and infection.
Stepan et al. found that children who visited the emergency department with abdominal pain and tested positive for SARS-CoV-2 either six months before or three months after had a higher incidence of irritable bowel syndrome (IBS) than those who tested negative (91.3% vs. 54.5%, p = 0.044) [44].
Ghoshal et al. reported the incidence of new post-infection IBS, uninvestigated dyspepsia (UD) and IBS-UD overlap to be 5.3%, 2.1% and 1.8%, respectively (n = 280), in those who tested positive for SARS-CoV-2 six months prior. Kaplan–Meier analysis showed a higher probability of developing such illnesses following SARS-CoV-2 exposure vs. in healthy controls [50]. Austhof et al. reported the incidence of post-infection IBS to be 20.4% in a smaller study of 49 participants who completed a survey at six months, with half of these meeting the Rome IV diagnostic criteria for IBS [54]. In another study, multivariate logistic regression identified the presence of nausea and diarrhoea during acute SARS-CoV-2 infection as predictors for the development of IBS (OR 4.00 95% CI: 1.01–15.84 and OR 5.64 95% CI: 1.21–26.31, respectively) [67].
Xu et al. ascertained the incident diagnoses of gastro-oesophageal reflux disease, peptic ulcer disease, acute pancreatitis, functional dyspepsia, acute gastritis, IBS and cholangitis in a cohort 30 days post-acute COVID-19 and a contemporary and pre-pandemic control cohort. The hazard ratio for a composite of all incident diagnoses was 1.37 (95% CI: 1.33–1.14), with cholangitis exhibiting the highest hazard of 2.02 (1.55–2.63). The COVID-19 cohort also displayed a higher hazard for abnormal coagulation studies and deranged liver function tests (HR 1.59 95% CI: 1.52–1.65 and HR 1.30 95% CI: 1.28–1.32) [66]. In a larger study, Ma et al. also reported a higher hazard for FGIDs, peptic ulcer disease, gastro-oesophageal reflux disease, gallbladder disease, severe liver disease, non-alcoholic fatty liver disease and pancreatic disease [72].
Two studies reported the incidence of autoimmune-mediated gastrointestinal illness following previous SARS-CoV-2 exposure. One study reported a hazard of 1.40 (95%CI 1.02–1.90) for IBD. Another reported a hazard of 1.78 (95%CI: 1.72–1.84) for IBD and 2.68 (95%CI: 2.51–2.85) for coeliac disease, the latter adjusted for healthcare-seeking behaviour [69,72].
4. Discussion
In this systematic review of 45 studies and 2,224,790 patients, we identified a relatively low (median = 5.2%) and variable (IQR 9.3; 1.2–10.5, n = 36 studies) prevalence of persistent gastrointestinal symptoms following SARS-CoV-2 infection. Prevalence estimates for persistent symptoms of any duration ranged from 0.2% to 24.1% for seven studies judged to be at low risk of bias, with a median follow-up time of 18 weeks. A higher rate of incident gastrointestinal illness, including functional disorders, motility disorders, autoimmune-mediated illness and hepatic and biliary disorders, were also observed after SARS-CoV-2 infection.
We found extensive inter-study heterogeneity, as expected when synthesising data from multiple observational studies across a variety of settings in changing circumstances. The studies with the highest methodological quality indicated that substantial numbers of individuals may be susceptible to persistent post-COVID gastrointestinal symptoms. We identified a higher pooled prevalence of diarrhoea, abdominal pain, nausea and vomiting in adults previously exposed to SARS-CoV-2 compared with controls. However, we do not report a reliable pooled effect estimate due to inter-study heterogeneity. Given the rapid spread of SARS-CoV-2 globally and recent UK estimates of post-COVID syndrome prevalence of 3.3%, a substantial proportion of those with chronic gastrointestinal complaints in the general population may be attributable to SARS-CoV-2 [3,75].
The prevalence of post-COVID syndrome may vary depending on the SARS-CoV-2 variant and the healthcare-seeking behaviour of the population. COVID-19 illness severity and hospitalisation rates are known to differ between the original wild-type SARS-CoV-2 and later variants [76,77]. As such, SARS-CoV-2 variant epochs may account for much of the variation in symptom prevalence over time and place that we found in this review. One study in our review reported the SARS-CoV-2 vaccination rate; however, this was not used as a covariate in any analysis [56]. Healthcare-seeking behaviour was also known to change throughout the COVID-19 pandemic; healthcare avoidance may result in an underestimation of the true frequency of gastrointestinal events in a population, particularly for studies requiring an in-person clinical review [78].
Of the studies reporting the incidence of gastrointestinal illness post-COVID, one found that, in children attending the emergency department with functional abdominal pain, those with historical SARS-CoV-2 infection were significantly more likely to be diagnosed with IBS than abdominal migraine compared with pre-pandemic controls [44]. This finding is in keeping with the hypothesis that SARS-CoV-2 infection may trigger post-infection functional gastrointestinal symptoms, whereas the aetiology of abdominal migraine is more likely to be non-infectious [79]. Similarly, four studies reported a higher incidence of IBS between three and six months after COVID-19; however, one study in our review did not find any significant difference.
In contrast, a cohort study of children assessing self-reported symptoms suggested that previous SARS-CoV-2 infection protects against persistent diarrhoea and nausea one month after subsequent COVID-19 diagnosis. Although younger child age has been shown to be protective against COVID-19 mortality, this study did not consider that SARS-CoV-2-positive children and their families were likely to be isolated from other causes of infectious diarrhoea during the study period [52,80].
Two studies in our review reported an association between SARS-CoV-2 infection and the development of autoimmune-mediated gastrointestinal illness. This may partly be explained by the molecular mimicry hypothesis, suggesting that SARS-CoV-2 dysregulates the hosts’ humoral immune system as a key factor in inducing autoimmunity in predisposed individuals [81]. Given the lag time between SARS-CoV-2 infection and the development and diagnosis of illnesses such as IBD and coeliac disease, it is possible that the increased incidence of such illnesses may not be evident for several years.
It was not possible to accurately estimate the impact of prior SARS-CoV-2 exposure on the future risk of gastrointestinal infections based on the data presented in our review. Although limited, current research suggests that rates of Clostridium difficile infection are no different between SARS-CoV-2-exposed and -unexposed patients [82]. One study in our review reported no significant association between previous SARS-CoV-2 infection and hospitalisation for gastrointestinal infections in older adults. Ascertaining cases of gastrointestinal infection in the general population is complicated, however, by the low frequency of testing and self-limiting symptoms, with relatively few cases presenting to secondary healthcare. It is also likely that non-pharmaceutical interventions (NPIs) intended to reduce COVID-19 transmission have also significantly reduced the transmission and case rates of enteric infections [83]. Therefore, if there is any excess risk and burden of enteric disease associated with SARS-CoV-2 infection, this would not be detected in these studies and may warrant further longitudinal follow-up and investigation.
Most studies did not control for antibiotic exposure during acute SARS-CoV-2 infection, despite up to three-quarters of COVID-19 patients receiving antibiotics early in the pandemic [84]. One study included in our review found that individuals treated with antibiotics during acute infection were more likely to have persistent diarrhoea than those who did not; another identified antibiotic exposure as the strongest predictor for post-COVID gastrointestinal sequelae [47,49]. Interestingly, Ghoshal et al. reported that all patients with persistent dysphagia received either oral or intravenous antibiotics during acute COVID-19 [50]. None of these studies, however, reported the incidence of bacterial co-infection in patients who received antibiotics.
Although not included in our initial extraction criteria, post hoc analysis identified only two studies reporting the incidence of low mood among patients with persistent post-COVID gastrointestinal symptoms, or vice-versa. Both studies did not find any significant associations between persistent gastrointestinal symptoms and symptoms of low mood, anxiety and sleep disturbance [49,68]. This is surprising considering that a higher prevalence of mental health conditions has been reported among those with functional gastrointestinal disorders during COVID-19 lockdowns and emerging evidence of mechanisms linking depression and gut health [85,86].
5. Limitations
Our review aimed to estimate the prevalence of persistent post-COVID gastrointestinal symptoms, but we faced several methodological challenges. Post-COVID syndrome is a dynamic condition that relapses and remits over time, so point prevalence estimates may not capture its true burden [87]. Half of the studies included were based on hospital cohorts of patients suffering more severe disease, which may overestimate symptom prevalence due to ascertainment bias. Our review supports the observations that post-COVID syndrome is more common in patients with a history of severe COVID-19 [37,47]. Hospitalised patients would generally be more likely to suffer comorbidities, including presentations with gastrointestinal symptoms [88]. Finally, six cross-sectional studies included in our review may be at a higher risk of recall bias, given the nature of recalling details of COVID-19 infection and providing a clinical history of gastrointestinal complaints retrospectively [30,31,55,63,70,71].
6. Conclusions
Our review found a generally low prevalence of persistent gastrointestinal symptoms up to eighteen months after COVID-19 recovery. However, most studies lacked comparator groups, so we could not determine whether this differs from background rates of gastrointestinal illness in the general population. We did identify—albeit from limited data—that individuals previously exposed to SARS-CoV-2 may be more likely to develop gastrointestinal illnesses, including IBS, dyspepsia, hepatic and biliary disease and autoimmune-mediated illnesses, than the general population. Significant heterogeneity between studies overall prevented us from providing reliable pooled estimates of long-lasting gastrointestinal consequences of SARS-CoV-2 infection. Ideally, COVID-19 studies would have included prospective observation of SARS-CoV-2-infected participants for the development of gastrointestinal complaints, accounting for vaccination, antimicrobial use, variant epochs and public health interventions. Given the established links between gut dysbiosis and a wide range of viral infections, new studies should also monitor the excess risk of enteric infections after the removal of COVID-related NPIs, and future pandemic preparedness would do well to include proactive surveillance of gastrointestinal infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15081625/s1, File S1: Search terms used for OVID MedLine, SCOPUS, Europe PubMed Central and medRxiv, Figure S1: Funnel plot of study bias for five studies reporting an odds ratio for persistent diarrhoea, Figure S2: Funnel plot of study bias for six studies reporting an odds ratio for persistent nausea and vomiting, Figure S3: Funnel plot of study bias for four studies reporting an odds ratio for persistent taste and smell disorders, Figure S4: Funnel plot of study bias for five studies reporting an odds ratio for persistent abdominal pain, Table S1: Risk of bias assessment, including justification for studies deemed to be of moderate or low quality.
Author Contributions
Conceptualisation, D.H. and I.B.; study design and methodology, D.H., I.B., N.M.V. and M.J.H.; literature and reference search, N.M.V. and M.J.H.; writing—preparation of the original draft, M.J.H.; analysis, quality assessment, analysis, tables and figure preparation, L.B., N.M.V. and M.J.H.; supervision, D.H., D.C., I.B., A.J.E. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the National Institute for Health and Care Research (NIHR) Health Protection Research Unit in Gastrointestinal Infections, a partnership between UK Health Security Agency, the University of Liverpool and the University of Warwick. The views expressed are those of the author(s) and not necessarily those of the NIHR, UK Health Security Agency or the Department of Health and Social Care. A.J.E. received support from the NIHR HPRU in Emergency Preparedness and Response at King’s College London. D.H. was funded by an NIHR post-doctoral fellowship (PDF-2018-11-ST2-006). I.B. was supported by an NIHR senior investigator award. Funding number: NIHR-200910.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data produced in the present work are contained in the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 18 July 2023).
- National Institute for Health and Care Excellence. Overview|COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/NG188 (accessed on 11 April 2023).
- Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK–Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/5january2023 (accessed on 11 April 2023).
- Mair, M.; Singhavi, H.; Pai, A.; Singhavi, J.; Gandhi, P.; Conboy, P.; Baker, A.; Das Frcs, S. A Meta-Analysis of 67 Studies with Presenting Symptoms and Laboratory Tests of COVID-19 Patients. Laryngoscope 2021, 131, 1254–1265. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Kline, A.; Elgaml, A.; Aboutaleb, M.H.; Salim, M.M.; Omar, M.; Munshi, R.; Mankowski, N.; Hussein, M.H.; Attia, A.S.; et al. Gastroenterology manifestations and COVID-19 outcomes: A meta-analysis of 25,252 cohorts among the first and second waves. J. Med. Virol. 2021, 93, 2740–2768. [Google Scholar] [CrossRef]
- Graham, G.; Taegtmeyer, M.; Lewis, J.; Subramanian, S. Gastrointestinal symptoms involvement in hospitalised COVID-19 patients in Liverpool, UK: A descriptive cross-sectional, single-centre study. Clin. Med. 2021, 21 (Suppl. S2), 23–24. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, E.S.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Chowdhury, M.; Singh, A.K.; Ma, K.; Kumar, A.; Ranjan, P.; Desai, D.; Wig, N. Clinical predictors of long COVID-19 and phenotypes of mild COVID-19 at a tertiary care centre in India. Drug Discov. Ther. 2021, 15, 156–161. [Google Scholar] [CrossRef]
- Meringer, H.; Mehandru, S. Gastrointestinal post-acute COVID-19 syndrome. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 345–346. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, X.; Fu, W.; Xiang, F.; He, X.; Yang, B.; Wang, X.; Ma, W.-L. Gut Microbiota Dysbiosis Correlates with Abnormal Immune Response in Moderate COVID-19 Patients with Fever. J. Inflamm. Res. 2021, 14, 2619–2631. [Google Scholar] [CrossRef]
- Hussain, I.; Cher, G.L.Y.; Abid, M.B. Role of Gut Microbiome in COVID-19: An Insight Into Pathogenesis and Therapeutic Potential. Front. Immunol. 2021, 12, 765965. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, C.; Faiq, M.A.; Pareek, V.; Narayan, R.K. SARS-CoV-2 Infectivity Vis-A-Vis Human Gut and Mesentery: Pathogenic Implications for COVID-19. SSRN Electron. J. 2020, 93, 1343–1350. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, G.Y.; Myoung, J.; Kim, S.-J.; Ahn, D.-G. Robust and persistent SARS-CoV-2 infection in the human intestinal brush border expressing cells. Emerg. Microbes Infect. 2020, 9, 2169–2179. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Fikree, A.; Byrne, P. Management of functional gastrointestinal disorders. Clin. Med. 2021, 21, 44–52. [Google Scholar] [CrossRef]
- Ballou, S.; Bedell, A.; Keefer, L. Psychosocial impact of irritable bowel syndrome: A brief review. World. J. Gastrointest. Pathophysiol. 2015, 6, 120–123. [Google Scholar] [CrossRef]
- Fancourt, D.; Steptoe, A.; Bu, F. Long-term psychological consequences of long Covid: A propensity score matching analysis comparing trajectories of depression and anxiety symptoms before and after contracting long Covid vs. short Covid. MedRxiv 2022. [Google Scholar] [CrossRef]
- MigliaMigliavaca, C.B.; Stein, C.; Colpani, V.; Munn, Z.; Falavigna, M. Prevalence Estimates Reviews––Systematic Review Methodology Group (PERSyst). Quality assessment of prevalence studies: A systematic review. J. Clin. Epidemiol. 2020, 127, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Halverson, C.C.; Bailey, C.; Ennis, J.A.; Cox, E.E. Nursing surveillance of respiratory adverse events among hospitalized adults: A systematic review to guide evidence-based practice. Worldviews Evid. Based Nurs. 2022, 19, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.; Cheng, C.P.W.; Fong, T.K.H.; Sharew, N.T.; Anders, R.L.; Xiang, Y.T.; Lam, S.C. Psychological impact on healthcare workers, general population and affected individuals of SARS and COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1004558. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Islam, M.S.; Ferdous, M.Z.; Islam, U.S.; Mosaddek, A.S.M.; Potenza, M.N.; Pardhan, S. Treatment, Persistent Symptoms, and Depression in People Infected with COVID-19 in Bangladesh. Int. J. Environ. Res. Public Health 2021, 18, 1453. [Google Scholar] [CrossRef]
- Khodeir, M.M.; Shabana, H.A.; Rasheed, Z.; Alkhamiss, A.S.; Khodeir, M.; Alkhowailed, M.S.; Alharbi, S.; Alsoghair, M.; Alsagaby, S.A.; Al Abdulmonem, W. COVID-19: Post-recovery long-term symptoms among patients in Saudi Arabia. PLoS ONE 2021, 16, e0260259. [Google Scholar] [CrossRef]
- Qamar, M.A.; Martins, R.S.; Dhillon, R.A.; Tharwani, A.; Irfan, O.; Suriya, Q.F.; Rizwan, W.; Khan, J.A.; Zubairi, A.b.S. Residual symptoms and the quality of life in individuals recovered from COVID-19 infection: A survey from Pakistan. Ann. Med. Surg. 2022, 75, 103361. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, M.-F.; Agier, L.; Martineau, C.; Philipponneau, M.; Romand, D.; Masdoua, V.; Behar, M.; Nesseler, C.; Achamrah, N.; Laubé, V.; et al. Food intake and weight loss of surviving inpatients in the course of COVID-19 infection: A longitudinal study of the multicenter NutriCoviD30 cohort. Nutr. Burbank 2022, 93, 111433. [Google Scholar] [CrossRef]
- Gérard, M.; Mahmutovic, M.; Malgras, A.; Michot, N.; Scheyer, N.; Jaussaud, R.; Nguyen-Thi, P.-L.; Quilliot, D. Long-Term Evolution of Malnutrition and Loss of Muscle Strength after COVID-19: A Major and Neglected Component of Long COVID-19. Nutrients 2021, 13, 3964. [Google Scholar] [CrossRef] [PubMed]
- Damanti, S.; Cilla, M.; Cilona, M.; Fici, A.; Merolla, A.; Pacioni, G.; De Lorenzo, R.; Martinenghi, S.; Vitali, G.; Magnaghi, C.; et al. Prevalence of Long COVID-19 Symptoms After Hospital Discharge in Frail and Robust Patients. Front. Med. 2022, 9, 834887. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, F.; Güneri, F.D.; Kardeş, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, M.; Baouche, H.; Gomis, S.; Lassalle, M.; Couchoud, C.; Registry, T.R. Long-lasting clinical symptoms 6 months after COVID-19 infection in the French national cohort of patients on dialysis. J. Nephrol. 2022, 35, 787–793. [Google Scholar] [CrossRef]
- Wu, Q.; Ailshire, J.A.; Crimmins, E.M. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci. Rep. 2022, 12, 11647. [Google Scholar] [CrossRef]
- Faycal, A.; Ndoadoumgue, A.; Sellem, B.; Blanc, C.; Dudoit, Y.; Schneider, L.; Tubiana, R.; Valantin, M.-A.; Seang, S.; Palich, R.; et al. Prevalence and factors associated with symptom persistence: A prospective study of 429 mild COVID-19 outpatients. Infect. Dis. Now 2022, 52, 75–81. [Google Scholar] [CrossRef]
- Robineau, O.; Wiernik, E.; Lemogne, C.; de Lamballerie, X.; Ninove, L.; Blanché, H.; Deleuze, J.-F.; Ribet, C.; Kab, S.; Goldberg, M.; et al. Persistent symptoms after the first wave of COVID-19 in relation to SARS-CoV-2 serology and experience of acute symptoms: A nested survey in a population-based cohort. Lancet Reg. Health Eur. 2022, 17, 100363. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.-L.; Wang, X. Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418. [Google Scholar] [CrossRef]
- Xie, X.; Sheng, L.; Han, C.; Jin, Y.; Bai, T.; Lin, R.; Ding, Z.; Hou, X. Features of capsule endoscopy in COVID-19 patients with a six-month follow-up: A prospective observational study. J. Med. Virol. 2022, 94, 246–252. [Google Scholar] [CrossRef]
- Penner, J.; Abdel-Mannan, O.; Grant, K.; Maillard, S.; Kucera, F.; Hassell, J.; Eyre, M.; Berger, Z.; Hacohen, Y.; Moshal, K.; et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. Lancet Child. Adolesc. Health 2021, 5, 473–482. [Google Scholar] [CrossRef]
- Stepan, M.D.; Cioboata, R.; Vintilescu, B.; Vasile, C.M.; Osman, A.; Ciolofan, M.S.; Popescu, M.; Petrovici, I.L.; Zavate, A.C. Pediatric Functional Abdominal Pain Disorders following COVID-19. Life 2022, 12, 509. [Google Scholar] [CrossRef]
- Comelli, A.; Viero, G.; Bettini, G.; Nobili, A.; Tettamanti, M.; Galbussera, A.A.; Muscatello, A.; Mantero, M.; Canetta, C.; Boneschi, F.M.; et al. Patient-Reported Symptoms and Sequelae 12 Months After COVID-19 in Hospitalized Adults: A Multicenter Long-Term Follow-Up Study. Front. Med. 2022, 9, 834354. [Google Scholar] [CrossRef]
- Attauabi, M.; Dahlerup, J.F.; Poulsen, A.; Hansen, M.R.; Vester-Andersen, M.K.; Eraslan, S.; Prahm, A.P.; Pedersen, N.; Larsen, L.; Jess, T.; et al. Outcomes and Long-Term Effects of COVID-19 in Patients with Inflammatory Bowel Diseases–A Danish Prospective Population-Based Cohort Study with Individual-Level Data. J. Crohns Colitis 2021, 16, jjab192. [Google Scholar] [CrossRef]
- Liptak, P.; Duricek, M.; Rosolanka, R.; Ziacikova, I.; Kocan, I.; Uhrik, P.; Grendar, M.; Hrnciarova, M.; Bucova, P.; Galo, D.; et al. Gastrointestinal sequalae months after severe acute respiratory syndrome corona virus 2 infection: A prospective, observational study. Eur. J. Gastroenterol. Hepatol. 2022, 34, 925. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Noviello, D.; Costantino, A.; Muscatello, A.; Bandera, A.; Consonni, D.; Vecchi, M.; Basilisco, G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol. Motil. 2022, 34, e14187. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U.; Rahman, M.M.; Mathur, A.; Rai, S.; Akhter, M.; Mostafa, T.; Islam, M.S.; Haque, S.A.; Pandey, A.; et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A case–control study. J. Gastroenterol. Hepatol. 2022, 37, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Galván-Tejada, C.E.; Herrera-García, C.F.; Godina-González, S.; Villagrana-Bañuelos, K.E.; Amaro, J.D.D.L.; Herrera-García, K.; Rodríguez-Quiñones, C.; Zanella-Calzada, L.A.; Ramírez-Barranco, J.; de Avila, J.L.R.; et al. Persistence of COVID-19 Symptoms after Recovery in Mexican Population. Int. J. Environ. Res. Public Health 2020, 17, 9367. [Google Scholar] [CrossRef]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef]
- Fernández-Plata, R.; Higuera-Iglesias, A.-L.; Torres-Espíndola, L.M.; Aquino-Gálvez, A.; Cruz, R.V.; Camarena, A.; Alderete, J.C.; García, J.R.; Alvarado-Vásquez, N.; Briseño, D.M.; et al. Risk of Pulmonary Fibrosis and Persistent Symptoms Post-COVID-19 in a Cohort of Outpatient Health Workers. Viruses 2022, 14, 1843. [Google Scholar] [CrossRef]
- Austhof, E.; Bell, M.L.; Riddle, M.S.; Catalfamo, C.; McFadden, C.; Cooper, K.; Walter, E.S.; Jacobs, E.; Pogreba-Brown, K. Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: Results from the Arizona CoVHORT. Epidemiol. Infect. 2022, 150, e136. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.; Israel, M.; Yehoshua, I.; Azuri, J.; Hoffman, R.; Shahar, A.; Reuveni, M.M.; Grossman, Z. Long COVID symptoms in Israeli children with and without a history of SARS-CoV-2 infection: A cross-sectional study. BMJ Open 2023, 13, e064155. [Google Scholar] [CrossRef] [PubMed]
- Bin Ahn, B.; Choi, S.H.; Yun, K.W. Non-neuropsychiatric Long COVID Symptoms in Children Visiting a Pediatric Infectious Disease Clinic After an Omicron Surge. Pediatr. Infect. Dis. J. 2023, 42, e143. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Kamolratanakul, S.; Leelahavanichkul, A.; Ariyanon, W.; Chinpraditsuk, S.; Saelim, R.; Vadcharavivad, S.; Phumratanaprapin, W.; Wilairatana, P. Gastrointestinal manifestations of long-term effects after COVID-19 infection in patients with dialysis or kidney transplantation: An observational cohort study. World. J. Gastroenterol. 2023, 29, 3013–3026. [Google Scholar] [CrossRef]
- Da Costa e Silva, G.R.; Moura Winny, É.A.; dos Santos, K.C.; Gomes, D.O.; Bandeira, G.N.; Guimarães, R.A.; Rosso, C.F.W.; Bazilio, G.S.; Leite, V.R.M.C.; Caetano, K.A.A.; et al. Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 1483. [Google Scholar] [CrossRef]
- Dagher, H.; Chaftari, A.-M.; Subbiah, I.M.; Malek, E.A.; Jiang, Y.; Lamie, P.; Granwehr, B.; John, T.; Yepez, E.; Borjan, J.; et al. Long COVID in cancer patients: Preponderance of symptoms in majority of patients over long time period. eLife 2023, 12, e81182. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Torres-Macho, J.; Guijarro, C.; Martín-Guerrero, J.D.; Pellicer-Valero, O.J.; Plaza-Manzano, G. Trajectory of Gastrointestinal Symptoms in Previously Hospitalized COVID-19 Survivors: The Long COVID Experience Multicenter Study. Viruses 2023, 15, 1134. [Google Scholar] [CrossRef]
- Fischer, A.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Staub, T.; Nazarov, P.V.; Ollert, M.; Fagherazzi, G. Long COVID Symptomatology After 12 Months and Its Impact on Quality of Life According to Initial Coronavirus Disease 2019 Disease Severity. Open Forum Infect. Dis. 2022, 9, ofac397. [Google Scholar] [CrossRef]
- Golla, R.; Vuyyuru, S.; Kante, B.; Kumar, P.; Thomas, D.M.; Makharia, G.; Kedia, S.; Ahuja, V. Long-term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 789–796. [Google Scholar] [CrossRef]
- Imoto, W.; Yamada, K.; Kawai, R.; Imai, T.; Kawamoto, K.; Uji, M.; Kanda, H.; Takada, M.; Ohno, Y.; Ohtani, H.; et al. A cross-sectional, multicenter survey of the prevalence and risk factors for Long COVID. Sci. Rep. 2022, 12, 22413. [Google Scholar] [CrossRef]
- Karuna, S.; A Gallardo-Cartagena, J.; Theodore, D.; Hunidzarira, P.; Montenegro-Idrogo, J.; Hu, J.; Jones, M.; Kim, V.; De La Grecca, R.; Trahey, M.; et al. Post-COVID symptom profiles and duration in a global convalescent COVID-19 observational cohort: Correlations with demographics, medical history, acute COVID-19 severity and global region. J. Glob. Health 2023, 13, 06020. [Google Scholar] [CrossRef]
- Sedik, R.N.M. The clinical course and outcomes of SARS-CoV-2 virus infection in children: A 24-week follow-up study in Sulaimaniyah, Iraq. BMC Pediatr. 2023, 23, 303. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Yamamoto, A.; Masaoka, T.; Homma, K.; Matsuoka, T.; Takemura, R.; Wada, M.; Sasaki, J.; Kanai, T.; Amagai, M.; et al. Early symptoms preceding post-infectious irritable bowel syndrome following COVID-19: A retrospective observational study incorporating daily gastrointestinal symptoms. BMC Gastroenterol. 2023, 23, 108. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Xie, Y.; Zeng, F.; Chen, S.; Chen, R.; Zhang, X.; Huang, S.; Li, D.; Bai, F. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A prospective follow-up cohort study. BMC Infect. Dis. 2023, 23, 422. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chen, T.Y.-T.; Wang, S.-I.; Hung, Y.-M.; Chen, H.-Y.; Wei, C.-C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.V.; Gella, V.; Radhakrishna, M.; Kumar, V.J.; Chatterjee, R.; Kulkarni, A.; Reddy, D.N. Post-COVID-19 symptoms are not uncommon among recovered patients-A cross-sectional online survey among the Indian population. MedRxiv 2021. [Google Scholar] [CrossRef]
- Fatima, G.; Bhatt, D.; Idrees, J.; Khalid, B.; Mahdi, F. Elucidating Post-COVID-19 manifestations in India. MedRxiv 2021. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Wei, R.; Dai, W.; Zeng, R.; Luo, D.; Jiang, R.; Wu, H.; Zhuo, A.; Yang, Q.; et al. Risks of digestive diseases in long COVID: Evidence from a large-scale cohort study. MedRxiv 2023. [Google Scholar] [CrossRef]
- Andersson, N.W.; Thiesson, E.M.; Lassaunière, R.; Hansen, J.V.; Hviid, A. SARS-CoV-2 infection and post-acute risk of non-Covid-19 infectious disease hospitalizations: A nationwide cohort study of Danish adults aged ≥50 years. MedRxiv 2023. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Hammer, J.; von Wulffen, M.; Shah, A.; Hoelz, H.; Kutyla, M.; Burger, D.; Martin, N.; Gurusamy, S.R.; et al. The Validity of a New Structured Assessment of Gastrointestinal Symptoms Scale (SAGIS) for Evaluating Symptoms in the Clinical Setting. Dig. Dis. Sci 2017, 62, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Daines, L.; Zheng, B.; Pfeffer, P.; Hurst, J.R.; Sheikh, A. A clinical review of long-COVID with a focus on the respiratory system. Curr. Opin. Pulm. Med 2022, 28, 174–179. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Tang, X.; He, D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Front. Public Health 2021, 9, 775224. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Splinter, M.J.; Velek, P.; Ikram, M.K.; Kieboom, B.C.T.; Peeters, R.P.; Bindels, P.J.E.; Wolters, F.J.; Leening, M.J.G.; de Schepper, E.I.T.; Licher, S. Prevalence and determinants of healthcare avoidance during the COVID-19 pandemic: A population-based cross-sectional study. PLoS Med. 2021, 18, e1003854. [Google Scholar] [CrossRef]
- Mani, J.; Madani, S. Pediatric abdominal migraine: Current perspectives on a lesser known entity. Pediatr. Health Med. Ther. 2018, 9, 47–58. [Google Scholar] [CrossRef]
- Khera, N.; Santesmasses, D.; Kerepesi, C.; Gladyshev, V.N. COVID-19 mortality rate in children is U-shaped. Aging 2021, 13, 19954–19962. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Granata, G.; Petrosillo, N.; Al Moghazi, S.; Caraffa, E.; Puro, V.; Tillotson, G.; Cataldo, M.A. The burden of Clostridioides difficile infection in COVID-19 patients: A systematic review and meta-analysis. Anaerobe 2022, 74, 102484. [Google Scholar] [CrossRef]
- Love, N.K.; Elliot, A.J.; Chalmers, R.M.; Douglas, A.; Gharbia, S.; McCormick, J.; Hughes, H.; Morbey, R.; Oliver, I.; Vivancos, R.; et al. Impact of the COVID-19 pandemic on gastrointestinal infection trends in England, February-July 2020. BMJ Open 2022, 12, e050469. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Alzahrani, M.A.; Alshamrani, A.S.; Ahmasani, I.M.; Alahmari, F.S.; Asiri, A.H.; Alshehri, A.M.; Alsamghan, A.S.; Awadalla, N.J. Coronavirus disease 2019 pandemic stress and its effects on irritable bowel syndrome patients in Saudi Arabia. Medicine 2020, 99, e23711. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.-S.; Huang, J.; Wong, Y.-Y.; Wong, G.L.-H.; Yip, T.C.-F.; Chan, R.N.-Y.; Chau, S.W.-H.; Ng, S.-C.; Wing, Y.-K.; Chan, F.K.-L. Epidemiology, Symptomatology, and Risk Factors for Long COVID Symptoms: Population-Based, Multicenter Study. JMIR Public Health Surveill. 2023, 9, e42315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).